Abstract
Despite widespread clinical use in the treatment of type 2 diabetes, the impact of sulfonylurea therapy on cardiovascular outcomes remains uncertain. Studies of naturally occurring genetic variation can be used to anticipate the expected clinical consequences of a pharmacological therapy. A common missense variant in the gene encoding a component of the sulfonylurea receptor (ABCC8 p.A1369S) promotes closure of the target channel of sulfonylurea therapy and is associated with increased insulin secretion, thus mimicking the effects of sulfonylurea therapy. Using individual-level data from 120,286 participants in the UK Biobank and summary association results from four large-scale genome-wide association studies, we examined the impact of this variant on cardiometabolic traits, type 2 diabetes, and coronary heart disease. The p.A1369S variant was associated with a significantly lower risk of type 2 diabetes (odds ratio [OR] 0.93; 95% CI 0.91, 0.95; P = 1.2 × 10−11). The variant was associated with increased BMI (+0.062 kg/m2; 95% CI 0.037, 0.086; P = 8.1 × 10−7) but lower waist-to-hip ratio adjusted for BMI, a marker of abdominal fat distribution. Furthermore, p.A1369S was associated with a reduced risk of coronary heart disease (OR 0.98; 95% CI 0.96, 0.99; P = 5.9 × 10−4). These results suggest that, despite a known association with increased weight, long-term sulfonylurea therapy may reduce the risk of coronary heart disease.
Introduction
Sulfonylurea therapy remains a recommended adjunctive therapy to lifestyle modifications and metformin in the treatment of type 2 diabetes on the basis of low cost and efficacy in improving glycemic control (1,2). However, the impact of sulfonylurea therapy on the risk of coronary heart disease or other cardiovascular outcomes remains uncertain. Sulfonylurea initiation has been linked to an increased risk of cardiovascular events in observational studies, an effect some have attributed to weight gain (3,4). Importantly, owing to approval and marketing many years before the U.S. Food and Drug Administration’s call in 2008 for rigorous cardiovascular risk assessment of new antidiabetes therapies (5), an adequately powered cardiovascular outcomes trial was never performed.
In the absence of gold-standard randomized clinical trial data, studies of naturally occurring genetic variation have emerged as a tool to anticipate clinical consequences of a therapy. For example, a common variant near the HMGCR gene, the target of statins, was associated with decreased LDL cholesterol levels and cardiovascular event risk but an increased risk of type 2 diabetes (6,7). This finding was borne out in large clinical trials in which statin therapy led to reduced LDL cholesterol levels and cardiovascular events but an increase in incident type 2 diabetes (8).
Sulfonylureas promote insulin release from pancreatic β-cells via binding to a hetero-octameric complex, with four subunits encoded by the ATP Binding Cassette Subfamily C Member 8 gene (ABCC8) and four by the Potassium Voltage-Gated Channel Subfamily J Member 11 (KCNJ11). The A amino acid allele at p.A1369S in ABCC8 has been associated with higher risk of type 2 diabetes (9,10); the reciprocal S amino acid allele consequently lowers risk of type 2 diabetes, promotes closure of the ATP-sensitive potassium channel in vitro (11), and is associated with higher insulin secretion after a glucose load (10), thus mimicking the effects of pharmacological sulfonylurea therapy. Here, we aimed to determine the impact of such genetic variation on cardiometabolic traits and risk of coronary heart disease.
Research Design and Methods
Study Design, Data Sources, and Study Participants
We used individual-level data from 120,286 individuals of European ancestry from the UK Biobank, a large population-based cohort (12). We supplemented this individual-level data with summary-level statistics from four genome-wide association study (GWAS) consortia examining anthropometric traits, glycemic traits, type 2 diabetes, and coronary heart disease, all predominantly containing individuals of European descent.
