Supplemental Digital Content is available in the text
Keywords: Ki-67, Nasal-type natural killer/T-cell lymphoma, Phosphatase and tensin homolog
Abstract
Nasal-type natural killer/T-cell (NK/T-cell) lymphoma is a more aggressive sub-group of non-Hodgkin lymphoma, which is more common in Asia. The phosphatase and tensin homolog (PTEN) was originally discovered as a candidate tumor suppressor mutated and lost in various cancers. However, its clinical value and role in NK/T-cell lymphoma remain to be further explored. In the present study, we analyzed PTEN protein expression in 60 cases of human NK/T-cell lymphoma tissues and 40 cases of control nasal mucosa tissues specimens by immunohistochemical analysis. As a result, positive rate of PTEN protein expression in NK/T-cell lymphoma tissues (33.3%) is significantly lower than that of control nasal mucosa tissues (85.0%) (P < .01). However, no significant association was found between PTEN protein expression and sex, age, tumor location, clinical staging (Ann Arbor staging), or serum lactate dehydrogenase level (P > .05). Instead, PTEN protein was inversely corrected with Ki-67 expression, indicating a functional role in PTEN in human NK/T-cell lymphoma (P < .05). For clinical outcomes, PTEN positive rate significantly increased in objective response group (CR+PR) (43.5%) compared with SD+PD group (18.9%). Furthermore, overexpression of PTEN contributed to chemotherapy sensitivity to different doses of cisplatin (DDP) in human NK/T-cell lymphoma SNK-6 cells. These results suggest that PTEN may regulate chemotherapy sensitivity of NK/T-cell lymphoma and contribute to clinical outcomes. These findings indicate PTEN to be a potential target anti-tumor therapeutics for NK/T-cell lymphoma.
1. Introduction
Nasal-type natural killer (NK)/T-cell lymphoma is a rare type of aggressive non-Hodgkin lymphoma (NHL) which mainly localizes to the upper aerodigestive tract, most commonly in the nose.[1,2] Occasional cases present in the skin, salivary gland, testis, and gastrointestinal tract. It is always associated with Epstein-Barr virus. Occurring worldwide, it is more common in Asian and Latin American countries, and more frequent in middle-aged adult men.[3–5]
Although the prevalence rate is not high, NK/T-cell lymphoma shows to be more clinically aggressive than other NHLs. Many patients lost the best therapeutic time window as the advanced or metastatic disease at the time of diagnose.[2,6,7] The 5-year survival rates remain relatively low while their mortality rates remain extremely high. In the absence of effective treatment, the median survival for advanced-stage NK/T-cell lymphoma is only 6 to 12 months.[4,5,7–9] In clinical practice, several important molecular factors have been investigated and show clinical value in therapy such as P53, P21, MMP-1, k-ras, MYC family, and ABCC4.[10–16] However, there is still an unmet medical need in discovery of the NK/T-cell lymphoma pathogenesis in clinic.
The phosphatase and tensin homolog (PTEN) was originally discovered as a candidate tumor suppressor mutated and lost in various cancers. The major role of PTEN is the buffering molecular of PI3K signaling pathway.[17,18] Yet recent studies point to additional novel, lipid phosphatase-independent functions that may contribute to its tumor-suppressive activity.[17,19] The function of PTEN in various tumor has been reported. Previous report indicated that NOTCH signaling and PI3K/AKT pathway act synergistically to maintain oncogenic activity in T-cell acute lymphoblastic leukemia.[20,21] PI3K/AKT was found activated in microarray analysis of NK/T cell lymphoma (NKTCL), and nuclear expression of phosphorylated-AKT was found in the nucleus of most NKTCL samples.[22] Data on PTEN pathway in Chinese population and its clinical value, are relatively limited.
