Abstract
Recent work has renewed interest in therapies targeting the renin-angiotensin system (RAS) to improve β-cell function in type 2 diabetes. Studies show that generation of angiotensin-(1–7) by ACE2 and its binding to the Mas receptor (MasR) improves glucose homeostasis, partly by enhancing glucose-stimulated insulin secretion (GSIS). Thus, islet ACE2 upregulation is viewed as a desirable therapeutic goal. Here, we show that, although endogenous islet ACE2 expression is sparse, its inhibition abrogates angiotensin-(1–7)–mediated GSIS. However, a more widely expressed islet peptidase, neprilysin, degrades angiotensin-(1–7) into several peptides. In neprilysin-deficient mouse islets, angiotensin-(1–7) and neprilysin-derived degradation products angiotensin-(1–4), angiotensin-(5–7), and angiotensin-(3–4) failed to enhance GSIS. Conversely, angiotensin-(1–2) enhanced GSIS in both neprilysin-deficient and wild-type islets. Rather than mediating this effect via activation of the G-protein–coupled receptor (GPCR) MasR, angiotensin-(1–2) was found to signal via another GPCR, namely GPCR family C group 6 member A (GPRC6A). In conclusion, in islets, intact angiotensin-(1–7) is not the primary mediator of beneficial effects ascribed to the ACE2/angiotensin-(1–7)/MasR axis. Our findings warrant caution for the concurrent use of angiotensin-(1–7) compounds and neprilysin inhibitors as therapies for diabetes.
Introduction
The renin-angiotensin system (RAS) is a major regulator of blood pressure and fluid balance, with its role in mediating other physiological effects recently being the subject of intense investigation. The classic view is that angiotensinogen is hydrolyzed by renin to produce angiotensin I, which is subsequently hydrolyzed by ACE to generate angiotensin II. Angiotensin II is biologically active, primarily binding angiotensin II receptor type I (AT1) to mediate its effects. This cascade is known as the ACE/angiotensin II/AT1 axis.
Discovery of more components of the RAS has seen the classic model evolve, wherein the ACE2/angiotensin-(1–7)/Mas receptor (MasR) axis was proposed. This involves the generation of angiotensin-(1–7) directly from angiotensin I or angiotensin II by ACE2 or indirectly from angiotensin-(1–9) (1–3). Angiotensin-(1–7) binds the G-protein–coupled receptor (GPCR) MasR (4) to elicit responses that can counteract those of the ACE/angiotensin II/AT1 axis.
Another peptidase capable of generating angiotensin-(1–7) is neprilysin (5–7). Kinetic studies of peptide cleavage showed that neprilysin more efficiently hydrolyzed angiotensin I to angiotensin-(1–7) compared with ACE2 (1). Also, angiotensin-(1–9) was cleaved preferentially by neprilysin to generate angiotensin-(1–7), which was further cleaved by neprilysin to several smaller peptides (1,6,8). Whether these small peptides bind the MasR remains unknown.
The existence of a local RAS has been reported in many tissues, including the pancreatic islet (9). The islet RAS plays important roles in regulating local blood flow, insulin biosynthesis and secretion, and β-cell survival (10,11). Previously, we demonstrated that neprilysin is expressed in islets (12). Despite this, the effects of angiotensin-(1–7) on islet function have been attributed to intact angiotensin-(1–7) per se, rather than neprilysin-derived degradation products. Moreover, emphasis has been on ACE2 as the primary enzyme governing the generation of angiotensin-(1–7) and its beneficial effects; however, whether activity and localization of endogenous ACE2 within the islet is sufficient to promote insulin secretion is unclear. Here, we used islets from neprilysin-deficient mice to determine whether lack of neprilysin activity limits the ability of angiotensin-(1–7) to promote insulin secretion. We identify the cellular localization of endogenous islet ACE2, compare this to the known expression pattern of neprilysin, and establish whether ACE2 inhibition also affects angiotensin-(1–7) action.
Research Design and Methods
Animals
C57BL/6.NEP−/− mice (originally from Dr. Lu, Harvard Medical School, Boston, MA) (13) and C57BL/6.NEP+/+ controls were from our colony at VA Puget Sound Health Care System in Seattle, WA. At the time of islet isolation, 16-h fasting plasma glucose levels were similar in C57BL/6.NEP+/+ versus C57BL/6.NEP−/− mice (male: 95.6 ± 4.1 vs. 96.6 ± 2.7 mg/dL; female: 85.6 ± 6.3 vs. 94.5 ± 3.1 mg/dL; n = 8–11). Studies were approved by the VA Puget Sound Health Care System Institutional Animal Care and Use Committee.
Peptides, Agonists, and Inhibitors
Angiotensin-(1–7) was purchased from Sigma-Aldrich (St. Louis, MO). Angiotensin-(5–7), angiotensin-(1–2), and angiotensin-(3–4) were from AnaSpec (Fremont, CA), and angiotensin-(1–4) from GenScript (Piscataway, NJ). The MasR agonist AVE0991 was from MedChem Express (Monmouth Junction, NJ) and antagonist A779 from Abcam (Cambridge, MA). The ACE2 inhibitor DX600 was from Phoenix Pharmaceuticals (Burlingame, CA).
Islet Isolation and Culture
Islets were isolated from 10- to 12-week-old female and male mice as previously described (14). RPMI 1640 media (Gibco #22400; Life Technologies Corporation, Grand Island, NY) containing 11.1 mmol/L glucose was used for all cultures. After overnight recovery, islets were cultured for 48 h in media plus one of the following: angiotensin-(1–7), angiotensin-(1–4), angiotensin-(5–7), angiotensin-(1–2), angiotensin-(3–4), or vehicle. A concentration of 1 nmol/L was used because dose-response studies showed this to be the lowest effective concentration of each peptide (data not shown). A 48-h culture period was chosen as it is the typical paradigm used to mimic long-term exposure of islets to factors seen under normal/diabetic conditions. In some experiments, the ACE2 inhibitor DX600 was added to islets for 48-h culture at a dose (10 μmol/L) previously shown (15) to maximally inhibit ACE2 activity in mouse tissues. In other experiments, the MasR antagonist A779 (1 μmol/L) was added to islets 20 min prior to the addition of angiotensin-(1–2) or the MasR agonist AVE0991 (0.1 μmol/L). For these A779 experiments, the culture period is as indicated in the Results section.
In a subset of experiments, freshly isolated islets were transfected with the following small interfering RNA (siRNA) oligonucleotides: 10 nmol/L or 25 nmol/L si-Scramble (catalog #4390843; Thermo Fisher, Waltham, MA); 10 nmol/L si-GPCR Family C Group 6 Member A (GPRC6A) (combination of 5 nmol/L siRNA [catalog #s102233] plus 5 nmol/L siRNA [catalog #s102235]; Thermo Fisher); 25 mmol/L si-MasR (catalog #s69599; Thermo Fisher); or lipofectamine vehicle (Lipofectamine RNAiMAX Reagent; Invitrogen, Carlsbad, CA) in RPMI 1640 media containing 11.1 mmol/L glucose. After 72 h, islets were transferred to fresh media and recovered for up to 72 h. After recovery, islets were cultured for 48 h in the presence of 1 nmol/L angiotensin-(1–2) or vehicle. This was followed by static insulin secretion measurements as described below. The degree of GPRC6A or MasR knockdown was similar immediately after 72-h transfection versus that at the time of insulin secretion assessment. Insulin secretion data from Lipofectamine vehicle versus si-Scramble–treated islets were similar.
