Abstract
The study was to comprehensively compare the postoperative outcome and imaging parameter characters in a short/middle period between the percutaneous endoscopic lumbar discectomy (PELD) and the internal fixation of bone graft fusion (the most common form is posterior lumbar interbody fusion [PLIF]) for the treatment of adjacent segment lumbar disc prolapse with stable retrolisthesis after a previous lumbar internal fixation surgery.
In this retrospective case-control study, we collected the medical records from 11 patients who received PELD operation (defined as PELD group) for and from 13 patients who received the internal fixation of bone graft fusion of lumbar posterior vertebral lamina decompression (defined as control group) for the treatment of the lumbar disc prolapse combined with stable retrolisthesis at Department of Spine Surgery, the Third Hospital of Hebei Medical University (Shijiazhuang, China) from May 2010 to December 2015. The operation time, the bleeding volume of perioperation, and the rehabilitation days of postoperation were compared between 2 groups. Before and after surgery at different time points, ODI, VAS index, and imaging parameters (including Taillard index, inter-vertebral height, sagittal dislocation, and forward bending angle of lumbar vertebrae) were compared.
The average operation time, the blooding volume, and the rehabilitation days of postoperation were significantly less in PELD than in control group. The ODI and VAS index in PELD group showed a significantly immediate improving on the same day after the surgery. However, Taillard index, intervertebral height, sagittal dislocation in control group showed an immediate improving after surgery, but no changes in PELD group till 12-month after surgery. The forward bending angle of lumbar vertebrae was significantly increased and decreased in PELD and in control group, respectively.
PELD operation was superior in terms of operation time, bleeding volume, recovery period, and financial support, if compared with lumbar internal fixation operation. Radiographic parameters reflect lumber structure changes, which could be observed immediately after surgery in both methods; however, the recoveries on nerve function and pain relief required a longer time, especially after PLIF operation.
Keywords: adjacent segment lumbar disc prolapse, lumbar internal fixation, percutaneous endoscopic lumbar discectomy, stable retrolisthesis
1. Introduction
Posterior lumbar interbody fusion (PLIF) used to be a widely employed approach for treating lumbar disc herniation and low back pain caused with intervertebral disc source, but adjacent segment disc prolapse (ASDP) is a long-term complication after a previous surgery of lumbar fusion and internal fixation or PLIF.[1–3] Whether there is only a simple adjacent segment herniation or degeneration, leading to the subsequent lumbar stenosis, may not cause the severe clinical manifestations. However, whether there is the stable retrolisthesis of the merged segmental lumbar vertebra, which leads to the lumbar intervertebral foramen further stenosis, would cause the severe nerve damage that results in the pain on back and legs and the movement difficulty. Traditional treatment for ASDP is to perform a secondary surgery (repeated open surgery), including the bone graft fusion and internal fixation of lumbar posterior vertebral lamina decompression (such as posterior lumbar interbody fusion, PLIF).[4] Internal fixation usually immobilizes the spine with implants such as metallic screws and rods/plates, or interbody cages, leading to the bony bridge healing across the 2 vertebrae. However, repeated open surgery of internal fixation has been associated with complications, such as tissue scarring and adjacent segment degeneration caused with further damage to the vertebral shift.[5,6] An alternative approach is percutaneous endoscopic lumbar discectomy (PELD), which is operated through intact tissue and can reduce the repeated damage to the posterior and para-spinal structures,[7–10] and is, therefore, a kind of minimal trauma surgery. Although the advantage of PELD has been studied, including pain relief, less damage to ligamentous structures, faster rehabilitation, and shorter hospital stay, more studies of PELD on the basis of clinical experience, indications, long-term postoperative effects and complications, and technique improvement need to be widely investigated.[11–13]
Radiography is a noninvasive and convenient way to monitor the adjacent segment disc changes on a short/middle-term basis after PELD or PLIF operation. The short/middle-term radiographic changes may predict a long-term prognosis. However, fewer reports showed the clinical outcome of PELD combined with its corresponding radio-imaging. Moreover, the focused study content varied from one to another.[14] Thus, details on the clinical outcomes of any operation and its corresponding radiographic parameter changes need to be widely studied to obtain a comprehensive and profound understanding on both PELD and PLIF operation. To this aim, we comprehensively compare the post-operative clinical outcome and imaging characters between PELD and PLIF for the treatment of adjacent segment lumbar disc protrusion with stable retrolisthesis after a previous lumbar internal fixation operation.