In the present study, we identified PTEN to be down-regulated in human NK/T-cell lymphoma tissues compared with control nasal mucosa tissues. Additionally, PTEN inversely corrects with Ki-67 and shows significant relationship with clinical outcome. Thus, this result indicates PTEN to be a functional therapeutic target for treatment of human NK/T-cell lymphoma.
2. Materials and methods
2.1. Clinical tissues
A total of 60 pairs of human NK/T-cell lymphoma tissues and 40 pairs of control nasal mucosa tissues specimens were collected from the first affiliated hospital of Zhengzhou University. The age and sex were matched in the study subjects (P > .05). The study was approved by the Research Ethics Committee of the Hospital.
2.2. Evaluation of PTEN immunohistochemical staining
PTEN expression was examined by immunohistochemical staining. Monoclonal Mouse Anti-Human PTEN (Dako, Denmark M362729–2) was applied in the study. The expression was assessed by 2 independent pathologists with no knowledge of the patient characteristics, and any discrepancies were resolved by consensus. The mean ± SEM percentage value of 2 cores was considered representative of 1 tumor. The intensity of PTEN staining was evaluated using the following criteria: (−) = no yellow; (+) = yellow or orange; (++) = brown or dark brown. Only yellow, orange, and brown staining were considered a positive result.
2.3. Cell culture and reagents
SNK-6 cells were cultured in Dulbecco Modified Eagle Medium (DMEM) (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Life Technologies) at 37 °C and 5% CO2. 293T cells were also cultured in DMEM plus 10% FBS at 37 °C and 5% CO2. Cis-platinum was from Yunnan Biovalley Pharmaceutical Co. LTD (Yunnan, China).
2.4. RNA isolation and qRT-PCR analysis
Total RNA was isolated using Trizol (Life Technologies) and reverse transcribed by FastQuant RT Kit (with gDNase) (TIANGEN BIOTECH, Beijing, China) according to the manufacturer instructions. The sequences of gene-specific PCR primers were: 5′-ACC ATA ACC CAC CAC AGC-3′ and 5′-CAG TTC GTC CCT TTC CAG-3′ for PTEN, 5′-CAT GAG AAG TAT GAC AAC AGC CT-3′, and 5′-AGT CCT TCC ACG ATA CCA AAG T-3′ for glyceraldehydes-3-phosphate dehydrogenase (GAPDH). Then, qRT-PCR was performed on the Applied Biosystems 7500 Fast Real-Time PCR System (ABI, CA USA) using the SYBR Green Real-Time PCR Master Mix Kit (Applied Biosystems) according to the manufacturer's instructions. Expression of PTEN was normalized to GAPDH.
2.5. Vector construction and lentivirus packaging
The pMD18-T-simple-PTEN plasmid which contained PTEN cDNA was purchased from Sino Biological (Beijing, China). The pCHD-CMV-MCS-EF1-copGFP plasmid was purchased from System Biosciences (Mountain View, Canada). The coding sequence of PTEN was amplified using pMD18-T-simple-PTEN as template by PCR technique and subcloned into the EcoR I and BamH I sites of the pCHD-CMV-MCS-EF1-copGFP plasmid. Three shRNAs were designed to target PTEN using the BLOCK-iT RNAi Designer (Thermo Fisher Scientific, Waltham, MA) and a scrambled shRNA was used as a control.
The gene ID of PTEN in NCBI is NM_000314. The shRNA target was designed through the website: http://rnaidesigner.lifetechnologies.com/rnaiexpress/. The sequences were listed as in supplemental data the followings while synthesis of shRNA was performed by Shanghai Biotech. The detailed sequences were shown in Supplemental Table 1
Paired shRNA oligos were cloned into the HpaI and XhoI sites of the Pll3.7 vector (Addgene, Cambridge, MA). For lentivirus packing, HEK293T cells were cotransfected with the constructs described above and the lentivirus packaging plasmids. The supernatant of lentivirus was collected, filtered with 0.45 μm membrane filter to remove cell debris, and ready for infection of SNK-6 cells.