Insulin Secretion and Content
Insulin secretion in response to 2.8 mmol/L (basal) or 20 mmol/L (stimulated) glucose was measured in static incubations as previously described (14), wherein both C57BL/6.NEP+/+ and C57BL/6.NEP−/− islets treated with either vehicle or angiotensin peptide were studied simultaneously for each n. Each angiotensin peptide was studied independently. For 48-h cultured islets, 2.8 or 20 mmol/L glucose-induced insulin secretion was measured in the absence of angiotensin peptides. In a subset of experiments, basal and glucose-induced insulin secretion were measured in the presence of 1 nmol/L angiotensin-(1–7) or angiotensin-(1–2) in islets that were isolated and recovered without angiotensin peptide exposure overnight. Islet insulin content was measured after acid-ethanol extraction. Insulin concentrations were determined using the Insulin (Mouse) Ultrasensitive ELISA (Alpco, Salem, NH).
Real-time Quantitative RT-PCR
Expression of islet GPRC6A, MasR and CasR mRNA was determined by real-time quantitative RT-PCR using the TaqMan system (ABI Prism 7500; Applied Biosystems, Carlsbad, CA), as previously described (12). GPRC6A (Mm00467618_m1), MasR (Mm00434823_s1), and CasR (Mm00443375_m1) gene expression mixes from Applied Biosystems were used with eukaryotic 18S rRNA (Hs99999901_s1) as the endogenous control.
Western Blotting
Islet protein (20–35 µg/well) separated by SDS-PAGE was transferred to polyvinylidene fluoride membranes, which were probed with polyclonal rabbit anti–phosphorylated AKT (pAKT) antibody (1:500; Cell Signaling Technology, Danvers, MA), anti-GPRC6A antibody (1:1,000; MyBioSource, San Diego, CA), or anti-MAS1 (1:500; Abcam). Stripped membranes were reprobed with polyclonal rabbit anti-AKT antibody (1:500; Cell Signaling Technology) or anti-β-actin (1:2,000; Sigma-Aldrich). Secondary antibody was goat anti-rabbit IgG horseradish peroxidase (1:50,000; Dako, Carpinteria, CA). Bands were visualized on a FluorChem M system using enhanced chemiluminescence and quantified using ImageJ software.
Mass Spectrometry
Angiotensin-(1–7), angiotensin-(1–4), and A779 at 40 μmol/L and recombinant human neprilysin (Reprokine Ltd, Valley Cottage, NY) at 0.8 μmol/L in 20 mmol/L ammonium acetate buffer (pH 6.5) were combined in equal volumes to yield final concentrations of 20 μmol/L peptide and 0.4 μmol/L enzyme and were incubated at 37°C. Samples were taken at 0, 0.75, 1.5, 3, 6, 12, 24, and 48 h, and the reactions were quenched by the addition of trifluoroacetic acid (TFA; 0.5%) and analyzed by liquid chromatography–time-of-flight mass spectrometry. Digestion products were separated on a Zorbax-SB-C18 column by a sequence of increasingly steep linear gradients from 0.1% acetic acid/0.1% TFA in 98% water/2% methanol to 0.1% acetic acid/0.1% TFA in 4% water/96% methanol. Mass spectrometry was performed in singlicate on at least two independent occasions. The extraction ion current area for each degradation product was computed and expressed as a percentage of the total ion count at each time point.
Histology
Frozen sections (7–10 μm) of OCT-embedded, formalin-fixed human pancreas from normal subjects were purchased from Zyagen (San Diego, CA). Frozen, OCT-embedded pancreas sections (5 μm) were obtained from C57BL/6.NEP+/+ mice after perfusion fixation and overnight postfixing in 10% neutral-buffered formalin. For both human and mouse pancreata, nonspecific immunoreactivity was blocked with up to 10% normal goat and/or donkey serum. Sections were incubated overnight with rabbit polyclonal anti-ACE2 antibody (1:100; Santa Cruz Biotechnology, Dallas, TX). Either Cy3-conjugated donkey anti-rabbit IgG or biotinylated goat anti-rabbit IgG followed by Alexa Fluor 488 streptavidin was then applied. To visualize β, α, δ, and pancreatic polypeptide (PP) cells, sections were then incubated for an hour with guinea pig anti-insulin (1:50; Dako), mouse anti-glucagon (1:2,000; Sigma-Aldrich), goat anti-somatostatin (1:250; Santa Cruz Biotechnology), or goat anti-PP (1:500; Novus Biologicals, Littleton, CO) antibodies, respectively. Sections were subsequently incubated for an hour with either Cy3-conjugated goat or donkey anti-IgG antibodies or biotinylated donkey anti-goat IgG followed by Alexa Fluor 488 streptavidin. To visualize cell nuclei, sections were counterstained with Hoechst 33258 stain (2 μg/mL). Secondary antibody controls were performed by substituting primary antibodies with the same amount of normal serum from the same species.
Statistical Analyses
Data are presented as mean ± SEM for the number of experiments indicated. Statistical significance was determined using ANOVA with post hoc analysis, the nonparametric Mann-Whitney U test, or paired Student t test. A P value <0.05 was considered to be statistically significant.
Results
Neprilysin Mediates the Ability of Angiotensin-(1–7) to Promote Insulin Secretion
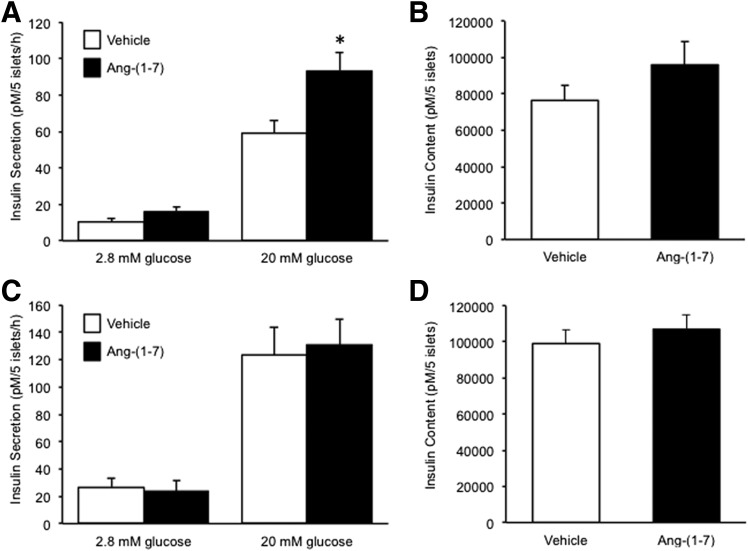
Angiotensin-(1–7) has been shown to enhance insulin secretion (16–18). To determine whether neprilysin activity is required for this effect, basal (2.8 mmol/L glucose) and glucose-stimulated (20 mmol/L glucose) insulin secretion was assessed after a 48-h culture of C57BL/6.NEP+/+ or C57BL/6.NEP−/− islets in the absence and presence of angiotensin-(1–7). In C57BL/6.NEP+/+ islets, angiotensin-(1–7) exposure did not alter basal secretion but did potentiate glucose-stimulated insulin secretion (GSIS) (Fig. 1A). However, the exposure of C57BL/6.NEP−/− islets to angiotensin-(1–7) did not result in changes in basal or GSIS (Fig. 1C). Islet insulin content was not different in either C57BL/6.NEP+/+ or C57BL/6.NEP−/− islets cultured with angiotensin-(1–7) (Fig. 1B and D).
Figure 1.
