Abstract
Rationale:
Little is known about optic radiation (OR) injury following aneurysmal subarachnoid hemorrhage (SAH). In the current study, we report on a patient who showed a visual field defect and injury of the OR following aneurysmal SAH, using diffusion tensor tractography (DTT).
Patient concerns:
At 4 weeks from onset, when a 62-year old female started rehabilitation, she complained of a visual field defect. Peripheral field defects were detected on both eyes using the Humphrey visual field test.
Diagnoses:
The patient underwent aneurysm clipping for a ruptured aneurysm in the left posterior communicating artery and extraventricular drainage (the left prefrontal approach) for subarachnoid hemorrhage. She also underwent conservative management for intracerebral hemorrhage in the left internal capsule detected at 2 days after onset.
Interventions:
DTT data were acquired at 4 weeks after onset
Outcomes:
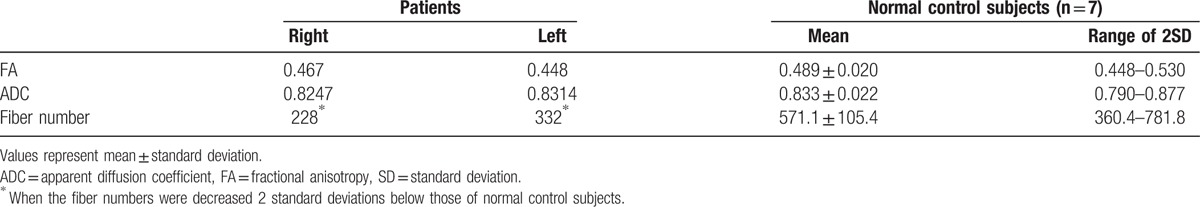
Regarding DTT parameters, fiber numbers of both ORs of the patient were decreased over 2 standard deviations of that of 7 age- and sex-matched normal subjects normal control subjects. However, the value of fractional anisotropy was similar to that of normal control subjects. On the configuration of the OR of the patient, both ORs were thinner than those of normal control subjects.
Lessons:
Injury of the OR was demonstrated in a patient with a visual field defect following aneurysmal SAH, using DTT.
Keywords: diffusion tensor imaging, optic radiation, subarachnoid hemorrhage, visual field defect
1. Introduction
Aneurysmal subarachnoid hemorrhage (SAH), caused by rupture of a cerebral artery aneurysm, can accompany various neurological sequelae, including visual impairment.[1,2] Visual field defect is one of the visual problems observed in patients with aneurysmal SAH; this symptom has been reported in approximately 50% of patients with aneurysmal SAH.[3,4] Visual field defect is a typical clinical manifestation of optic radiation (OR) injury.[5] Diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), enables 3-dimensional construction and evaluation of the OR.[6] Many studies using DTT have reported on injury of the OR in various brain pathologies including intracerebral hemorrhage and traumatic brain injury.[7–11] However, no study on injury of the OR in patients with aneurysmal SAH has been reported.
In the current study, we report on a patient who showed a visual field defect and injury of the OR following aneurysmal SAH.
2. Case report
A 62-year old female underwent aneurysm clipping for a ruptured aneurysm in the left posterior communicating artery and extraventricular drainage (the left prefrontal approach) for subarachnoid hemorrhage at the neurology department of a university hospital (Fig. 1A). She also underwent conservative management for intracerebral hemorrhage in the left internal capsule, which is not involved in the pathway of OR, detected at 2 days after onset. At 4 weeks from onset, she was transferred to the rehabilitation department of another university hospital to undergo rehabilitation and complained of a visual field defect although there was no problem on both eyes. Peripheral field defects were detected on both eyes using the Humphrey visual field test (Fig. 1B). Seven age- and sex-matched normal subjects (mean age, 59.7 years; range, 51–66 years) were enrolled in this study. All participants provided written, informed consent and the local ethics committee approved the study protocol.
Figure 1.