2.6. Proliferation assays
SNK-6 cells were seeded in 96-well plates with 10,000 cells per well. Cell proliferation was measured by Cell Counting Kit-8 (CCK-8) colorimetric assay (Dojindo Laboratories, Gaithersburg, Japan) posttransduction of lenti-virus. The cellular growth density was measured per 24 hours by OD450 using the microplate reader over the course of 5 days. This experiment repeated 3 times so as to get sufficient data. Growth inhibitory rate was calculated as the following formula: (absorbance of control group − absorbance of treatment group)/absorbance of control group × 100%.
2.7. Statistical analysis
Statistical comparisons were made using the ANOVA analysis where appropriate. Results were considered significant in all experiments at ∗P < .05, ∗∗P < .01, and ∗∗∗P < .005. Data were expressed as the mean ± SD.
3. Results
3.1. PTEN levels are down-regulated in human NK/T-cell lymphoma clinical samples
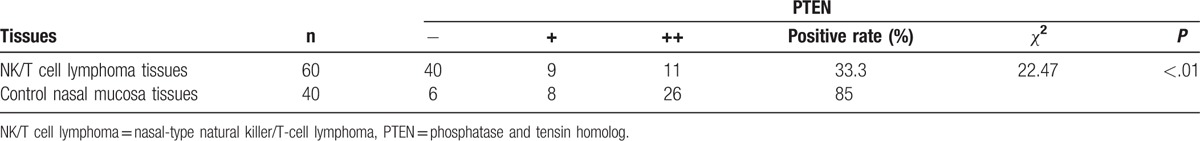
To investigate PTEN protein expression in clinical samples, 60 cases of human NK/T-cell lymphoma tissues and 40 cases of control nasal mucosa tissues specimens embedded in paraffin underwent immunohistochemical analysis (Fig. 1). The relative expression level of PTEN was scored by 2 independent pathologists. As a result, only 20 of 60 (33.3%) NK/T-cell lymphoma tissues were positive for PTEN protein expression, whereas 34 of 40 (85.0%) control nasal mucosa tissues were positive for PTEN protein expression (Table 1). Chi-square analysis showed that χ2 = 22.47 with P < .01. These findings suggest that PTEN protein expression is down-regulated in human NK/T-cell lymphoma clinical samples.
Figure 1.
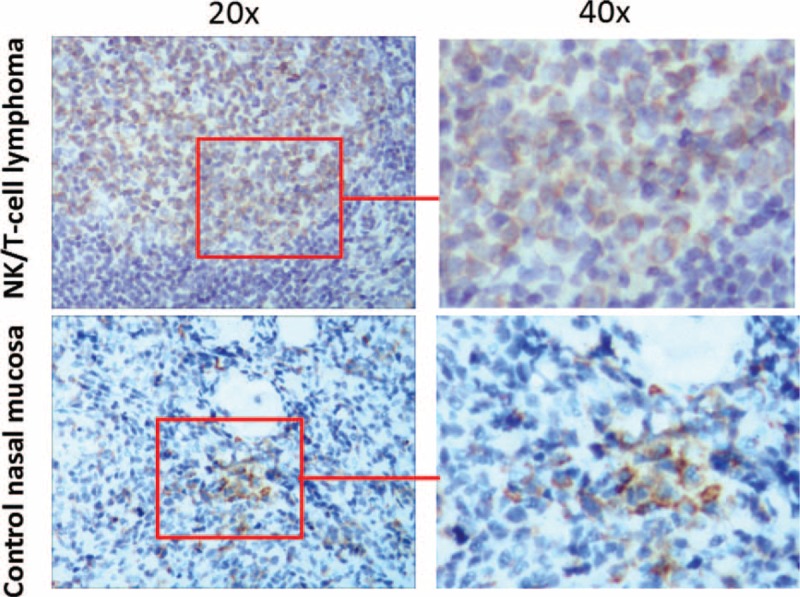
PTEN expression in clinical samples. PTEN protein expression in human NK/T-cell lymphoma tissues and control nasal mucosa tissues were examined by immunochemistry staining. (A) Human NK/T-cell lymphoma tissues; (B) control nasal mucosa tissues. NK/T-cell = nasal-type natural killer/T-cell, PTEN = phosphatase and tensin homolog.