The ABCC8 p.A1369S variant (rs757110) is inherited as a haplotype with a p.E23K variant (rs5219) in KCNJ11 (the additional subunit composing the ATP-sensitive potassium channel) with individuals inhering the S allele in ABCC8 almost always inheriting the E allele in KCNJ11 (r2 = 0.98 between the two variants in Europeans, and r2 = 1.0 in Africans and East Asians). Because of this coinheritance, genetics studies were unable to determine which of the two variants was causally linked to channel activity (10). However, subsequent in vitro analyses demonstrated the S allele of ABCC8 p.A1369S to be the causal variant in sensitivity to gliclazide and promoting closure of the ATP-sensitive potassium channel (not p.E23K in KCNJ11) (13). Therefore, in our primary analysis, we examined the effect of ABCC8 p.A1369S, a mutation that mimics sulfonylurea therapy, on type 2 diabetes, coronary heart disease, and cardiometabolic traits. All traits were plotted in units of standard deviations to facilitate comparisons among traits but were displayed in clinical units for interpretation.
Using the UK Biobank cohort, we also examined whether ABCC8 p.A1369S is associated with other cardiovascular disease, including stroke, heart failure, and peripheral vascular disease, as well as a composite of these outcomes and coronary heart disease. Last, we conducted a phenome-wide association study for 32 additional diseases in UK Biobank, including endocrine, renal, urological, gastrointestinal, neurological, musculoskeletal, respiratory, and neoplastic disorders.
Genotyping and imputation was performed in UK Biobank as previously described (14). Individual-level genetic data were available from 120,286 individuals in UK Biobank, after excluding one related individual of each related pair of individuals, individuals whose genetic sex did not match self-reported sex, and 480 samples with an excess of missing genotype calls or more heterozygosity than expected.
Of these individuals, 41,397 were genotyped using the Affymetrix UK Biobank Lung Exome Variant Evaluation (UK BiLEVE) array, and 78,889 were genotyped using the Affymetrix UK Biobank Axiom Array. Phasing and imputation were performed centrally, by UK Biobank, using a reference panel combining UK10K and 1000 Genomes samples.
Statistical Analysis
The S amino acid of the ABCC8 p.A1369S variant (corresponding to the “A” DNA sequence allele of rs757110) has been associated with a lower risk of type 2 diabetes (9,10). We first sought to independently replicate the association of this variant with type 2 diabetes in the UK Biobank. After replication, we used this variant as an instrument to estimate the effect of genetic inhibition of the sulfonylurea receptor on cardiometabolic traits and coronary heart disease.
In the UK Biobank, we estimated the association of the ABCC8 p.A1369S with each outcome using logistic regression adjusting for age, sex, 10 principal components of ancestry, and a dummy variable for genotyping array. For the summary-level data, this approach is equivalent to the association of the ABCC8 p.A1369S variant with the trait or outcome of interest.
For our primary outcome, coronary heart disease, a P value of <0.05 was used to determine statistical significance. For our secondary analysis of cardiometabolic traits, which included eight traits, we set a Bonferroni-adjusted level of significance of P = 0.05/8 = 0.00625 (though this may be conservative for some traits, given the higher prior probability of association in an expected direction). For our phenome-wide association study of 32 phenotypes, we set a level of significance of P = 0.05/32 = 0.0016.
Imaging Analysis
Recent evidence suggests that excess abdominal fat increases the risk of coronary heart disease, even after adjustment for BMI (15). As pharmacological sulfonylurea therapy induces weight gain, we sought to examine whether ABCC8 p.A1369S was associated with abdominal fat, which may mediate the weight gain associated with pharmacological therapy. Of 120,286 participants with genotype data available in UK Biobank, 1,296 participants underwent dual energy X-ray absorptiometry (DEXA). Total fat mass and the ratio of abdominal (android) fat mass to peripheral (arm and leg) fat mass were estimated using full-body DEXA. We examined the association of the ABCC8 p.A1369S variant with these three measurements in this subset of 1,296 UK Biobank participants, after adjustment for age, sex, 10 principal components of ancestry, and a dummy variable for array type.
Analyses were conducted using R version 3.2. All results are reported relative to the S amino acid allele of ABCC8 p.A1369S (the “A” DNA sequence allele), which promotes closure of the ATP-sensitive potassium channel (13).
Results
ABCC8 p.A1369S genotype was available in 120,286 participants of UK Biobank, including 15,593 (13%) homozygous for the allele encoding A/A, 55,612 (46%) with A/S, and 49,081 (41%) with S/S. The mean age was 57 years and 56,936 (47%) participants were men (Supplementary Table 1).
