Abstract
The aim of the study was to estimate the rate of incidental cardiac findings (ICF) in patients undergoing noncardiac chest CT.
An experienced radiologist retrospectively reviewed 237 consecutive patients (147 males and 90 females with median age of 69 years) undergoing a noncardiac chest CT. ICF at targeted review were compared to those mentioned in original reports (χ2 test).
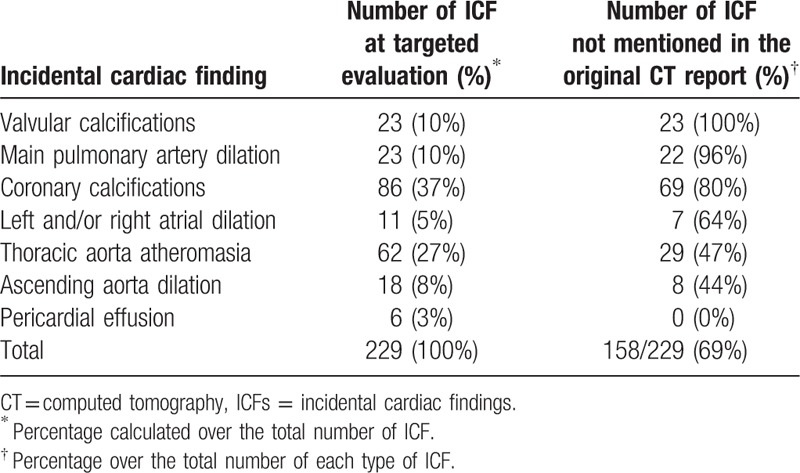
At review, ≥1 ICF was detected in 124/237 patients (52%), for a total of 229 ICF, 158 of them (69%) not originally mentioned. Valvular calcifications were unmentioned in 23/23 (100%) patients, main pulmonary artery dilation in 21/22 (96%), coronary calcifications in 69/86 (80%), right or left atrial dilation in 7/11 (64%), aortic atherosclerosis in 29/62 (47%), and ascending aorta dilatation in 8/18 (44%). All 6 pericardial effusions were originally mentioned. No association with sex (P ≥ .189); positive correlation with age (P < .001).
Half of patients undergoing noncardiac chest CT presented ≥1 ICF, independently from sex but increasing with age. Moreover, 69% of detectable ICFs were not originally mentioned.
Keywords: cardiovascular system, computed tomography, incidental findings, thorax
1. Introduction
An incidentaloma may be defined as “an incidentally discovered mass or lesion, detected by CT, MRI, or other imaging modality performed for an unrelated reason.”[1] Most of them are immediately recognized as benign and have no clinical relevance.[2] However, additional diagnostic examinations may be needed in a number of cases to reach a complete diagnosis.[3]
Noncardiac chest computed tomography (CT) is one of the most widely used imaging technique for the initial evaluation of thoracic disorders,[4] gaining a crucial role in medical practice.[5] The heart evaluation in nongated chest CT has been so far hampered by cardiac motion artifacts. However, recent improvements in multi-detector CT technology have shortened scanning times and decreased cardiac motion artifacts in such an extent that chest CT examinations without electrocardiographic gating allows now fairly good evaluation of the cardiac structures.[6–8] Thus, the detectability of pertinent reportable cardiac findings is substantially increased.
Incidental cardiac findings (ICFs) detected in noncardiac-dedicated chest CT examinations may be of some clinical importance, possibly representing an underlying cardiac condition, or are not specific instead. Indeed, cardiac conditions such as idiopathic and acquired cardiomyopathy, ischemic heart disease, and valvular disease may cause noncardiac symptoms such as dyspnea, chest pain, and hemoptysis that, in turn, prompt the request for a chest CT,[9] challenging noncardiac-dedicated radiologists that often tend to overlook the heart, thus likely missing ICFs.[10]
The prevalence of incidentalomas and their clinical relevance have been estimated in several studies, performed in different clinical settings.[3,11–18] The aim of our study was to qualitatively and quantitatively evaluate the distribution of ICFs in a series of patients undergoing a noncardiac chest CT.