Table 1.
PTEN expressions in clinical tissues.
3.2. PTEN levels inversely correct with Ki-67 expression
To further investigate the correction between PTEN protein expression and clinical characters, we collected the clinical information of the samples, such as sex, age, tumor location, clinical staging (Ann Arbor staging), and serum lactate dehydrogenase (LDH) level. However, as shown in Table 2, no significant association was found between PTEN protein expression and sex, age, tumor location, clinical staging (Ann Arbor staging), or serum LDH level (P > .05).
Table 2.
Correlation between PTEN and clinical characters.
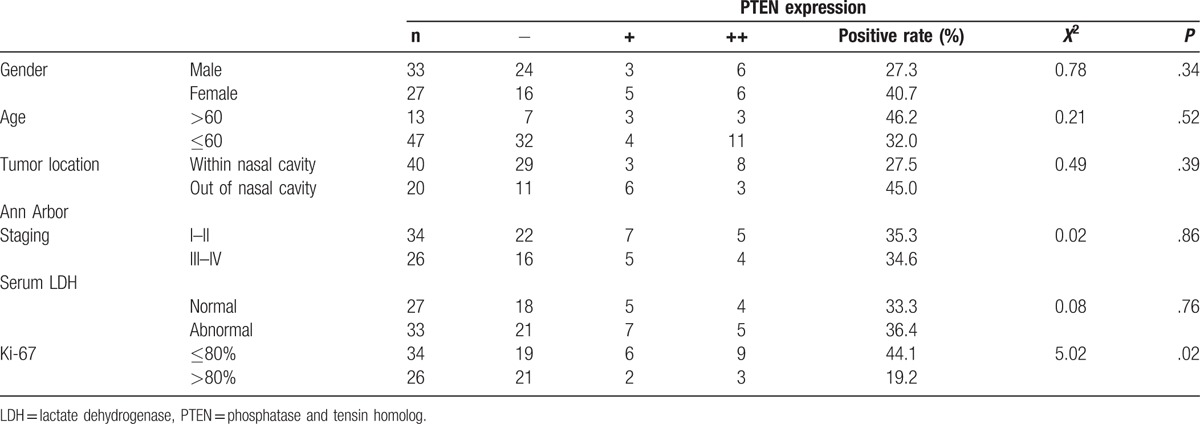
Ki-67 is a common biomarker for tumor growth and progression. We further analyze Ki-67 expression in the above human NK/T-cell lymphoma tissues and control nasal mucosa tissues specimens by immunohistochemical analysis (Supplemental Fig. 1). As shown in Table 2, PTEN protein was inversely corrected with Ki-67 expression, indicating a functional role in PTEN in human NK/T-cell lymphoma (P < .05).
3.3. PTEN levels correct with clinical therapeutic outcome
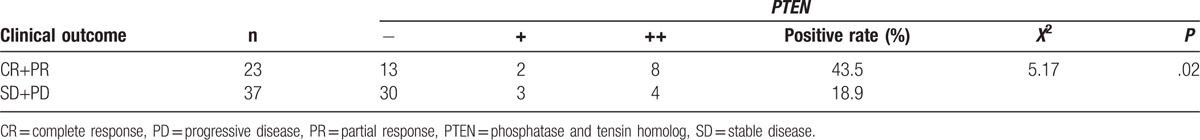
Given the potential functional role of PTEN in human NK/T-cell lymphoma, we analyze its expression with clinical outcome. Patients were follow-up for 1 year after therapy. CR (complete response), partial response (PR), stable disease (SD), and progressive disease (PD) were recorded. The objective response rate (CR+PR) was found in 23 of 60 cases. PTEN positive rate significantly increased in objective response group (CR+PR) (43.5%) compared with SD+PD group (18.9%). These results indicate that PTEN levels were correlated with clinical therapeutic outcome (P < .05) (Table 3).