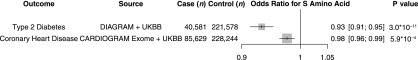
As in prior published results (9,10), the S allele at ABCC8 p.A1369S was associated with a lower risk of diabetes in UK Biobank participants (odds ratio [OR] 0.94; 95% CI 0.90, 0.97; P = 0.0015). A meta-analysis of the DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) and UK Biobank results showed ABCC8 p.A1369S to be associated with lower risk of type 2 diabetes at a genome-wide level of significance (OR 0.93; 95% CI 0.91, 0.95; P = 3.0 × 10−11) (Fig. 1).
Figure 1.
Association of ABCC8 p.A1369S with type 2 diabetes and coronary heart disease. For coronary heart disease, estimates were derived in UK Biobank (UKBB) using logistic regression, adjusted for age, sex, 10 principal components, and array type, and in Coronary ARtery DIsease Genome-wide Replication and Meta-analysis (CARDIOGRAM) Exome Consortium and were pooled using inverse-variance weighted fixed-effects meta-analysis. For type 2 diabetes, estimates were derived in UK Biobank using logistic regression, adjusted for age, sex, 10 principal components, and array type, and in DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium and were pooled using inverse-variance weighted fixed-effects meta-analysis. Data are given as OR [95% CI], unless otherwise stated.
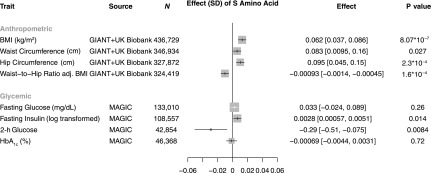
As predicted for a variant that mimics the pharmacological impact of a sulfonylurea, p.A1369S was associated with increased fasting insulin and a decrease in 2-h glucose as assessed via an oral glucose tolerance test (Supplementary Table 2 and Fig. 2). Although each of these associations was nominally significant, neither reached the prespecified Bonferroni-adjusted P value. Beyond glycemic traits, p.A1369S was associated with increased BMI (+0.062 kg/m2; 95% CI 0.037, 0.086; P = 8.1 × 10−7) and hip circumference (+0.095 cm; 95% CI 0.045, 0.15; P = 2.3 × 10−4) but lower waist-to-hip ratio adjusted for BMI, a marker of abdominal fat distribution (−0.00093; 95% CI −0.0014, −0.00045; P = 1.6 × 10−4) (Fig. 2). p.A1369S was not associated with unadjusted waist-to-hip ratio (−0.00012; 95% CI −0.00058, 0.00036; P = 0.64).
Figure 2.
Association of ABCC8 p.A1369S with cardiometabolic traits. Anthropometric traits are derived from inverse-variance weighted fixed-effects meta-analysis of the Genetic Investigation of ANthropometric Traits (GIANT) Consortium and UK Biobank and the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC). Data are given as effect [95% CI], unless otherwise stated.
Individual-level data in the UK Biobank, restricted to 119,913 participants in whom anthropometric data were available, confirmed an increased BMI among those with genotypes encoding the A/S or S/S genotypes (P for trend = 0.0075). Despite this increased overall BMI, these genotypes were associated with decreased central adiposity as assessed by waist-to-hip ratio adjusted for BMI (P for trend = 2.2 × 10−4) (Table 1). Direct imaging assessment of body fat mass using DEXA scanning was available in a subset of 1,296 participants. The S allele p.A1369S did not relate to total fat mass but was nominally associated with a reduced ratio of android (abdominal) to peripheral fat mass, a direct measurement of abdominal fat distribution (P = 0.039) (Table 1).
Table 1.