2. Patients and methods
2.1. Patients
In this retrospective case-control study, we analyzed the medical records of 24 patients who received the secondary surgery on lumbar spine at Department of Spine Surgery, the Third Hospital of Hebei Medical University (Shijiazhuang, China) from May 2010 to December 2015. Each patient had received a previous lumbar internal fixation at our or other hospitals. All patients were diagnosed as adjacent segment lumbar disc prolapse, and stable retrolisthesis. Eleven patients received PELD operation (defined as PELD group) and 13 patients received the internal fixation of bone graft fusion of lumbar posterior vertebral lamina decompression (defined as control group).
The eligible criteria of patients’ grouping and of the records selection for the study were as follows. The inclusion criteria were for the patients with the nerve root pain on unilateral low extremity, and intermittent claudication; severer pain in the leg than in the lower back; poor prognosis with previously conserved treatment; adjacent segment lumbar disc protrusion and stable retrolisthesis after a previous lumbar internal fixation; consistence of the symptoms, physical signs, and radiography examination (Figs. 1 and 2), completed12-month follow-up record. Exclusion criteria were for patients with lumbar disc protrusion without stable retrolisthesis; and/or with cauda equine syndrome; and/or with nonobvious single sided leg pain; and/or with infection, tumor, or fracture; and/or with no possibility to complete follow-up; who cannot tolerate surgery because of dysfunction in hematopoiesis or blot clotting, or severe cardiovascular diseases.
Figure 1.
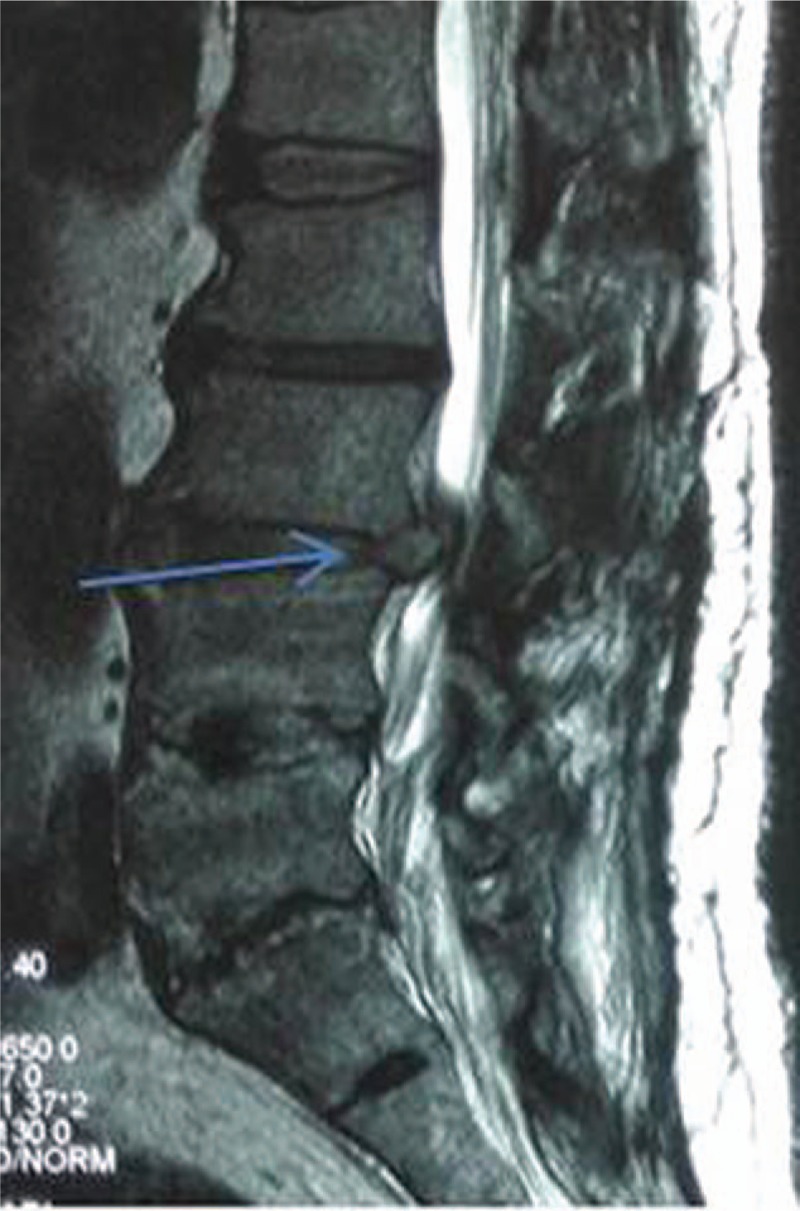
MRI of lateral position taken preoperatively showed a stable retrolisthesis of lumbar 3 after a previous internal fixation. Patient was a male, 50 years old. Arrow pointed the L3 retrolisthesis. MRI = magnetic resonance imaging.