Angiotensin-(1–7) promotes insulin secretion only in islets expressing neprilysin. Insulin secretion in response to 2.8 or 20 mmol/L glucose and insulin content from C57BL/6.NEP+/+ (A and B; n = 8–9) and C57BL/6.NEP−/− (C and D; n = 6) islets after a 48-h culture in the absence or presence of 1 nmol/L angiotensin-(1–7). Data are the mean ± SEM. *P < 0.05 vs. vehicle.
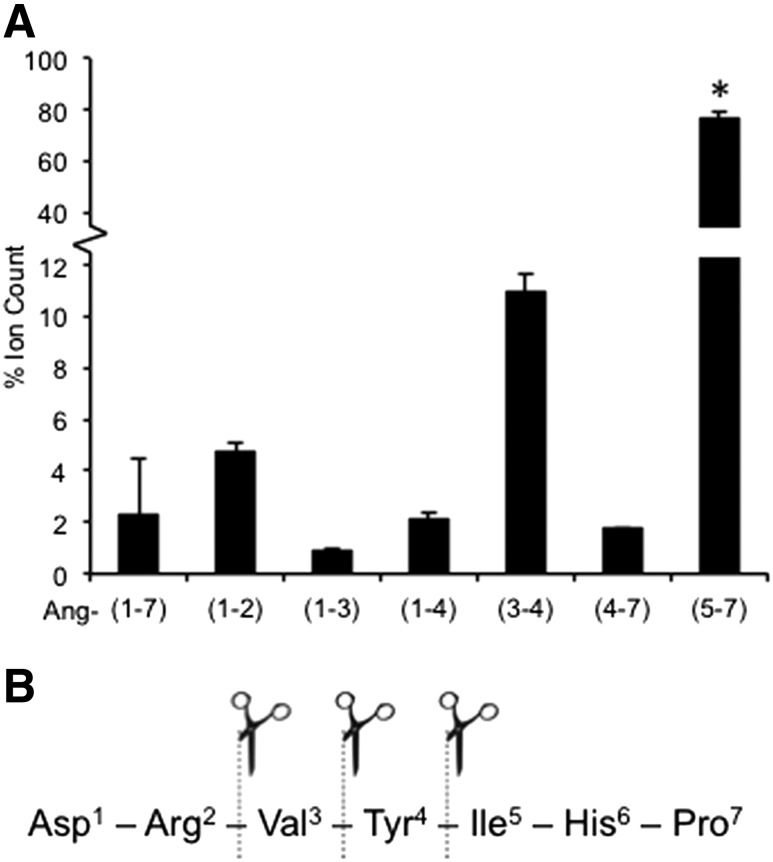
Since neprilysin can cleave angiotensin-(1–7) into smaller peptides and one or more of these may be potentiating GSIS in C57BL/6.NEP+/+ islets, we performed mass spectrometry to identify the neprilysin-derived products of angiotensin-(1–7). When angiotensin-(1–7) was incubated with neprilysin for 48 h to mirror the period of islet culture, six peptide products were generated, corresponding to three cleavage sites (Fig. 2A and B). Time-course experiments demonstrated that the generation of the six products occurred rapidly (i.e., within 45 min) and that the relative abundance of each peptide product did not change significantly over time (data not shown). The most abundant peptide was angiotensin-(5–7), which is consistent with previous reports that neprilysin primarily cleaves angiotensin-(1–7) between amino acids 4 (Tyr) and 5 (Ile) (8). The least abundant peptide was angiotensin-(1–3), accounting for <1.0% of the total ion count at 48 h.
Figure 2.
Angiotensin-(1–7) is cleaved into several smaller peptides by neprilysin. Angiotensin-(1–7) and neprilysin were coincubated, and samples taken at various time points up to 48 h were analyzed by mass spectrometry. A: Extraction ion current areas for each peptide are expressed as a percentage of the total ion count at 48 h (n = 2). B: Based on mass spectrometry data, three neprilysin cleavage sites within angiotensin-(1–7) were identified. Data are the mean ± SEM. *P < 0.05 vs. all other cleavage products.
Angiotensin-(1–4), but Not Angiotensin-(5–7), Enhances Insulin Secretion in the Presence of Neprilysin
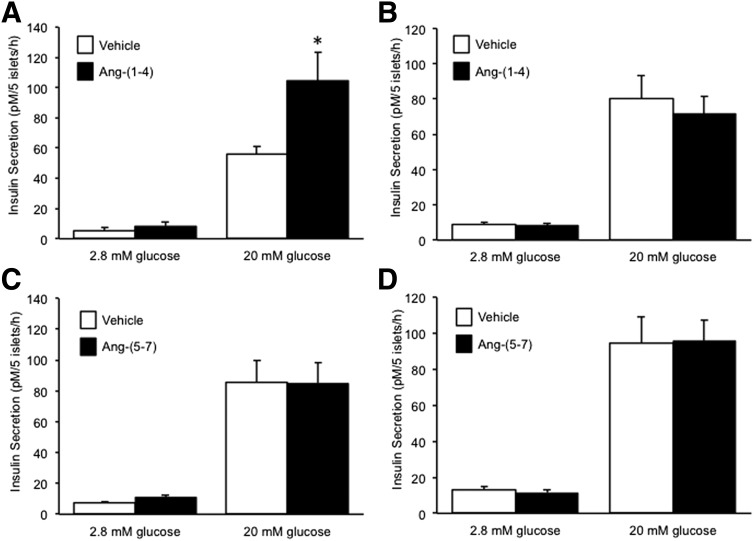
To determine whether the neprilysin-derived products of angiotensin-(1–7) hydrolysis between residues 4 and 5 were insulinotropic, C57BL/6.NEP+/+ or C57BL/6.NEP−/− islets were cultured in the absence and presence of angiotensin-(1–4) or angiotensin-(5–7) for 48 h and insulin secretion was assessed. With angiotensin-(1–4), basal insulin secretion was unaltered in C57BL/6.NEP+/+ islets whereas GSIS was enhanced (Fig. 3A), and in C57BL/6.NEP−/− islets both basal secretion and GSIS were unaltered (Fig. 3B). These data argue that further cleavage of angiotensin-(1–4) is required to promote GSIS. With angiotensin-(5–7), basal secretion and GSIS were unchanged in both C57BL/6.NEP+/+ (Fig. 3C) and C57BL/6.NEP−/− (Fig. 3D) islets.
Figure 3.
Angiotensin-(1–4) but not angiotensin-(5–7) promotes insulin secretion only in islets expressing neprilysin. Insulin secretion in response to 2.8 or 20 mmol/L glucose from islets after a 48-h culture in the absence or presence of 1 nmol/L angiotensin-(1–4) (A: C57BL/6.NEP+/+, n = 6; B: C57BL/6.NEP−/−, n = 8) or 1 nmol/L angiotensin-(5–7) (C: C57BL/6.NEP+/+, n = 7; D: C57BL/6.NEP−/−, n = 7). Data are the mean ± SEM. *P < 0.05 vs. vehicle.
Given that angiotensin-(1–4) enhanced GSIS only in islets expressing neprilysin, we determined by mass spectrometry whether it could be further cleaved by neprilysin into smaller bioactive products. After a 48-h incubation of angiotensin-(1–4) with neprilysin, two dipeptides were detected corresponding to cleavage of angiotensin-(1–4) between amino acids 2 (Arg) and 3 (Val) (data not shown).