Adjusted measurements of body fat and distribution in participants in UK Biobank by ABCC8 genotype
| ABCC8 p.A1369S | P for trend | |||
|---|---|---|---|---|
| A/A (n = 15,538) | A/S (n = 55,452) | S/S (n = 48,923) | ||
| Participants (n) | 15,538 | 55,452 | 48,923 | |
| BMI (kg/m2) | 27.48 | 27.52 | 27.58 | 0.0075 |
| Waist-to-hip ratio | 0.8758 | 0.8753 | 0.8748 | 0.073 |
| Waist-to-hip ratio adjusted for BMI | 0.8763 | 0.8755 | 0.8745 | 2.2 × 10−4 |
| A/A (n = 185) |
A/S (n = 618) |
S/S (n = 483) |
P for trend | |
| Total fat mass (kg) | 26.4 | 25.9 | 26.1 | 0.83 |
| Ratio of abdominal fat mass to peripheral fat mass | 0.252 | 0.245 | 0.239 | 0.039 |
| Ratio of abdominal fat mass to peripheral fat mass adjusted for BMI | 0.250 | 0.246 | 0.239 | 0.031 |
The S allele inhibits the ATP-dependent potassium channel, mimicking the effects of sulfonylurea therapy. Fat mass was estimated using full-body DEXA. Estimates of each genotype were derived in UK Biobank, with adjustment for age, sex, 10 principal components, and array type, and standardization to the mean of these covariates.
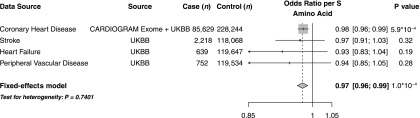
Overall, the ABCC8 p.A1369S variant was associated with a reduced risk of coronary heart disease (OR 0.98; 95% CI 0.96, 0.99; P = 5.9 × 10−4) (Fig. 1) and a reduced risk of a composite cardiovascular disease phenotype (OR 0.97; 95% CI 0.96, 0.99; P = 1.0 × 10−4) (Fig. 3). Although the associations of p.A1369S with stroke, heart failure, or peripheral vascular disease did not reach statistical significance, effects were directionally consistent with protection without evidence for significant heterogeneity across phenotypes (P heterogeneity = 0.74) (Fig. 3). In a phenome-wide association study in UK Biobank, ABCC8 p.A1369S was not associated with 32 other diseases, including other endocrine disorders and cancer (Supplementary Fig. 1 and Supplementary Table 3).
Figure 3.
Association of ABCC8 p.A1369S with cardiovascular disease. For coronary heart disease, estimates were derived in UK Biobank (UKBB) using logistic regression, adjusted for age, sex, 10 principal components, and array type, and in Coronary ARtery DIsease Genome-wide Replication and Meta-analysis (CARDIOGRAM) Exome Consortium and were pooled using inverse variance-weighted fixed-effects meta-analysis. For stroke, heart failure, and peripheral vascular disease, estimates were derived in UK Biobank using logistic regression, adjusted for age, sex, 10 principal components, and array type. Data are given as OR [95% CI], unless otherwise stated.
Discussion
The ABCC8 p.A1369S missense variant is common in the population and is known to mimic the pharmacological impact of sulfonylurea therapy. We confirm and extend previous observations of a reduced risk of type 2 diabetes and elevated BMI among individual carriers of the S allele, consistent with the effects of pharmacological sulfonylurea therapy (16). Furthermore, we demonstrate that, despite an increased BMI, carriers of the S amino acid have a more favorable body fat distribution and are protected from coronary heart disease.
These results permit several conclusions. First, these results suggest that pharmacological inhibition of the sulfonylurea receptor may reduce the risk of coronary heart disease and cardiovascular disease more broadly over the long term. Each copy of the S allele at ABCC8 p.A1369S was associated with a 6.9% lower risk of type 2 diabetes, a 2.4% lower risk of coronary heart disease, and a 2.5% lower risk of cardiovascular disease. This cardioprotective effect was noted despite an association with increased BMI and may relate to improved glycemic profile, a more favorable body fat distribution (15), or other pathways that could not be characterized in this study. The magnitude of association of the p.A1369S variant in ABCC8 with coronary heart disease (OR 0.96 per 10% reduction in risk of diabetes) is similar to a similar recent report involving a missense variant in the gene encoding GLP1R (OR 0.95 per 10% reduction in risk of diabetes) (17). This suggestion of cardioprotection derived from a genetic study was confirmed in subsequently completed cardiovascular outcome trials involving the GLP-1 analogs liraglutide and semaglutide (18,19). Although currently available clinical trial data for sulfonylurea therapy are underpowered to determine impact on cardiovascular outcomes (20), we provide evidence that pharmacological sulfonylurea therapy may similarly lead to cardiovascular benefit.