Figure 2.

Before PELD operation, X-ray imaging of lateral position showed the 3 inserted screws in L4-S1 from a previous PLIF operation, and the arrow showed the stable retrolisthesis at L3 from same patient in Figure 1. PELD = percutaneous endoscopic lumbar discectomy, PLIF = posterior lumbar interbody fusion.
2.2. Surgery procedures
For all the patients in the study, the lumbar segments were carefully examined by spine surgeons and by radiologists with computed tomography (CT) and magnetic resonance imaging (MRI) to identify the location that trigger the pain. The example (Figs. 1 and 2) showed a stable retrolisthesis of lumbar 3 after previous internal fixation, which was taken preoperatively. All surgeries were operated by 2 senior and experienced spine surgeons (Dr YS and Dr WZ). Patients in PELD group received the local anesthesia and the decompression of nucleus pulposus removal via nerve root hole expanded under the C arm X-ray perspective machine was performed: establishing a posterolateral work approach via the intervertebral foramen with TESSYE technology (Fig. 3A); puncture positioning was guided under the C arm X-ray perspective machine (C Arm X-ray Perspective Machine-SIREMOBIL Compact L, Siemens Shanghai Medical Equipment Ltd; Shanghai, China) step by step, parts of the bone on the ventral and the cuspidate articular process were abraded with an abrasive drilling, the intervertebral foramen was expanded to form a passageway, and nerve-root was decompressed; the protruded nucleus pulposus in the spinal canal was removed with a nucleus pulposus clamp through the passageway (Fig. 3B and C); the compressed epidural and the nerve root adhesion were released, salient fiber ring was ablated with radio-frequency electrode (Fig. 3D), and bleeding was stopped; after the compression from the nerve root and the spinal cord were completely removed (Fig. 4), the epidural throb could be immediately observed; we then asked the patients to raise the leg straight for testing; no protruded nucleus pulposus tissue and no active bleeding were confirmed with adjusting the different directions of detection, the passageway was pulled out and the incision was sutured.
Figure 3.

The procedure of PELD operation. A, Establishing a work channel on patient's posterolateral position. B, Operation field under the endoscopy. C, Nucleus pulposus clamp. D, Radio-frequency electrode.
Figure 4.

Fluoroscopic image was taken intraoperatively: removing disc in the process of PELD.
Local anesthesia: Kirschner wire was placed on the surface of the skin, the perspective was done in the anteroposterior position and in the lateral position of a patient under C arm X-ray perspective machine to identify the puncture position via the metal needle position. Then, infiltration anesthesia with 0.5% of lidocaine was injected into the skin and subcutaneous tissues of puncture points. If a patient was sensitive to the pain, some of 0.5% lidocaine was injected into deep soft tissue via a long syringe needle to strengthen anesthesia effect.