2. Methods
2.1. Study design and participants
The local Ethics Committee approved this retrospective study (Ethics Committee: Comitato Etico Ospedale San Raffaele; authorization number: 41/INT/2017). All 237 consecutive patients undergoing a noncardiac chest CT at our institution between February 2012 and August 2014 were reviewed. They were 147 males and 90 females with median age of 69 years (interquartile range [IQR] 61–76 years). No repeat examination was included.
2.2. CT protocol
All chest CT examinations were performed with a 16-slice (SOMATOM, Sensation Cardiac 16, Siemens Medical Solution, Erlangen, Germany) or 64-slice (SOMATOM Definition AS 64, Siemens Medical Solution, Erlangen, Germany) scanner with a tube voltage of 120 kVp, a pitch from 0.5 to 1.0, a squared field of view from 250 to 380 mm.
According to the clinical query, we used protocols with or without intravenous contrast agent administration (Iopamidol-370, Bracco Imaging SpA, Milan, Italy) administered using a power injector (EmpowerCTA Contrast Injection System, Bracco Diagnostic Inc., Monroe Township, NJ).
2.3. Image analysis
All CT examinations were originally reported by noncardiac radiologists. For the purpose of this study, an independent radiologist with 8 years of experience in cardiovascular CT, blinded to the original CT reports, re-evaluated all chest CT images with the purpose of detecting only ICFs. The distribution of ICFs observed at this targeted re-evaluation was compared with that derived from original CT reports. When the original report only mentioned “previously-known cardiac findings are unchanged” to refer to an already known ICF, we retrieved the second previous CT reports (or the third, the fourth, and so on) to retrieve what was the cited ICF. A second independent reader with 10 years of experience in cardiovascular CT evaluated a subset of 50 patients to estimate the inter-reader agreement.
Examples of ICFs included ascending aorta dilatation (>40 mm), pericardial effusion, coronary calcifications, thoracic aorta atherosclerosis, aortic or mitral valve calcifications, main pulmonary artery dilation (>25 mm), or left and/or right atrial dilation (>24 cm2). The inclusion of ICF concerning the main pulmonary artery and the ascending aorta among ICFs was due to the common consideration of those vascular segments as strictly related to the heart pathophysiology. For the sake of completeness, also ICFs regarding the aortic arch and the descending thoracic aorta were included. Ascending aorta dilatation and pericardial effusion were considered as clinically relevant ICFs, whereas other ICFs as not clinically relevant.
2.4. Statistical analysis
Distributions were given as counts and percentages. The rate of ICF not mentioned in the original CT report was calculated. This rate was associated with age category, sex and CT technology (number of slices) using the χ2 test. Odds ratios were calculated as well. Inter-reader agreement was estimated using the Cohen κ.
Statistical analysis was performed using SPSS for Windows (v. 21.0, SPSS Inc., Chicago, IL) and Excel (Microsoft Excel 2010, Redmond, WA). A P < .05 was considered statistically significant.
3. Results
Of 237 patients, 124 (52%) patients had at least 1 ICF at the targeted re-evaluation: 51 (41%) had 1 ICF, 47 (38%) 2 ICFs, 19 (15%) 3 ICFs, and 7 (6%) 4 ICFs, for a total of 229 ICFs (about 1.0 ICF per patient). Of these 229 ICFs, 158 (69%) were not mentioned in the original CT reports. Only 22 (9%) patients had an unenhanced chest CT.
Of 237 patients, 162 (68%) were studied with the 16-slice CT scanner, 89 of them (55%) showing at least 1 ICF at the targeted re-evaluation; the remaining 75 patients (32%) were studied with the 64-slice CT scanner, 35 of them (47%) showing at least 1 ICF. The number of slices of the CT scanner did not impact on the prevalence of such ICFs (P = .236).