The expected magnitude of cardioprotection afforded by sulfonylurea therapy can be modeled based on previous clinical trial data. In the UK Prospective Diabetes Study (UKPDS) trial, pharmacological sulfonylurea therapy reduced the risk of an HbA1c >7% at 3 years by 33% (16). Standardizing the genetic estimates observed in this study to a 33% reduction in risk of diabetes, these results would suggest that pharmacological sulfonylurea therapy would increase BMI by 0.34 kg/m2 but reduce the risk of coronary heart disease by 13%. The 13% predicted reduction in risk of coronary heart disease with sulfonylurea therapy is identical to the 13% reduction in risk of coronary heart disease observed in a recent trial of liraglutide (18).
A genetic predisposition to decreased risk of type 2 diabetes is known to associate with protection from coronary heart disease as well (21). In order to determine whether the magnitude of impact of p.A1369S in ABCC8 with coronary heart disease could be fully explained by its effect of type 2 diabetes, we compared the observed effect to that previously noted in a study of 26 type 2 diabetes genetic variants free of pleiotropy (21). In this study, a 33% lower risk of diabetes is associated with a 4% lower risk of coronary heart disease (OR 0.96; 95% CI 0.98, 0.94). By contrast, for the p.A1369S variant, a 33% lower risk of diabetes is associated with a 13% lower risk of coronary heart disease (OR 0.87; 95% CI 0.81, 0.94; P interaction = 0.03). This indicates that degree of risk reduction for the p.A1369S variant on coronary heart disease exceeds that which would be predicted based on the average impact of type 2 diabetes associated variant at other genetic loci.
Second, these results highlight the potential importance of examining body fat distribution in addition to BMI as measurements of adiposity. As expected based on clinical experience with pharmacological sulfonylurea therapy (4), the ABCC8 p.A1369S variant was associated with increased BMI. However, estimates based both on anthropometric assessment and direct quantification using DEXA imaging suggest that the variant is associated with reduced abdominal adiposity. Although the impact on improved body fat distribution was modest, it may account for a portion of the reduced risk of coronary heart disease. Furthermore, these genetic results suggest that therapies that increase BMI may not increase coronary heart disease risk if weight gain is mediated by fat accumulation in peripheral tissues (22).
This study has several limitations. First, these results are based on the lifelong impact of a constitutive genetic variant within the sulfonylurea receptor. However, pharmacological therapy with sulfonylurea is initiated later in life and leads to more dramatic inhibition of the ATP-sensitive potassium channel and upregulation of insulin secretion. As such, our genetic modeling approach may not have fully captured adverse on-target effects of sulfonylurea therapy, such as hypoglycemia, or other off-target effects. Second, our estimates of the association of the ABCC8 p.A1369S variant with cardiometabolic traits and coronary heart disease were derived in the general population; the association of the ABCC8 p.A1369S variant with traits and coronary heart disease among individuals with type 2 diabetes (in whom pharmacological sulfonylurea therapy is currently implemented) may differ. Third, the associations of ABCC8 p.A1369S with stroke, peripheral vascular disease, and heart failure remained underpowered to detect statistically significant associations. Finally, the majority of participants in the UK Biobank and included GWAS consortia were of European ancestry; future analyses will seek to validate these observations in other ethnicities as genetic data becomes available.
In conclusion, genetic variation mimicking the impact of sulfonylurea therapy is associated with reduced risk of both type 2 diabetes and coronary heart disease.
Supplementary Material
Article Information
Funding. D.K. is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (T32 HL007734). P.N. is supported by a John S. LaDue Memorial Fellowship at Harvard Medical School. S.K. is supported by a research scholar award from the Massachusetts General Hospital, the Donovan Family Foundation, and National Institutes of Health (R01 HL127564). A.V.K. is supported by a John S. LaDue Memorial Fellowship at Harvard Medical School and a KL2/Catalyst Medical Research Investigator Training Award from Harvard Catalyst funded by the National Institutes of Health (TR001100).