Table 3.
Correction between PTEN and therapeutic outcome.
3.4. PTEN regulates human NK/T-cell lymphoma SNK-6 cell chemotherapy resistance to cisplatin (DDP)
We further explored the biological function(s) of PTEN down-regulation in human lymphoma SNK-6 cells. The mRNA expression of PTEN was analyzed by real-time PCR. PTEN expressions both at mRNA and protein levels were significantly down-regulated in SNK-6 cells compared with normal NK cells (Fig. 2A and C). Stable over-expression or down-expression of PTEN in SNK-6 cells were set up using lentivirus transfection (Fig. 2B and D). As shown in Fig. 2E, over-expression of PTEN in SNK-6 cells significantly inhibited cell growth compared with control group (P < .01). When treated with different doses of cisplatin (DDP) (0.03125–8 μg/mL), overexpression of PTEN contributed to chemotherapy sensitivity to DDP (Fig. 2F) after treatment for 24 hours compared with control group (P < .01). These results suggest that PTEN regulates chemotherapy resistance to DDP in human NK/T-cell lymphoma SNK-6 cells.
Figure 2.
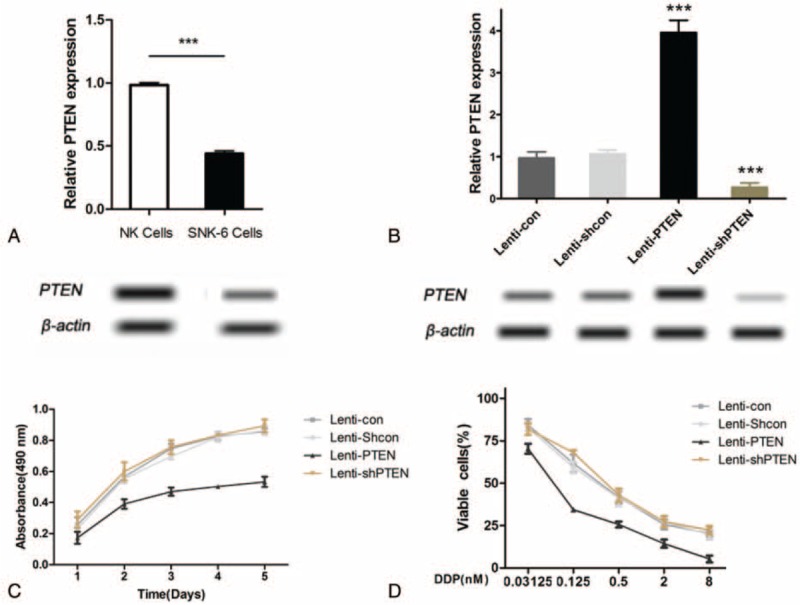
PTEN regulates chemotherapy resistance to DDP in human NK/T-cell lymphoma SNK-6 cells. (A) The mRNA expression of PTEN in NK cells and SNK-6 cells were measured by real-time PCR. (B) Stable overexpression of PTEN in SNK-6 cells was carried out through lentivirus transfection while knockdown of its expression was performed using shRNA. The mRNA expression of PTEN was measured by real-time PCR. (C) The protein expression of PTEN in NK cells and SNK-6 cells were measured by Western blot. (D) The protein expression of PTEN in overexpression and knowdown SNK-6 along with control cells were measured by Western blot. (E) Cells were cultured for 5 days and cell proliferation was examined by CCK-8. (F) Cells were treated with DDP (0.03125–8 μg/mL) for 24 hours. Cell viability was examined by CCK-8. Lenti-con (cells transfected with control vector for PTEN overexpression); Lenti-Shcon (cells transfected with control shRNA vector); Lenti-PTEN (cells transfected with vector for PTEN overexpression); Lenti-Shcon (cells transfected with shRNA vector). CCK-8 = cell counting Kit-8, DDP = different doses of cisplatin, NK/T-cell = nasal-type natural killer/T-cell, PTEN = phosphatase and tensin homolog.