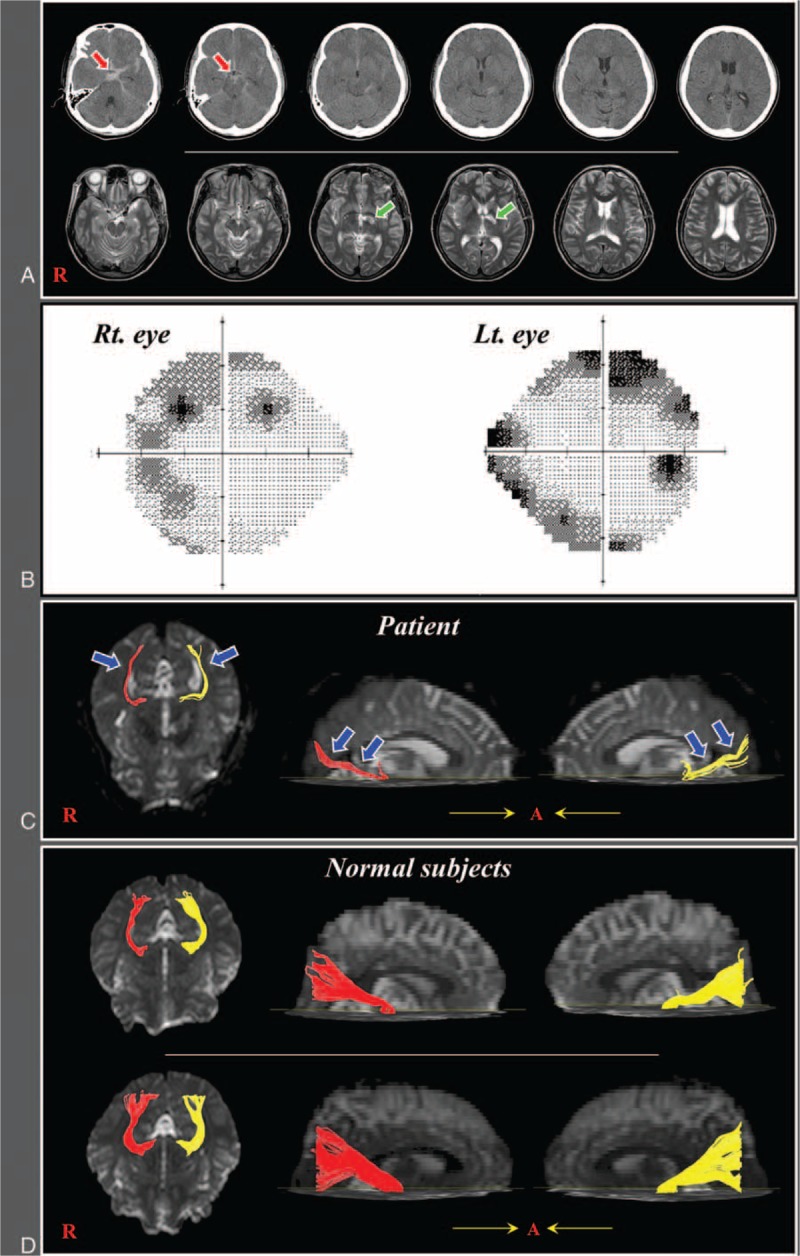
(A) Brain CT images at onset show subarachnoid hemorrhage and T2-weighted MR images at 4 weeks after onset show a leukomalactic lesion in the left internal capsule. (B) The Humphrey visual field test of the patients shows a peripheral field defect in both eyes. (C) Diffusion tensor tractography images of the optic radiation in the patient (red color: right optic radiation, yellow color: left optic radiation). (D) Diffusion tensor tractography images of the optic radiation in 2 control subjects (62-year and 60-year old females).CT = computed tomography, MR = magnetic resonance.