Angiotensin-(1–2) Enhances Insulin Secretion Independent of Islet Neprilysin Activity
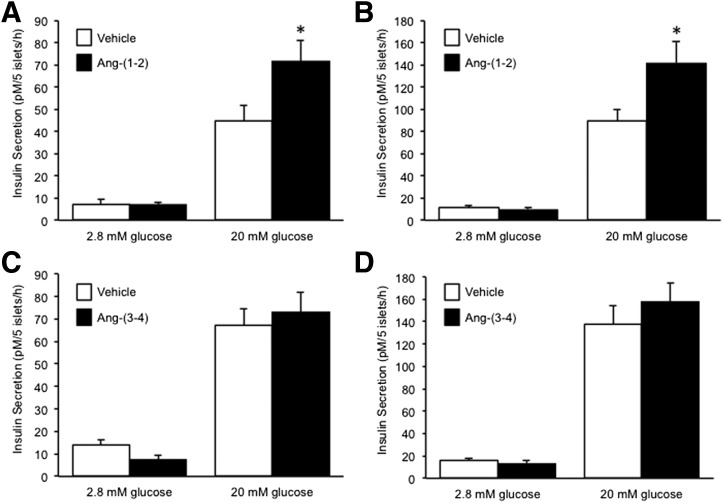
Next, we tested whether either angiotensin-(1–2) and/or angiotensin-(3–4) were able to promote insulin secretion. After a 48-h culture of C57BL/6.NEP+/+ islets in the presence of angiotensin-(1–2), basal secretion was unchanged, whereas GSIS was increased by 60% (Fig. 4A). Similarly in C57BL/6.NEP−/− islets, angiotensin-(1–2) enhanced GSIS by 60% without altering basal secretion (Fig. 4B). In contrast, neither basal secretion nor GSIS were affected when C57BL/6.NEP+/+ or C57BL/6.NEP−/− islets were cultured with angiotensin-(3–4) (Fig. 4C and D).
Figure 4.
Angiotensin-(1–2) but not angiotensin-(3–4) promotes insulin secretion in islets with or without neprilysin. Insulin secretion in response to 2.8 or 20 mmol/L glucose from islets after a 48-h culture in the absence or presence of 1 nmol/L angiotensin-(1–2) (A: C57BL/6.NEP+/+, n = 6; B: C57BL/6.NEP−/−, n = 6) or 1 nmol/L angiotensin-(3–4) (C: C57BL/6.NEP+/+, n = 6; D: C57BL/6.NEP−/−, n = 6). Data are the mean ± SEM. *P < 0.05 vs. vehicle.
Angiotensin-(1–2) Does Not Signal Through the MasR to Promote Insulin Secretion
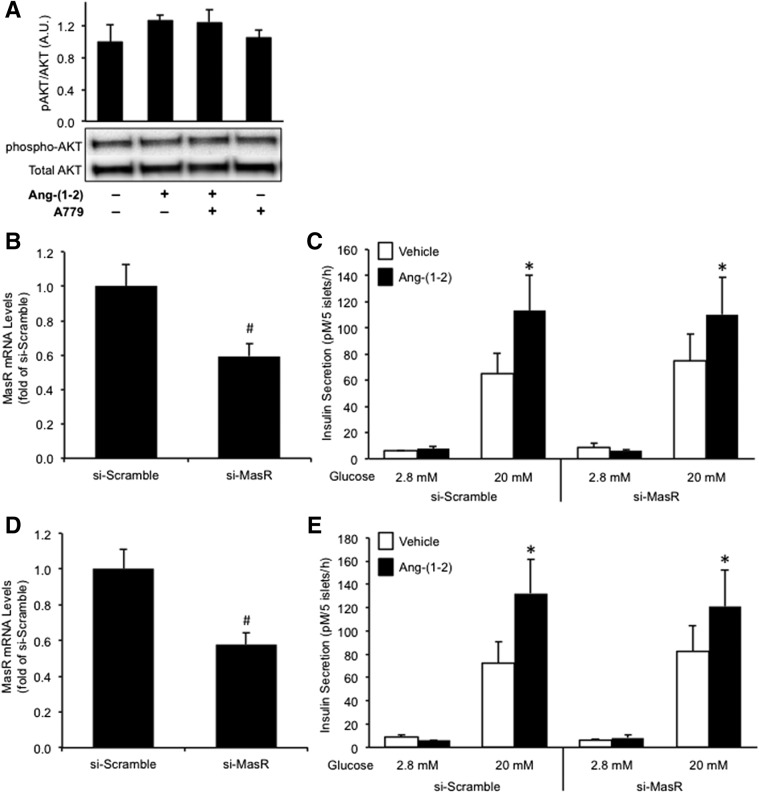
In order to establish whether angiotensin-(1–2) signals through the MasR to modulate insulin secretion, islets were pretreated with the MasR antagonist A779 or siRNA oligonucleotides to knockdown MasR prior to culture with angiotensin-(1–2).
As the amino acid sequence of A779 is identical to angiotensin-(1–7) with the exception of a proline to d-alanine substitution at position 7, we first sought to determine whether it was similarly cleaved by neprilysin. Mass spectrometry revealed that after a 48-h incubation of neprilysin with A779, several smaller products were detectable indicating four cleavage sites (Supplementary Fig. 1 and Supplementary Table 1). One of these products was angiotensin-(1–2); thus, we tested whether a 48-h incubation of C57BL/6.NEP+/+ islets with A779 would enhance insulin secretion. Indeed, A779 increased GSIS by 30% (Supplementary Fig. 2). Additionally, A779 potentiated the insulin response of C57BL/6.NEP+/+ islets to angiotensin-(1–2) (data not shown). Next, we tested whether A779 would alter insulin secretion in the absence of neprilysin. Incubation of C57BL/6.NEP−/− islets with A779 resulted in increased GSIS after a 48-h incubation but not after a 12-h incubation (data not shown). Thus, experiments using A779 and angiotensin-(1–2) were performed in C57BL/6.NEP−/− islets only, with a 12-h culture period.
After a 12-h culture of C57BL/6.NEP−/− islets with angiotensin-(1–2), we evaluated protein levels of pAKT as a measure of signaling downstream of the MasR. pAKT and total AKT levels were unchanged with angiotensin-(1–2) compared with vehicle treatment (Fig. 5A) despite GSIS being increased (124.4 ± 14.7 vs. 87.8 ± 10.6 pmol/L/5 islets/h; P = 0.02, n = 9). Similarly, A779 pretreatment of islets did not affect either pAKT or total AKT levels (Fig. 5A). To confirm that we could detect an increase in pAKT upon MasR activation, we generated a positive control in which C57BL/6.NEP−/− islets were treated with a known MasR agonist, AVE0991, for 12 h. AVE0991 significantly increased pAKT levels (pAKT/AKT: vehicle 1.0 ± 0.2-fold vs. AVE0991 1.8 ± 0.6-fold; P = 0.04, n = 6), an effect that could not be blocked by pretreatment with A779. A potential explanation for the latter may be that a longer exposure to A779 is required; however, given the caveats of using A779 over a longer time frame, we used an alternate method to establish whether angiotensin-(1–2) signals through the MasR to increase GSIS.
Figure 5.
Angiotensin-(1–2) does not signal through the MasR to promote insulin secretion. pAKT and total AKT protein levels (A: n = 3–5) from C57BL/6.NEP−/− islets cultured in the absence or presence of 1 nmol/L angiotensin-(1–2), with or without pretreatment with 1 μmol/L A779. Western blot images are representative, with quantification of all experiments shown in the graphs. MasR mRNA levels in C57BL/6.NEP+/+ (B; n = 9) and C57BL/6.NEP−/− (D; n = 8) islets transfected with either si-Scramble or si-MasR for 72 h. Insulin secretion in response to 2.8 or 20 mmol/L glucose from transfected C57BL/6.NEP+/+ (C; n = 7) and C57BL/6.NEP−/− (E; n = 5) islets after a 48-h culture in the absence or presence of 1 nmol/L angiotensin-(1–2). Data are the mean ± SEM. #P < 0.001 vs. si-Scramble; *P < 0.05 vs. vehicle 20 mmol/L glucose.