4. Discussion
PTEN, is one of the most common tumor suppressors in human malignancies. The importance of PTEN as a tumor suppressor is further supported by the fact that cancer-specific human mutations, functional connections to oncogenic signaling, and robust animal models through multiple mechanisms contribute substantially to human cancer.[17,19,21] Biochemically, PTEN is a phosphatase that de-phosphorylates phosphatidylinositol-[3, 4, 5]-tri-phosphate (PIP3), the lipid product of class I phosphoinositide 3-kinase (PI3K) regulating AKT pathway. The continued elucidation of the roles of nuclear PTEN will mediate the expressions of various tumor functional genes, and regulate diverse cellular processes, including metabolism, survival, proliferation, apoptosis, growth, and cell migration.[17,23] Unsurprisingly, loss of PTEN has a substantial impact on multiple aspects of cancer development. In fact, several cancer types have less or loss of PTEN expression, such as cervical cancer, prostate cancer, lung cancer, mesothelioma, and lymphoma.[19,21] Absence of PTEN expression occurs in T-cell acute lymphoblastic leukemia (T-ALL). T-ALL patients had a PTEN mutation rate of 27% and a PTEN deletion rate of 9%. According to diffuse large B-cell lymphoma, PTEN was lost in 11% of the patients.[24,25] In the present study, we found that PTEN showed negative expression in 68% of the NK/T-cell lymphoma tissues, which is consist with its down-regulation in many tumors. In addition, Ki-67 inversely expressed with PTEN. Ki-67 is a biomarker for tumor growth. The increase of Ki-67 may promote tumor cell proliferation. Although we identified the inverse expression between PTEN and Ki-67, the mechanism remained unknown. PTEN is strongly associated with and functions through p-PI3K and p-AKT signal.[26,27] We also examine the influence of PTEN on p-PI3K and p-AKT pathway in human tissues. As shown in Supplemental Fig. 2, p-PI3K and p-AK expressions were reduced in PTEN-high sample compared with PTEN-low sample. Thus, p-PI3K and p-AK may be involved in the effect of PTEN in NK/T-cell lymphoma tissues.
Complete loss of PTEN protein expression is significantly associated with advanced cancer and poor outcome. For NK/T-cell lymphoma, we found that PTEN expression significantly corrects with objective response group (CR+PR) compared with SD+PD group, indicating a better clinical therapeutic outcome (P < .05). However, around 68% of NK/T-cell lymphoma tissues didn’t express PTEN. The increase of PTEN may benefit the therapeutic effect. We next overexpressed PTEN in human lymphoma SNK-6 cells. Similar to our hypothesis, overexpression of PTEN increase the chemotherapy sensitivity to DDP. This may partially explain the relationship between PTEN expression in tissues and therapeutic outcome. These findings provide support for restoring PTEN as a potential gene therapy, as it may provide therapeutic benefits for treating human NK/T-cell lymphoma. Thus, PTEN may become a therapeutic target for drug development.
However, this study has a number of limitations. First, the sample size is still limited. A larger scale of study will provide more convincing results on PTEN expression and the association with clinical characters. Secondly, we only use immunohistochemical staining to investigate PTEN expression. If we could obtain flesh sample to examine mRNA expression by RT-PCR and protein expression by Western blot, it would provide more supportive evidence. Thirdly, in vivo animal study will provide more evidences on the role of PTEN in NK/T-cell lymphoma.