For patients in control group, patients were in prone position, and received general anesthesia (inhalation) with mechanical ventilation (that is the endotracheal intubation). The originally surgical scar and the subcutaneous scar tissue on the back middle of lumbar region were incised (cautions: because the rear vertebral lamina had been removed in a previous surgery, we should be extra careful when operative field was revealed to avoid the cerebrospinal fluid leakage by dura damage); all previously inserted nuts, screw rods, and pedicle screws were removed out, adjacent impaired segments were exposed, and pedicle screws were inserted again; similar to the previous surgery: excising the spines and lamina, exposing the dura and the nerve root, separating the tissue adhesion; the nerve roots were protected with brain cotton slices, the dura was protected and taken away with a nerve retractor; fiber ring was excised, nucleus pulposus in the damaged intervertebral disc was removed with a nucleus pulposus clamp, the cartilage endplate was resectioned, then a bone graft was implanted into intervertebral space, and a right size of cage (intervertebral fusion, which was filled with autologous bone block) was inserted; the brain cotton slices were taken out, bleeding was flushed, the same side screw was connected with screw rods, drainage tube was placed after stitching; spontaneous breathing in patients was restored, when all vital signs were stable, the endotracheal intubation was removed.
2.3. Evaluation of postoperative outcome and radiography
Postoperative improvements of clinical symptoms and radiography were evaluated on the same day of operation, and at 3- and 12-month follow-up. The contents included X-ray examination of lumbar instability with Flexion-Extension Position of antero/posterior and lateral position film, MRI image for lumbar stability determination. The Oswestry Disability Index (ODI) was important for measuring degree of disability and estimating quality of life in a person with low back pain. The Visual Analogue Scale (VAS) for pain evaluation, measurement of lumbar spinal motion, and other clinical evidence was also examined and recorded.
All the imaging data and clinical records were discussed by 5 experienced surgeons and radiologists.
2.4. Data analysis
Qualitative data were present as frequency (%), quantitative data were present as mean ± standard deviation. Comparisons of qualitative data between 2 groups were analyzed with the Fisher exact probability method, and comparisons of quantitative data between 2 groups were analyzed with t test when data following the normal distribution, otherwise, with Wilcoxon 2 sample test when the data un-following the normal distribution. Repetitive measure analysis of variance was used at different time points for preoperative and postoperative indexes between groups. At each time, paired t test or Wilcoxon signed rank test was used for comparisons before and after operation. However, multivariate analysis for predicting and sensitivity analyses were not applied in the study because of the small size of the samples and no variables of missing value. All data were analyzed with statistical software SAS9.3. P value less than .05 was considered significant.
3. Results
There were 13 male and 11 female of the 24 patients with mean age of 56.5 (range from 32 to 73) years old. There were 9 cases with merger of L4-S1, leading to L3-L4 disc herniation and prolapse; 7 cases with merger L4-L5, leading to L3-L4 disc herniation and prolapse; 6 cases with merger L5-S1, leading to L4-L5 disc herniation and prolapse; and 2 cases with merger L3-L5, leading to L2-L3disc herniation and prolapse.
The apparent characteristics shown in Table 1 were shorter operation time, less bleeding loss in perioperation, and faster postoperative recovery in PELD than in control group (103.6 ± 9.2 vs 181.2 ± 22.8 min; 62.3 ± 9.6 vs 766.2 ± 103.7 mL; 1.3 ± 0.4 vs 8 ± 2.3 days; respectively, all P < .01). Patients in PELD group also spent significantly less days (4.9 ± 0.8 vs 14.2 ± 1.3 days) and corresponding cost (2.8 ± 0.1 vs 5.6 ± 0.8 ¥10 K, respectively, all P < .01) in hospitalization, approximately 1/3 of hospital stay and about half of the cost compared with the control group (Table 1).
Table 1.
Comparisons of basic information between 2 groups.

The ODI and VAS index were utilized to evaluate the surgery results. There are 3 apparent characteristics observed in Table 2. First, the improvements on both ODI and VAS index were, indeed, obtained at any time point after surgery in both groups. Second, at each time point, the improvements on ODI and VAS index were better in PELD than in Control group. Finally, the maximal recovery on ODI was obtained on the same day after surgery only in PELD group. For example, ODI index was significantly reduced from preoperative 62.9 ± 12.9 to postoperative 10.1 ± 6.0% in PELD group, while from preoperative 67.1 ± 12.6 to postoperative 34.2 ± 10.6 in control group.
Table 2.
Pre- and postoperation VAS and ODI index (mean ± sd).