We transfected islets for 72 h with siRNA to knock down MasR then assessed GSIS after a 48-h exposure to angiotensin-(1–2). C57BL/6.NEP+/+ and C57BL/6.NEP−/− islets transfected with si-MasR showed an ∼40% decrease in MasR mRNA (Fig. 5B and D) and an ∼30% decrease in MasR protein (si-Scramble 1.00 ± 0.06-fold vs. si-MasR 0.69 ± 0.05-fold; P < 0.01, n = 8) levels, compared with si-Scramble. As expected, a 48-h exposure to angiotensin-(1–2) increased GSIS in si-Scramble–treated C57BL/6.NEP+/+ and C57BL/6.NEP−/− islets (Fig. 5C and E). Knockdown of MasR failed to affect this increase in GSIS. Together, these data suggest that angiotensin-(1–2) is not acting through the MasR to enhance insulin secretion.
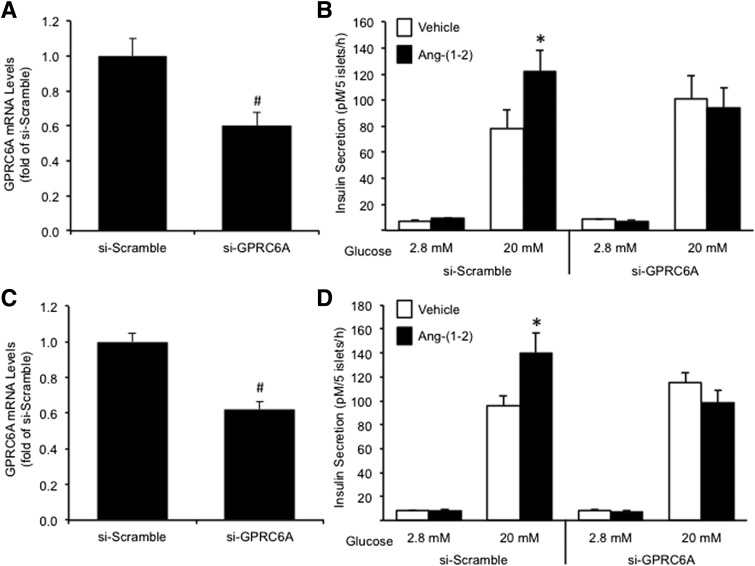
Angiotensin-(1–2) Signals Through GPRC6A to Promote Insulin Secretion
Another GPCR shown to be involved in mediating insulin secretion in response to amino acids and small peptides is GPRC6A (19). We tested whether angiotensin-(1–2) signals through GPRC6A to promote GSIS. C57BL/6.NEP+/+ and C57BL/6.NEP−/− islets were transfected with siRNA to knock down GPRC6A as described above for MasR, resulting in GPRC6A mRNA and protein levels that were decreased by ∼40% (Fig. 6A and C) and ∼30% (si-Scramble 1.00 ± 0.19-fold vs. si-GPRC6A 0.69 ± 0.10-fold; P < 0.05, n = 9), respectively, compared with si-Scramble (Fig. 6A and C). Again, a 48-h exposure to angiotensin-(1–2) increased GSIS in si-Scramble–treated C57BL/6.NEP+/+ and C57BL/6.NEP−/− islets (Fig. 6B and D). In contrast, the knockdown of GPRC6A attenuated the increase in GSIS seen with angiotensin-(1–2) in both genotypes, suggesting angiotensin-(1–2) acts, at least in part, via GPRC6A to enhance insulin secretion. Basal secretion was unchanged by siRNA treatment or angiotensin-(1–2) culture (Fig. 6B and D). Further, GPRC6A knockdown per se (i.e., in the absence of angiotensin-[1–2]) did not alter GSIS after a 48-h culture compared with si-Scramble. Although off-target effects of GPRC6A knockdown cannot be completely excluded, mRNA levels of another closely related receptor, Ca2+-sensing receptor (CaSR), in islets treated with si-GPRC6A were unchanged compared with si-Scramble (data not shown), suggesting that any off-target effects may be minimal.
Figure 6.
Angiotensin-(1–2) promotes insulin secretion via GPRC6A. GPRC6A mRNA levels in C57BL/6.NEP+/+ (A; n = 9) and C57BL/6.NEP−/− (C; n = 10) islets transfected with either si-Scramble or si-GPRC6A for 72 h. Insulin secretion in response to 2.8 or 20 mmol/L glucose from transfected C57BL/6.NEP+/+ (B; n = 9) and C57BL/6.NEP−/− (D; n = 10) islets after a 48-h culture in the absence or presence of 1 nmol/L angiotensin-(1–2). Data are the mean ± SEM. #P < 0.0001 vs. si-Scramble; *P < 0.05 vs. vehicle 20 mmol/L glucose.
Short-term Exposure of Islets to Angiotensin-(1–7) or Angiotensin-(1–2) Does Not Potentiate Insulin Secretion
To determine whether angiotensin-(1–7) and angiotensin-(1–2) also enhance GSIS after short-term exposure, we performed static insulin secretion experiments wherein isolated and overnight recovered C57BL/6.NEP+/+ and C57BL/6.NEP−/− islets were coincubated with one of these peptides (1 nmol/L) plus either 2.8 or 20 mmol/L glucose for 1 h. After short-term exposure, neither basal secretion nor GSIS was altered in islets coincubated with 1 nmol/L angiotensin-(1–7) or angiotensin-(1–2) versus vehicle (Supplementary Fig. 3).