2.1. Diffusion tensor imaging
DTI data were acquired at 4 weeks after onset using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera. Imaging parameters were as follows: acquisition matrix=96×96; reconstructed to matrix=192×192matrix; field of view=240×240 mm2; TR = 10,398ms; TE = 72ms; EPI factor=59; b=1000 s/mm2; NEX = 1; slice gap = 0 mm and a slice thickness of 2.5 mm. Eddy current-induced image distortions were removed using affine multi-scale 2-dimensional registration in the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl).[12] DTI-Studio software (CMRM, Johns Hopkins Medical Institute, Baltimore, MD) was used for reconstruction of ORs.[13] For the OR, the seed region of interest (ROI) was placed on the lateral geniculate body on the color map, and the target ROI was placed on the bundle of OR at the end portion between the lateral geniculate body and the occipital pole with the option of a CUT operation.[6,9,14] The CUT operation was used for reconstruction of the OR between seed ROI and target ROI by eliminating other redundant fiber trajectories. Fiber tracking was started at the center of a seed voxel with a fractional anisotropy (FA) of > 0.15 and ended at a voxel with a fiber assignment of > 0.15 and a tract turning-angle of < 70°.[14] FA, apparent diffusion coefficient (ADC), and fiber number of the OR were measured in both hemispheres. DTI parameter values showing more than 2 standard deviations of that of normal control values were defined as abnormal.
Regarding DTT parameters, Table 1 summarizes FA, ADC, and fiber number of the ORs of the patient and normal control subjects. Fiber numbers of both ORs of the patient were decreased over 2 standard deviations of that of normal control subjects. However, FA and ADC values were similar to that of normal control subjects. On the configuration of the OR of the patient, both ORs were thinner (blue arrows) than those of normal control subjects (Fig. 1C and D).
Table 1.
Diffusion tensor image parameters of patient and normal control subjects.
3. Discussion
In this study, we attempted to demonstrate injury of the OR in a patient who showed a peripheral field defect of both eyes on a visual field test. On DTT parameters, the fiber number of the OR in this patient was decreased in both hemispheres, whereas the FA value was similar to that of normal control subjects in both hemispheres. The FA value represents the degree of directionality of microstructures (e.g., axons, myelin, and microtubules), whereas the fiber number is determined by the number of neural fibers contained within the neural tract.[15,16] Therefore, the decrement of fiber number without significant change of FA value in both ORs indicates injury of both ORs. The thinning of both ORs appears to be additional evidence of both OR injury. We believe that these results on DTT coincided with the peripheral field. A few studies on the pathogenic mechanism of neural injury in SAH have been reported.[17,18] In 2007, Liu et al[17] reported that SAH may cause global mild vasogenic edema in white matter and deep gray matter, which could not be detected on conventional MRI, but was detected by measuring the apparent diffusion coefficient value in the subacute stage of SAH. In 2013, Sabri et al[18] investigated the mechanism of brain injury in SAH, and they reported that SAH induced excitotoxicity and apoptosis of brain cell. This result indicated that SAH can cause injury to white matter by excitotoxicity, which is located near the arachnoid space. Considering the results of the previous studies, we can assume that SAH may cause injury to the OR near the arachnoid space in the occipital lobe. However, follow up DTI studies from acute to chronic stage should be encouraged for clarification of the pathogenic mechanism of injury of the OR following SAH.
Many studies have reported on visual impairment in patients with SAH.[3,4,17,19] Most of these studies focused on visual problems caused by the lesion in the visual pathway, except for the OR, such as the eye, optic nerve, or optic chiasm.[3,19] To the best of our knowledge, only a few studies have reported results related to injury of the OR.[4,17] In 2007, Liu et al[17] reported that the apparent diffusion coefficient value in normal appearing white matter in the occipital lobe, as well as the whole cerebral lobe, except for the frontal lobe, was increased in patients with aneurismal SAH at subacute stage. In 2011, Obuchowska et al[4] reported detection of visual field defects in 50% of 23 patients with SAH treated with aneurismal clipping. The most frequent type of visual field defects were: constricted field (47.8%), multiple peripheral foci (26.1%), and superior field defect (17.4%). As a result, to the best of our knowledge, this is the first study to demonstrate injury of the OR in patients with aneurysmal SAH. However, because precise reconstruction of the anterior portion of the visual pathway using DTT is limited, we could not rule out injury of the anterior portion of the visual pathway such as optic nerve or optic chiasm.[6] In addition, we did not consider the body weight of subjects. Further studies to clarify the association of injury of the anterior visual pathway and visual field defect should be encouraged.
In conclusion, injury of the OR was demonstrated in a patient with a visual field defect following aneurysmal SAH, using DTT. We believe that DTI scanning can be recommended for patients who complain of a visual field defect following aneurysmal SAH. Conduct of further complementary studies involving larger case numbers is warranted, and further studies on other areas of the visual pathway in the brain would be necessary.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, FA = fractional anisotropy, OR = optic radiation, SAH = subarachnoid hemorrhage.