Duality of Interest. S.K. has received grants from Bayer Healthcare, Aegerion Pharmaceuticals, and Regeneron Pharmaceuticals and consulting fees from Merck, Novartis, Sanofi, AstraZeneca, Alnylam Pharmaceuticals, Leerink Partners, Noble Insights, Quest Diagnostics, Genomics PLC, and Eli Lilly and Company. S.K. holds equity in San Therapeutics and Catabasis Pharmaceuticals. A.V.K. has received consulting fees from Merck and Amarin. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.A.E. and A.V.K. conceived of the study, conducted the analysis, and wrote the initial draft of the manuscript. All other authors revised the manuscript for critical intellectual content. C.A.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0149/-/DC1.
See accompanying article, p. 2069.
References
- 1.American Diabetes Association Pharmacologic approaches to glycemic treatment. Sec. 8. In Standards of Medical Care in Diabetes—2017 Diabetes Care 2017;40(Suppl. 1):S64–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Hu Y, Ley SH, Rajpathak S, Hu FB. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014;37:3106–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemmingsen B, Schroll JB, Wetterslev J, et al. Sulfonylurea versus metformin monotherapy in patients with type 2 diabetes: a Cochrane systematic review and meta-analysis of randomized clinical trials and trial sequential analysis. CMAJ Open 2014;2:E162–E175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration Guidance for Industry Diabetes Mellitus — Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. U.S. Department of Health and Human Services, 2008 [Google Scholar]
- 6.Swerdlow DI, Preiss D, Kuchenbaecker KB, et al.; DIAGRAM Consortium; MAGIC Consortium; InterAct Consortium . HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 2015;385:351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016;375:2144–2153 [DOI] [PubMed] [Google Scholar]
- 8.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742 [DOI] [PubMed] [Google Scholar]
- 9.Gloyn AL, Weedon MN, Owen KR, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 2003;52:568–572 [DOI] [PubMed] [Google Scholar]
- 10.Florez JC, Burtt N, de Bakker PIW, et al. Haplotype structure and genotype-phenotype correlations of the sulfonylurea receptor and the islet ATP-sensitive potassium channel gene region. Diabetes 2004;53:1360–1368 [DOI] [PubMed] [Google Scholar]
- 11.Fatehi M, Raja M, Carter C, Soliman D, Holt A, Light PE. The ATP-sensitive K(+) channel ABCC8 S1369A type 2 diabetes risk variant increases MgATPase activity. Diabetes 2012;61:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamming KSC, Soliman D, Matemisz LC, et al. Coexpression of the type 2 diabetes susceptibility gene variants KCNJ11 E23K and ABCC8 S1369A alter the ATP and sulfonylurea sensitivities of the ATP-sensitive K(+) channel. Diabetes 2009;58:2419–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emdin CA, Khera AV, Natarajan P, et al.; CHARGE–Heart Failure Consortium; CARDIoGRAM Exome Consortium . Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J Am Coll Cardiol 2016;68:2761–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emdin C, Khera AV, Natarajan P, et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA 2017;317:626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group . Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 17.Scott RA, Freitag DF, Li L, et al.; CVD50 Consortium; GERAD_EC Consortium; Neurology Working Group of the Cohorts for Heart; Aging Research in Genomic Epidemiology (CHARGE); Alzheimer’s Disease Genetics Consortium; Pancreatic Cancer Cohort Consortium; European Prospective Investigation into Cancer and Nutrition–Cardiovascular Disease (EPIC-CVD); EPIC-InterAct; CHARGE Consortium; CHD Exome+ Consortium; CARDIOGRAM Exome Consortium . A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci Transl Med 2016;8:341ra76–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 20.Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2013;15:938–953 [DOI] [PubMed] [Google Scholar]
- 21.Ahmad OS, Morris JA, Mujammami M, et al. A Mendelian randomization study of the effect of type-2 diabetes on coronary heart disease. Nat Commun 2015;6:7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lotta LA, Gulati P, Day FR, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet 2017;49:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.