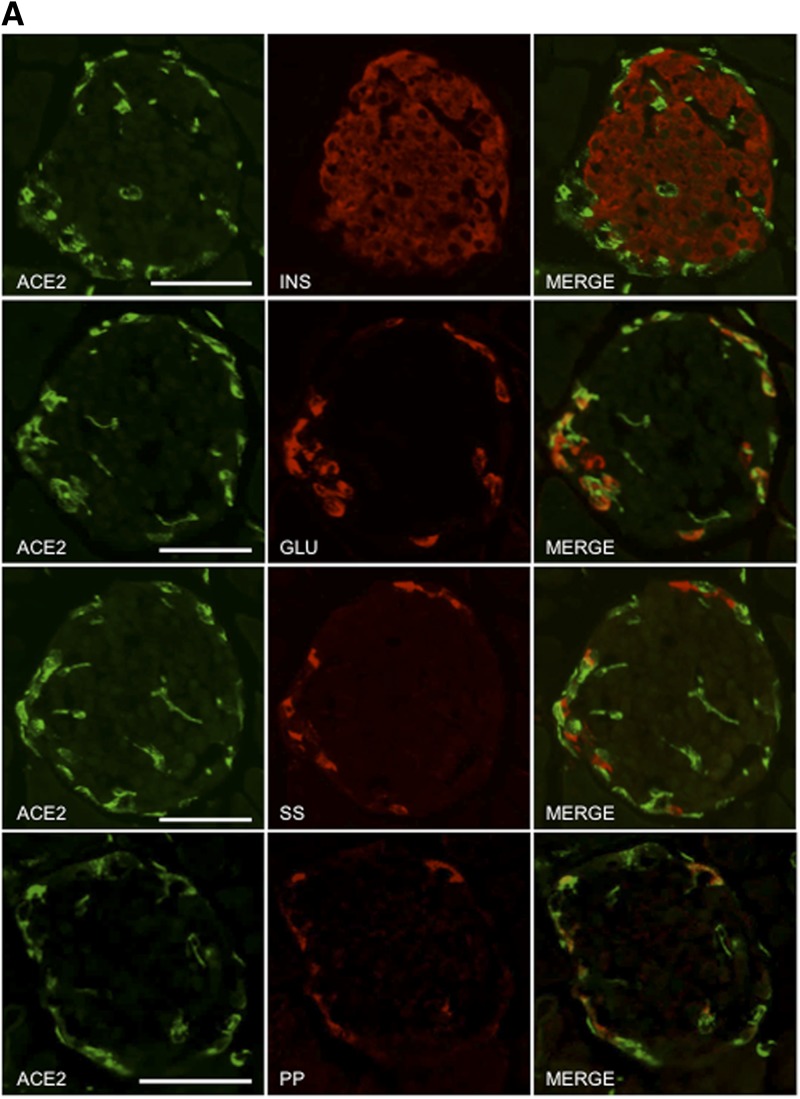
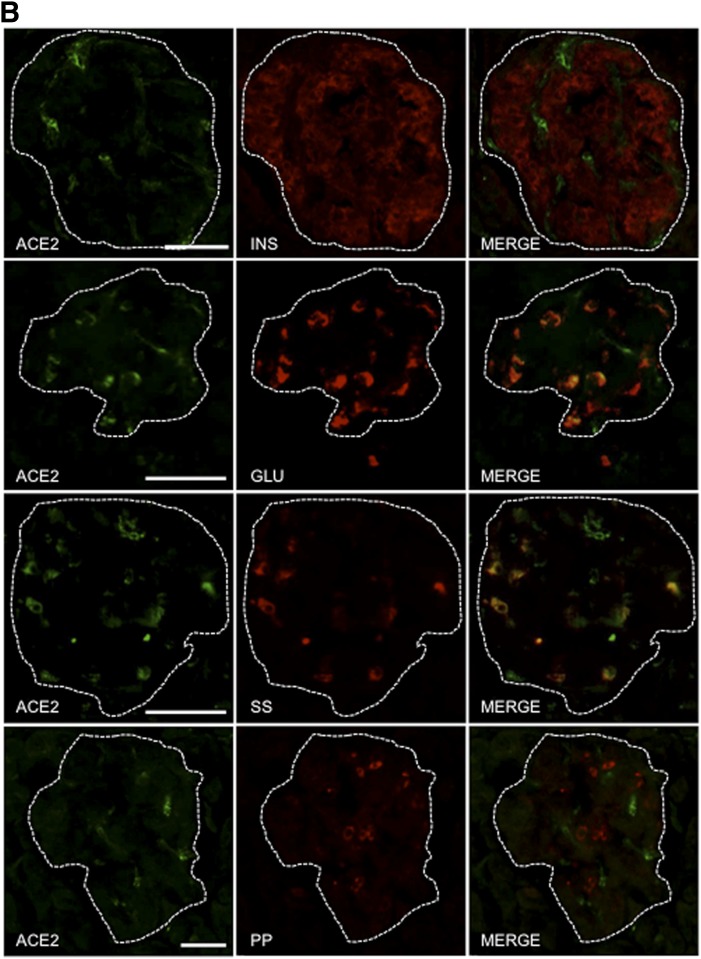
ACE2 Is Not Widely Expressed in Human or Mouse Islets but May Play a Paracrine Role in Modulating Angiotensin-(1–7)–Mediated Insulin Secretion
Both neprilysin and ACE2 are capable of generating angiotensin-(1–7), and neprilysin can further cleave angiotensin-(1–7) into smaller peptides (1,5,6,8). Previously, we showed that islet neprilysin is expressed in both β-cells and non–β-cells (12) and is thus ideally positioned to modulate insulin secretion. However, the expression pattern of islet ACE2 remains unclear. To determine the localization of ACE2 in islets, pancreas sections from normal healthy humans and C57BL/6.NEP+/+ mice were immunostained for ACE2, insulin, glucagon, somatostatin, and PP. Figure 7A and B shows that ACE2 expression was colocalized with glucagon, somatostatin, and PP, with the latter being seen in mouse islets only. Importantly, no colocalization was observed with insulin in either human or mouse islets, suggesting that ACE2 is not expressed in β-cells.
Figure 7.
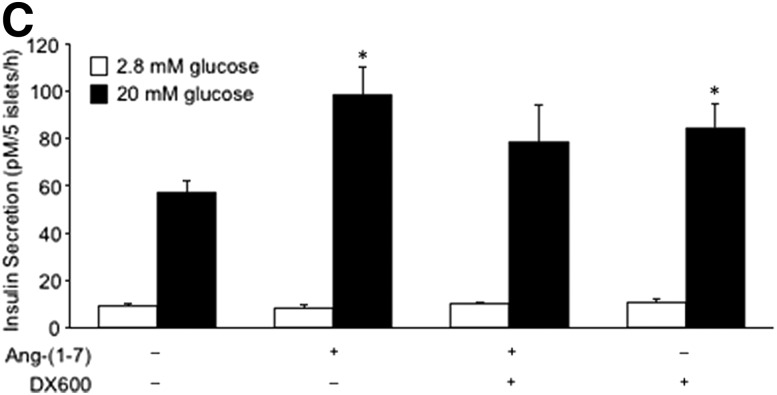
ACE2 is localized to non–β-cells and contributes to angiotensin-(1–7)–mediated potentiation of insulin secretion. Pancreas sections from C57BL/6.NEP+/+ mice (A) and humans (B) were immunostained for ACE2, insulin (INS), glucagon (GLU), somatostatin (SS), and PP. Merged images (MERGE) show colocalization (yellow) of ACE2 with glucagon, somatostatin, and PP, but not insulin. Islets are demarcated by the dashed line in panel B. Scale bars: 50 μm. C: Insulin secretion in response to 2.8 or 20 mmol/L glucose from C57BL/6.NEP+/+ islets cultured for 48 h in the absence or presence of 1 nmol/L angiotensin-(1–7) with or without the ACE2 inhibitor DX600 (10 μmol/L). Data are the mean ± SEM; n = 5. *P < 0.05 vs. vehicle 20 mmol/L glucose.
To determine whether ACE2 inhibition affects the ability of angiotensin-(1–7) to potentiate insulin secretion, we cocultured C57BL/6.NEP+/+ islets with angiotensin-(1–7) and the ACE2 inhibitor DX600 then determined GSIS. As expected, a 48-h exposure to angiotensin-(1–7) increased GSIS without changing basal insulin secretion (Fig. 7C). However, coculture of angiotensin-(1–7) with DX600 abrogated the effect of angiotensin-(1–7) to promote GSIS. Further, DX600 alone increased the insulin response to 20 mmol/L glucose.
Discussion
Emerging evidence suggests that the modulation of pancreatic RAS flux can improve glycemic control in diabetic conditions. Specifically, the ACE2/angiotensin-(1–7)/MasR axis has been identified as an important player, wherein the upregulation of ACE2 and/or angiotensin-(1–7) appears to be an attractive therapeutic strategy to treat type 2 diabetes (20). Here, we show that another RAS component, neprilysin, is critical in ensuring that angiotensin-(1–7) is capable of eliciting beneficial effects with respect to insulin secretion.