However, the radiography parameters after surgery neither showed the consistent improvement patterns with the ODI and VAS index, nor showed any similarity between 2 groups at any follow-up time point. The Taillard index inter-vertebral height, and the sagittal dislocation were significantly improved after surgery in control group (Taillard index: preoperative 10.02 ± 1.61 vs immediately postoperative 1.08 ± 1.2%; intervertebral height: preoperative 9.65 ± 0.8 vs immediately postoperative 11.62 ± 1.0 mm; sagittal dislocation:preoperative 3.51 ± 0.56 vs immediately postoperative 0.38 ± 0.4 mm in control group, respectively; all P < .01), but no changes in PELD group on the same day after operation, and at 3- and 12-month postoperation, if compared with preoperation (Table 3). The forward bending angle of lumbar vertebrae was significantly increased in PELD group but was significantly deceased in control group at each time point when compared with preoperation (Table 3). The apparent character of imaging parameter changes was that the changes could be obtained immediately on the same day after operation, then there were the tiny changes till 12-month postoperation (Table 3).
Table 3.
Radiographic parameter changes between 2 groups (mean ± sd).

4. Discussion
Our result showed the apparent advantages of the shorter operation time, less blood losing, and faster recovery in perioperation in patients of PELD group compared with patients of control group, the results were consistent with literature reports.[11–13] It could be explained that smaller surgical incision and field of PELD operation reduced injury for surrounding tissues, leading to the less bleeding and faster recovery. The faster recovery further shortened the hospitalization days and reduced the financial burden of the patients.
The ODI is currently considered the gold standard for measuring degree of disability and estimating quality of life in a person with low back pain and lumbar disc herniation [15]; the higher the percentage of ODI, the severer the dysfunction. The average ODI percentage was sharply decreased about 82.7% in PELD group, and 49.1% in control group on the same day after operation (Table 2); close to the report of decreased 79.7% in PELD and 69.4% in OLD group (open lumber micro discectomy),[12] different with the report of decreased about 50% in PELD, about 48% in OLD group at 6-month postoperative.[11] VAS score is the evaluation for the pain [16]; the higher the scores, the severer the pain. A similar pattern of VAS improvement was observed in both groups, which meant the decreased VAS scores required a period time; but the decreased pain was always more significant in PELD than in control group (Table 2). Ahn et al[11] reported similar results. Choi et al reported that the pain relief degree on back was better in PELD than in OLF group, but on legs was the same between 2 groups.[12]
The change patterns of radiography were not consistent with change patterns of ODL and VAS scores in both groups. For control group, the decreases on Taillard index and sagittal dislocation, and increase on intervertebral height after PLIF were the inevitable consequences, because PLIF operation reconstructed the preoperative impaired lumbers to restore to normal lumber structure. The decrease in the forward bending angle of lumbar vertebrae was also an expected consequence because PLIF sacrificed the segment joint activities, in which the segmental fusion after surgery limited the segmental activities. Further, the formed scar tissue limited the segmental activities, theoretically, resulting in the decrease of forward bending angle of lumbar.
Theoretically, PELD does not improve Taillard index, sagittal displacement, lumbar intervertebral height, and forward bending angle of lumbar vertebrae. However, because of the removal of nucleus pulposus, intervertebral height might be lost/reduced, a slight increase may also appear in sagittal displacement, and Taillard index and the mobility of intervertebral body may be increased slightly or unchanged. The results of the imaging parameters in this were well consistent with the theoretic speculation.
There are several limitations of the current study, including a small sample size and relatively short-term follow-up. Of note, recurrence after successful micro-endoscopic discectomy has been reported.[10,17] Long-term follow-up is important to observe complications, and potential recurrence of adjacent segment lumbar disc protrusion and stable retrolisthesis postoperations. Not each patient will benefit from the same procedure.[18] Studies to predict what can be expected, and who is most likely to benefit are needed for a personalized surgical procedure. Future studies with larger samples and long-term follow-up are needed, to further examine the benefits of PELD over open lumbar surgery.
In conclusion, PELD operation was superior in terms of operation time, bleeding volume, recovery period, and financial support, if compared with lumbar internal fixation operation. Radiographic parameters reflect lumber structure changes, which could be observed immediately after surgery in both methods; however, the recoveries on nerve function and pain relief required a longer time, especially after PLIF operation.