In summary, the present study identified PTEN to be downexpressed in human NK/T-cell lymphoma tissues and regulates chemotherapy sensitivity to DDP, suggesting a possible functional therapeutic target.
Supplementary Material
Footnotes
Abbreviations: CCK-8 = cell counting Kit-8, CR = complete response, DDP = different doses of cisplatin, DMEM = Dulbecco Modified Eagle Medium, FBS = fetal bovine serum, GAPDH = glyceraldehydes-3-phosphate dehydrogenase, NK/T-cell = nasal-type natural killer/T-cell, PD = progressive disease, PI3K = phosphoinositide 3-kinase, PIP3 = de-phosphorylates phosphatidylinositol [3, 4, 5]-tri-phosphate, PR = partial response, PTEN = phosphatase and tensin homolog, SD = stable disease, T-ALL = T-cell acute lymphoblastic leukemia.
XF and XZ have contributed equally to the study and should be considered as co-first authors.
This study was supported by grants from the Youth Foundation of the First Affiliated Hospital of Zhengzhou University (13Y0165) and General Program of the National Natural Science Foundation of China (81570203 and 81470364).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Lin TC, Chen SU, Chen YF, et al. Intramucosal variant of nasal natural killer (NK)/T cell lymphoma has a better survival than does invasive variant: implication on loss of E26 transformation-specific sequence 1 (ETS-1) and T-box expressed in T cells (T-bet) with invasion. Histopathology 2012;60:287–95. [DOI] [PubMed] [Google Scholar]
- [2].Harabuchi Y, Takahara M, Kishibe K, et al. Nasal natural killer (NK)/T-cell lymphoma: clinical, histological, virological, and genetic features. Int J Clin Oncol 2009;14:181–90. [DOI] [PubMed] [Google Scholar]
- [3].Tse E, Kwong YL. Nasal NK/T-cell lymphoma: RT, CT, or both. Blood 2015;126:1400–1. [DOI] [PubMed] [Google Scholar]
- [4].Sun L, Che K, Zhao Z, et al. Sequence analysis of Epstein-Barr virus (EBV) early genes BARF1 and BHRF1 in NK/T cell lymphoma from Northern China. Virol J 2015;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim SH, Yang WI, Min YH, et al. The role of the polycomb repressive complex pathway in T and NK cell lymphoma: biological and prognostic implications. Tumour Biol 2016;37:2037–47. [DOI] [PubMed] [Google Scholar]
- [6].Wang L, Wang JH, Wu-Xiao ZJ, et al. Lymphopenia during routine follow-up may predict relapse in patients with extranodal NK/T cell lymphoma. Tumour Biol 2015;36:1747–53. [DOI] [PubMed] [Google Scholar]
- [7].Radochova V, Radocha J, Nova M, et al. NK/T-cell lymphoma nasal type with an unusual clinical course. Indian J Dermatol Venereol Leprol 2014;80:564–6. [DOI] [PubMed] [Google Scholar]
- [8].Hamidah NH, Shahrom S, Siti AM, et al. Nasal type NK/T-cell lymphoma—diagnosis and treatment difficulties. Clin Ter 2014;165:139–42. [DOI] [PubMed] [Google Scholar]
- [9].Roy AD, Tuli IP, Joshi D. NK/T cell lymphoma with inverted papilloma: A rare coexistence. Australas Med J 2014;7:318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Meneses-Garcia A, Herrera J, Mohar A, et al. [Metalloproteinase (MMP-1, 2 and 11), tissue inhibitor of metalloproteinase-1 (TIMP-1), and p53 expression in nasal-type angiocentric T/NK-cell lymphoma: an immunohistochemical study]. Gac Med Mex 2005;141:291–6. [PubMed] [Google Scholar]