In mouse islets lacking neprilysin, we found that angiotensin-(1–7) failed to enhance GSIS. Similarly, its neprilysin-derived degradation products angiotensin-(1–4) and -(5–7) did not increase GSIS in neprilysin-deficient islets. Mass spectrometry analysis revealed that both angiotensin-(1–7) and angiotensin-(1–4) could be further cleaved by neprilysin to generate the dipeptide angiotensin-(1–2), which was insulinotropic in islets with or without neprilysin expression. These data argue that the ability of angiotensin-(1–7) to evoke an insulin secretory response is critically dependent on its neprilysin-mediated degradation to the bioactive angiotensin-(1–2) dipeptide. To our knowledge, this is the first report to show that intact angiotensin-(1–7) is not the primary mediator of beneficial effects ascribed to the ACE2/angiotensin-(1–7)/MasR axis.
The role of neprilysin in the RAS is often overlooked. Like ACE2, it can hydrolyze angiotensins I and II to generate angiotensin-(1–7). In fact, neprilysin hydrolyzes angiotensin I to angiotensin-(1–7) with greater efficiency than ACE2 (1). In comparing the expression pattern of neprilysin to ACE2 in islets, we (12) and others (21) have demonstrated that neprilysin is more widespread, because it is in both β-cells and non–β-cells. We show that ACE2 is limited to α, δ, and PP cells, which is in keeping with a recent report (22). Other histological reports do not clearly define the islet cell types expressing ACE2 (11,23–25). One study (26) showed that ACE2 colocalized with both insulin and somatostatin, but rarely with glucagon and PP, whereas another study (21) showed ACE2 colocalized primarily with insulin. Inconsistency in descriptions of islet ACE2 localization could be due to species-specific differences in ACE2 expression. We believe our observations in human pancreas help to clarify this. Importantly, we show that although islet ACE2 expression is sparser than neprilysin, it also plays a key role in modulating the β-cell response to exogenous angiotensin-(1–7). Specifically, when islet ACE2 activity is inhibited, angiotensin-(1–7) can no longer potentiate GSIS. To our knowledge, no studies have shown that ACE2 cleaves angiotensin-(1–7) as neprilysin does; thus, the molecular mechanism for its ability to promote angiotensin-(1–7)–mediated insulin secretion in a paracrine manner remains to be elucidated.
Few studies have interrogated the direct effect of angiotensin-(1–7) on modulating insulin secretion. In one study, short-term exposure of mouse islets to angiotensin-(1–7) was shown to increase both basal secretion and GSIS by 1.4-fold. However, when continuously infused in mice for 10 days, angiotensin-(1–7) failed to enhance the insulin response to oral glucose. Interestingly, when studied ex vivo, islets from these mice demonstrated increased GSIS even without further angiotensin-(1–7) exposure in culture (21). These findings were attributed to both MasR-dependent and MasR-independent mechanisms modulating insulin secretion via increased cAMP. In other studies using β-cells cocultured with islet endothelial cells (17) or diabetic rats (18), angiotensin-(1–7)–mediated improvements in GSIS were associated with enhanced islet endothelial cell function and pancreatic microcirculation. Given that our in vitro study design involved islet culture with peptides for up to 48 h and that the majority of endothelial cells are typically lost during this period in culture (27), it is unlikely that the islet microvasculature played a significant role in the increased insulin secretion we observed with angiotensin peptides.
Importantly, our findings suggest that angiotensin-(1–7) must be hydrolyzed by neprilysin to angiotensin-(1–2) in order to elicit an increase in insulin secretion. It has previously been shown that angiotensin-(1–7) can be cleaved to generate angiotensin-(1–4) and then angiotensin-(1–2) (5,8). There are no published studies demonstrating the ability of angiotensin-(1–2) to bind and activate the MasR. Although the effects of angiotensin-(1–7) have been proposed to be mediated mainly via the MasR (4), we did not find this to be the case with angiotensin-(1–2). Rather, we uncovered that another GPCR, GPRC6A, facilitates the insulinotropic actions of angiotensin-(1–2). GPRC6A is known to bind small peptides and l-α-amino acids, including l-arginine, to increase intracellular calcium concentrations (28–31) and insulin secretion (19,28,32). Thus, our findings contribute to the evolving model of the RAS (Fig. 8), wherein we propose that neprilysin, angiotensin-(1–2), and GPRC6A mediate, at least in part, the secretory response of islets to angiotensin-(1–7). This mechanism requires long-term (>1 h) exposure to angiotensin-(1–2), given that short-term (1 h) exposure failed to enhance GSIS. Long-term exposure raises the possibility that the effects of angiotensin-(1–2) on GSIS may be due to non–receptor-mediated events; however, we feel that this is unlikely given that GPRC6A knockdown abolished the potentiation of GSIS by angiotensin-(1–2).
Figure 8.
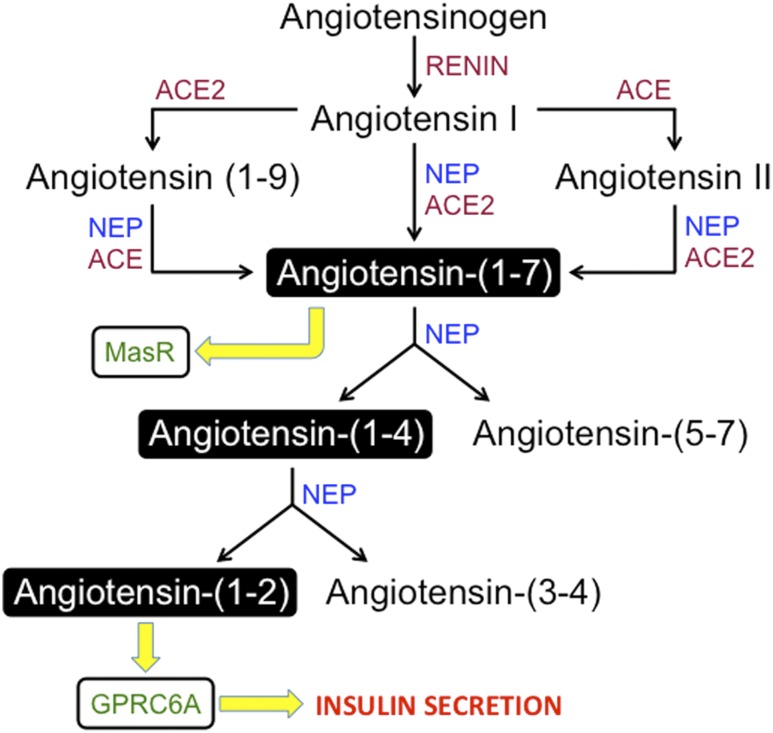
Proposed NEP/angiotensin-(1–2)/GPRC6A axis of the RAS. Angiotensinogen is cleaved to angiotensin I, which is further cleaved to angiotensin II, angiotensin-(1–9), and angiotensin-(1–7) by previously identified enzymes. Angiotensin-(1–7) has been shown to bind the MasR to mediate beneficial effects. In islets, we show that angiotensin-(1–7) is cleaved by neprilysin to angiotensin-(1–4) and angiotensin-(5–7). Angiotensin-(1–4) is further cleaved to angiotensin-(1–2) and angiotensin-(3–4) by neprilysin. Angiotensin-(1–2) binds GPRC6A to elicit an increase in insulin secretion.