Of 124 patients with at least 1 ICF, only 48 (39%) had a complete original CT report with no missing ICF, whereas 76 (61%) patients had at least 1 ICF not mentioned in the original CT report. Valvular calcifications were not mentioned in the original CT report in 23/23 patients (100%), main pulmonary artery dilation in 22/23 (96%), coronary calcifications in 69/86 (80%), left and/or right atrial dilation in 7/11 (64%), thoracic aorta atherosclerosis in 29/62 (47%), and ascending aorta dilatation in 8/18 (44%). All 6 pericardial effusions were mentioned in the original CT report. No cardiac tumors, thrombus, wires or any other ICF was found. Overall, patients showed a total of 14/24 (58%) clinically relevant ICFs not mentioned in the original report. These data are summarized in Table 1. Inter-reader agreement was perfect (Cohen κ=1) for thoracic aorta atherosclerosis, pericardial effusion, coronary calcification, and valvular calcification; it was almost perfect for ascending aorta dilatation (Cohen κ=0.834) and left and/or right atrial dilation (Cohen κ=0.790).
Table 1.
Distribution of incidental cardiac findings (ICF) observed in 237 patients.
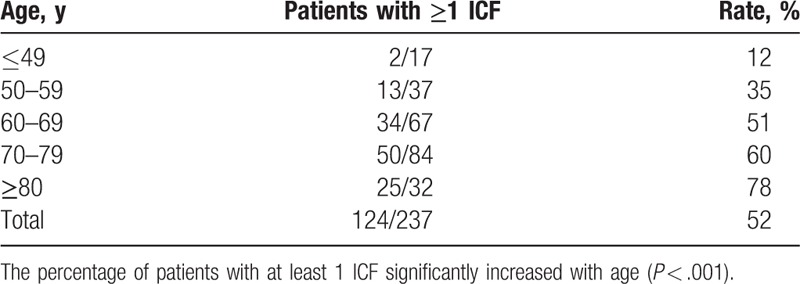
Although not statistically significant, a difference was observed between the rate of males with at least 1 ICF (57%) and of females (44%), for an odds ratio of 1.7 (P = .189). A significant positive correlation was found between age category and percentage of patients with at least 1 ICF (P < .001) (Table 2).
Table 2.
Rate of patients with at least 1 incidental cardiac finding (ICF) according to age category.
Examples of ICFs are shown in Figs. 1–7.
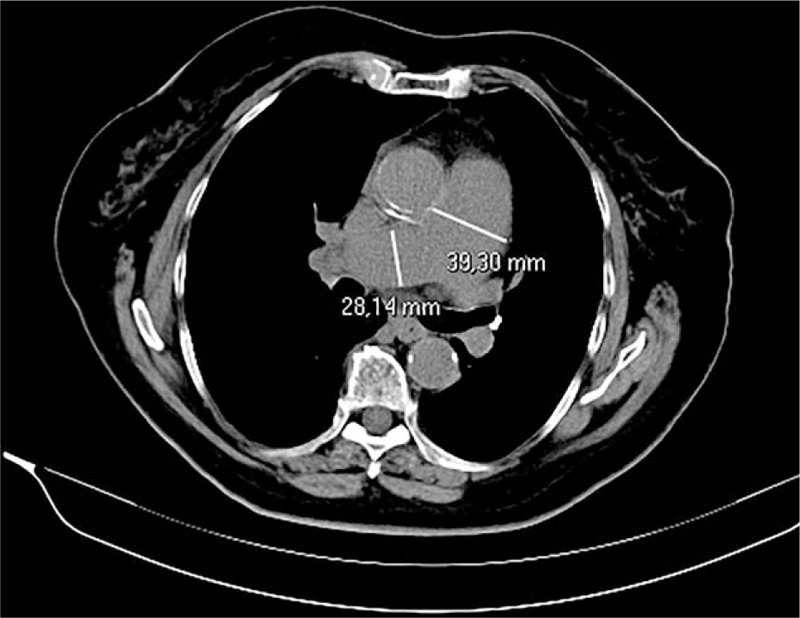
Figure 1.
Axial chest computed tomography showing a dilation of the right atrium in a patient with lung carcinoma. This incidental finding was not mentioned in the original report.