Funding: This work was supported by the Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
The authors have no conflicts of interest to disclose.
References
- [1].Sarner M, Rose FC. Clinical presentation of ruptured intracranial aneurysm. J Neurol Neurosurg Psychiatry 1967;30:67–70. [Google Scholar]
- [2].van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306–18. [DOI] [PubMed] [Google Scholar]
- [3].Tsementzis SA, Williams A. Ophthalmological signs and prognosis in patients with a subarachnoid haemorrhage. Neurochirurgia (Stuttg) 1984;27:133–5. [DOI] [PubMed] [Google Scholar]
- [4].Obuchowska I, Turek G, Mariak Z, et al. Late ophthalmological assessment of patients with subarachnoid hemorrhage and clipping of cerebral aneurysm. Acta Neurochir (Wien) 2011;153:2127–36. [DOI] [PubMed] [Google Scholar]
- [5].Mizrachi IB, Schmaier AH, Trobe JD. Homonymous hemianopia caused by occipital lobe infarction in heparin-induced thrombocytopenia and thrombosis syndrome. J Neuroophthalmol 2005;25:193–7. [DOI] [PubMed] [Google Scholar]
- [6].Hofer S, Karaus A, Frahm J. Reconstruction and dissection of the entire human visual pathway using diffusion tensor MRI. Front Neuroanat 2010;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yoshida M, Ida M, Nguyen TH, et al. Resolution of homonymous visual field loss documented with functional magnetic resonance and diffusion tensor imaging. J Neuroophthalmol 2006;26:11–7. [DOI] [PubMed] [Google Scholar]
- [8].Polonara G, Salvolini S, Fabri M, et al. Unilateral visual loss due to ischaemic injury in the right calcarine region: a functional magnetic resonance imaging and diffusion tension imaging follow-up study. Int Ophthalmol 2011;31:129–34. [DOI] [PubMed] [Google Scholar]
- [9].Seo JP, Choi BY, Chang CH, et al. Diffusion tensor imaging findings of optic radiation in patients with putaminal hemorrhage. Eur Neurol 2013;69:236–41. [DOI] [PubMed] [Google Scholar]
- [10].Seo YS, Kim SH, Jang SH. Bilateral homonymous quadrantanopsia due to optic radiation injury in a patient with traumatic brain injury. Am J Phys Med Rehabil 2015;94:e116. [DOI] [PubMed] [Google Scholar]
- [11].Jang SH, Seo JP. Damage to the optic radiation in patients with mild traumatic brain injury. J Neuroophthalmol 2015;35:270–3. [DOI] [PubMed] [Google Scholar]
- [12].Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–19. [DOI] [PubMed] [Google Scholar]
- [13].Jiang H, van Zijl PC, Kim J, et al. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 2006;81:106–16. [DOI] [PubMed] [Google Scholar]
- [14].Glass HC, Berman JI, Norcia AM, et al. Quantitative fiber tracking of the optic radiation is correlated with visual-evoked potential amplitude in preterm infants. AJNR Am J Neuroradiol 2010;31:1424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008;34:51–61. [DOI] [PubMed] [Google Scholar]
- [16].Jang SH, Jang WH. Change of the corticospinal tract in the unaffected hemisphere by change of the dominant hand following stroke: a cohort study. Medicine (Baltimore) 2016;95:e2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu Y, Soppi V, Mustonen T, et al. Subarachnoid hemorrhage in the subacute stage: elevated apparent diffusion coefficient in normal-appearing brain tissue after treatment. Radiology 2007;242:518–25. [DOI] [PubMed] [Google Scholar]
- [18].Sabri M, Lass E, Macdonald RL. Early brain injury: a common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Rres Treat 2013;2013:394036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hara N, Mukuno K, Ohtaka H, et al. Ischemic optic neuropathy associated with subarachnoid hemorrhage after rupture of anterior communicating artery aneurysm. Ophthalmologica 2003;217:79–84. [DOI] [PubMed] [Google Scholar]