Footnotes
Abbreviations: ASDP = adjacent segment disc prolapse, CT = computed tomography, MRI = magnetic resonance imaging, ODI = Oswestry Disability Index, PELD = percutaneous endoscopic lumbar discectomy, PLIF = posterior lumbar interbody fusion, VAS = Visual Analogue Scale.
The study was supported by Medical Scientific Research Key Project in Hebei Province (No. 20160600).
The authors have no conflicts of interest to disclose.
References
- [1].Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Asil K, Yaldiz C. Retrospective comparison of radiological and clinical outcomes of PLIF and TLIF techniques in patients who underwent lumbar spinal posterior stabilization. Medicine (Baltimore) 2016;95:e3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu H, Xu Y, Yang SD, et al. Unilateral versus bilateral pedicle screw fixation with posterior lumbar interbody fusion for lumbar degenerative diseases: a meta-analysis. Medicine (Baltimore) 2017;96:e6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Epstein NE. Older literature review of increased risk of adjacent segment degeneration with instrumented lumbar fusions. Surg Neurol Int 2016;7:S70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cinotti G, Roysam GS, Eisenstein SM, et al. Ipsilateral recurrent lumbar disc herniation. A prospective, controlled study. J Bone Joint Surg Br 1998;80:825–32. [DOI] [PubMed] [Google Scholar]
- [6].Ozgen S, Naderi S, Ozek MM, et al. Findings and outcome of revision lumbar disc surgery. J Spinal Disord 1999;12:287–92. [PubMed] [Google Scholar]
- [7].Eun SS, Lee SH, Sabal LA. Long-term follow-up results of percutaneous endoscopic lumbar discectomy. Pain Phys 2016;19:E1161–6. [PubMed] [Google Scholar]
- [8].Lee DY, Shim CS, Ahn Y, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for recurrent disc herniation. J Korean Neurosurg Soc 2009;46:515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoogland T, van den Brekel-Dijkstra K, Schubert M, et al. Endoscopic transforaminal discectomy for recurrent lumbar disc herniation: a prospective, cohort evaluation of 262 consecutive cases. Spine (Phila Pa 1976) 2008;33:973–8. [DOI] [PubMed] [Google Scholar]
- [10].Yao Y, Zhang H, Wu J, et al. Minimally invasive transforaminal lumbar interbody fusion versus percutaneous endoscopic lumbar discectomy: revision surgery for recurrent herniation after microendoscopic discectomy. World Neurosurg 2017;99:89–95. [DOI] [PubMed] [Google Scholar]
- [11].Ahn SS, Kim SH, Kim DW, et al. Comparison of outcomes of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for young adults: a retrospective matched cohort study. World Neurosurg 2016;86:250–8. [DOI] [PubMed] [Google Scholar]
- [12].Choi KC, Kim JS, Park CK. Percutaneous endoscopic lumbar discectomy as an alternative to open lumbar microdiscectomy for large lumbar disc herniation. Pain Phys 2016;19:E291–300. [PubMed] [Google Scholar]
- [13].Chen HC, Lee CH, Wei L, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar surgery for adjacent segment degeneration and recurrent disc herniation. Neurol Res Int 2015;2015:791943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee SH, Chung SE, Ahn Y, et al. Comparative radiologic evaluation of percutaneous endoscopic lumbar discectomy and open microdiscectomy: a matched cohort analysis. Mt Sinai J Med 2006;73:795–801. [PubMed] [Google Scholar]
- [15].Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976) 2000;25:2940–52. [DOI] [PubMed] [Google Scholar]
- [16].Behrend CJ, Schonbach EM, Vaccaro AR, et al. Maximum pain on visual analog scales in spinal disorders. Spine J 2016. [DOI] [PubMed] [Google Scholar]
- [17].Yao Y, Liu H, Zhang H, et al. Risk factors for the recurrent herniation after microendoscopic discectomy. World Neurosurg 2016;95:451–5. [DOI] [PubMed] [Google Scholar]
- [18].Mayer HM, Brock M. Percutaneous endoscopic lumbar discectomy (PELD). Neurosurg Rev 1993;16:115–20. [DOI] [PubMed] [Google Scholar]