- [11].Hongyo T, Hoshida Y, Nakatsuka S, et al. p53, K-ras, c-kit and beta-catenin gene mutations in sinonasal NK/T-cell lymphoma in Korea and Japan. Oncol Rep 2005;13:265–71. [PubMed] [Google Scholar]
- [12].Ye Z, Cao Q, Niu G, et al. p63 and p53 expression in extranodal NK/T cell lymphoma, nasal type. J Clin Pathol 2013;66:676–80. [DOI] [PubMed] [Google Scholar]
- [13].Huang X, Sun Q, Fu H, et al. Both c-Myc and Ki-67 expression are predictive markers in patients with extranodal NK/T-cell lymphoma, nasal type: a retrospective study in China. Pathol Res Pract 2014;210:351–6. [DOI] [PubMed] [Google Scholar]
- [14].Zhang X, Zhao L, Li X, et al. ATP-binding cassette sub-family C member 4 (ABCC4) is overexpressed in human NK/T-cell lymphoma and regulates chemotherapy sensitivity: Potential as a functional therapeutic target. Leuk Res 2015;39:1448–54. [DOI] [PubMed] [Google Scholar]
- [15].Hoshida Y, Hongyo T, Jia X, et al. Analysis of p53, K-ras, c-kit, and beta-catenin gene mutations in sinonasal NK/T cell lymphoma in northeast district of China. Cancer Sci 2003;94:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Takahara M, Kishibe K, Bandoh N, et al. P53, N- and K-Ras, and beta-catenin gene mutations and prognostic factors in nasal NK/T-cell lymphoma from Hokkaido, Japan. Hum Pathol 2004;35:86–95. [DOI] [PubMed] [Google Scholar]
- [17].Boosani CS, Agrawal DK. PTEN modulators: a patent review. Expert Opin Ther Pat 2013;23:569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nakanishi A, Kitagishi Y, Ogura Y, et al. The tumor suppressor PTEN interacts with p53 in hereditary cancer (Review). Int J Oncol 2014;44:1813–9. [DOI] [PubMed] [Google Scholar]
- [19].Tokuhira N, Kitagishi Y, Suzuki M, et al. PI3K/AKT/PTEN pathway as a target for Crohn's disease therapy (Review). Int J Mol Med 2015;35:10–6. [DOI] [PubMed] [Google Scholar]
- [20].Guo D, Teng Q, Ji C. NOTCH and phosphatidylinositide 3-kinase/phosphatase and tensin homolog deleted on chromosome ten/AKT/mammalian target of rapamycin (mTOR) signaling in T-cell development and T-cell acute lymphoblastic leukemia. Leuk Lymphoma 2011;52:1200–10. [DOI] [PubMed] [Google Scholar]
- [21].Palomero T, Dominguez M, Ferrando AA. The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell Cycle 2008;7:965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kucuk C, Hu X, Gong Q, et al. Diagnostic and biological significance of KIR expression profile determined by RNA-Seq in Natural Killer/T-Cell Lymphoma. Am J Pathol 2016;186:1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bertrand FE, McCubrey JA, Angus CW, et al. NOTCH and PTEN in prostate cancer. Adv Biol Regul 2014;56:51–65. [DOI] [PubMed] [Google Scholar]
- [24].Subramaniam PS, Whye DW, Efimenko E, et al. Targeting nonclassical oncogenes for therapy in T-ALL. Cancer Cell 2012;21:459–72. [DOI] [PubMed] [Google Scholar]
- [25].Zuurbier L, Petricoin ER, Vuerhard MJ, et al. The significance of PTEN and AKT aberrations in pediatric T-cell acute lymphoblastic leukemia. Haematologica 2012;97:1405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dongchan R, Chaeyoung L. Expression quantitative trait loci for PI3K/AKT pathway. Medicine 2017;96:e5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Han X, Ji Y, Zhao J, et al. Expression of PTEN and mTOR in pancreatic neuroendocrine tumors. Tumour Biol 2013;34:2871–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