A caveat for interpretation of our data are that it is unclear as to whether angiotensin-(1–7) is indeed cleaved to angiotensin-(1–2) by endogenous neprilysin in our islet culture system. Although our mass spectrometry data clearly show cleavage occurs with recombinant neprilysin in a “test tube” setting, confirmation of this in islets is hindered by a lack of the resolution required to detect a dipeptide like angiotensin-(1–2) in a highly complex biological sample. Future work will be required to resolve this issue. Another consideration for data interpretation relates to the lack of GSIS potentiation by angiotensin-(1–7) in neprilysin-deficient islets. One potential explanation for these data are that under control/vehicle conditions those islets secrete more insulin than wild-type islets. This may limit or mask the effect of angiotensin-(1–7) to potentiate GSIS, although we feel that this is unlikely because other peptides like angiotensin-(1–2) are still able to increase GSIS in neprilysin-deficient islets above levels seen with vehicle treatment. Finally, our mass spectrometry data on the cleavage of the MasR antagonist A779 warrant caution for its use in conditions where neprilysin is active. Its effect to enhance GSIS independent of MasR antagonism in islets after a 48-h culture, even in islets lacking neprilysin, coupled with the inability to block MasR agonist-mediated increases in pAKT over 12 h, limits its use in studying the role of MasR in β-cell function.
In summary, we show that both neprilysin and ACE2 are required for angiotensin-(1–7) to enhance insulin secretion in vitro. Neprilysin cleaves angiotensin-(1–7) to generate the insulinotropic angiotensin-(1–2) dipeptide, which acts, at least in part, through GPRC6A. With the recent development of therapeutic angiotensin-(1–7) and neprilysin inhibitors, our findings have significant clinical implications for the use of these compounds concurrently and thus warrant further investigation.
Supplementary Material
Article Information
Acknowledgments. The authors thank P. Bergquist, D. Hackney, M. Peters, A. Rahman, J. Wilkins-Gutierrez, and J. Willard from the Seattle Institute for Biomedical and Clinical Research for excellent technical support.
Funding. This work was supported by the National Institutes of Health/National Institute of General Medical Sciences grant GM-078114 to D.P.R., the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants DK-080945 and DK-098506 to S.Z., and the Department of Veterans Affairs. Histological studies and islet isolations were performed in part at the University of Washington’s DRC Cellular and Molecular Imaging and Cell Function Analysis Cores, respectively, which are supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant DK-017047.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.S.B. and B.M.B. designed and performed experiments, analyzed data, and edited the manuscript. M.W. performed experiments, analyzed data, and edited the manuscript. R.G., E.C., A.W., and B.R. performed experiments and analyzed data. D.P.R. contributed analytic tools, analyzed data, and edited the manuscript. S.Z. conceived and designed the study, performed experiments, analyzed data, and wrote the manuscript. S.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1318/-/DC1.
References
- 1.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 2004;383:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos RA, Brosnihan KB, Chappell MC, et al. . Converting enzyme activity and angiotensin metabolism in the dog brainstem. Hypertension 1988;11:I153–I157 [DOI] [PubMed] [Google Scholar]
- 3.Vickers C, Hales P, Kaushik V, et al. . Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 2002;277:14838–14843 [DOI] [PubMed] [Google Scholar]
- 4.Santos RA, Simoes e Silva AC, Maric C, et al. . Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A 2003;100:8258–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gafford JT, Skidgel RA, Erdös EG, Hersh LB. Human kidney “enkephalinase”, a neutral metalloendopeptidase that cleaves active peptides. Biochemistry 1983;22:3265–3271 [DOI] [PubMed] [Google Scholar]
- 6.Stephenson SL, Kenny AJ. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J 1987;241:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension 1992;19:692–696 [DOI] [PubMed] [Google Scholar]
- 8.Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1–7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol 2000;279:F841–F850 [DOI] [PubMed] [Google Scholar]
- 9.Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol 2007;580:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia 1998;41:127–133 [DOI] [PubMed] [Google Scholar]
- 11.Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes 2004;53:989–997 [DOI] [PubMed] [Google Scholar]
- 12.Zraika S, Hull RL, Udayasankar J, et al. . Identification of the amyloid-degrading enzyme neprilysin in mouse islets and potential role in islet amyloidogenesis. Diabetes 2007;56:304–310 [DOI] [PubMed] [Google Scholar]
- 13.Lu B, Gerard NP, Kolakowski LF Jr, et al. . Neutral endopeptidase modulation of septic shock. J Exp Med 1995;181:2271–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zraika S, Dunlop M, Proietto J, Andrikopoulos S. The hexosamine biosynthesis pathway regulates insulin secretion via protein glycosylation in mouse islets. Arch Biochem Biophys 2002;405:275–279 [DOI] [PubMed] [Google Scholar]
- 15.Pedersen KB, Sriramula S, Chhabra KH, Xia H, Lazartigues E. Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am J Physiol Regul Integr Comp Physiol 2011;301:R1293–R1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Yang Z, Yang H, et al. . Regulation of insulin sensitivity, insulin production, and pancreatic β cell survival by angiotensin-(1-7) in a rat model of streptozotocin-induced diabetes mellitus. Peptides 2015;64:49–54 [DOI] [PubMed] [Google Scholar]
- 17.Lu CL, Wang Y, Yuan L, Li Y, Li XY. The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects the function of pancreatic β cells by improving the function of islet microvascular endothelial cells. Int J Mol Med 2014;34:1293–1300 [DOI] [PubMed] [Google Scholar]
- 18.Yuan L, Li Y, Li G, Song Y, Gong X. Ang(1-7) treatment attenuates β-cell dysfunction by improving pancreatic microcirculation in a rat model of type 2 diabetes. J Endocrinol Invest 2013;36:931–937 [DOI] [PubMed] [Google Scholar]
- 19.Pi M, Wu Y, Lenchik NI, Gerling I, Quarles LD. GPRC6A mediates the effects of L-arginine on insulin secretion in mouse pancreatic islets. Endocrinology 2012;153:4608–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol 2009;302:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahr A, Wolke C, Maczewsky J, et al. . The angiotensin-(1-7)/Mas axis improves pancreatic β-cell function in vitro and in vivo. Endocrinology 2016;157:4677–4690 [DOI] [PubMed] [Google Scholar]
- 22.Pedersen KB, Chodavarapu H, Porretta C, Robinson LK, Lazartigues E. Dynamics of ADAM17-mediated shedding of ACE2 applied to pancreatic islets of male db/db mice. Endocrinology 2015;156:4411–4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes 2010;59:2540–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhabra KH, Xia H, Pedersen KB, Speth RC, Lazartigues E. Pancreatic angiotensin-converting enzyme 2 improves glycemia in angiotensin II-infused mice. Am J Physiol Endocrinol Metab 2013;304:E874–E884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu MJ, Yang JK, Lin SS, Ji XJ, Guo LM. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine 2008;34:56–61 [DOI] [PubMed] [Google Scholar]
- 26.Fang HJ, Yang JK. Tissue-specific pattern of angiotensin-converting enzyme 2 expression in rat pancreas. J Int Med Res 2010;38:558–569 [DOI] [PubMed] [Google Scholar]
- 27.Nyqvist D, Köhler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 2005;54:2287–2293 [DOI] [PubMed] [Google Scholar]
- 28.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res 2011;26:1680–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pi M, Faber P, Ekema G, et al. . Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 2005;280:40201–40209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem 2005;93:383–391 [DOI] [PubMed] [Google Scholar]
- 31.Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Bräuner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol 2005;67:589–597 [DOI] [PubMed] [Google Scholar]
- 32.Pi M, Kapoor K, Ye R, et al. . Evidence for osteocalcin binding and activation of GPRC6A in β-cells. Endocrinology 2016;157:1866–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.