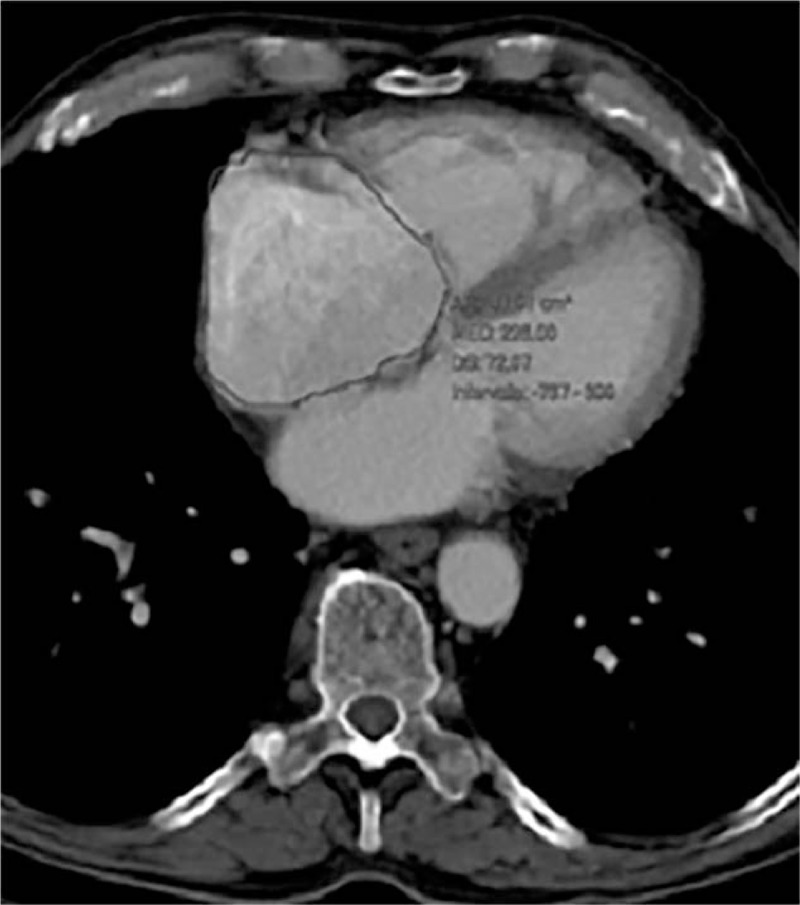
Figure 7.
Axial chest computed tomography showing main pulmonary artery dilation. This incidental finding was not mentioned in the original report.
Figure 2.
Axial chest computed tomography showing a thoracic aorta atherosclerosis. This incidental finding was not mentioned in the original report.
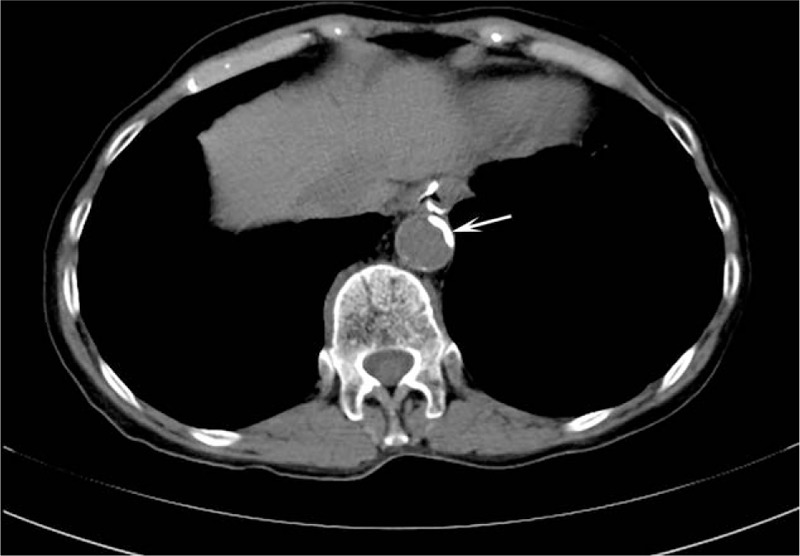
Figure 3.
Axial chest computed tomography showing a calcification of the left anterior descending artery and left circumflex artery. This incidental finding was not mentioned in the original report.
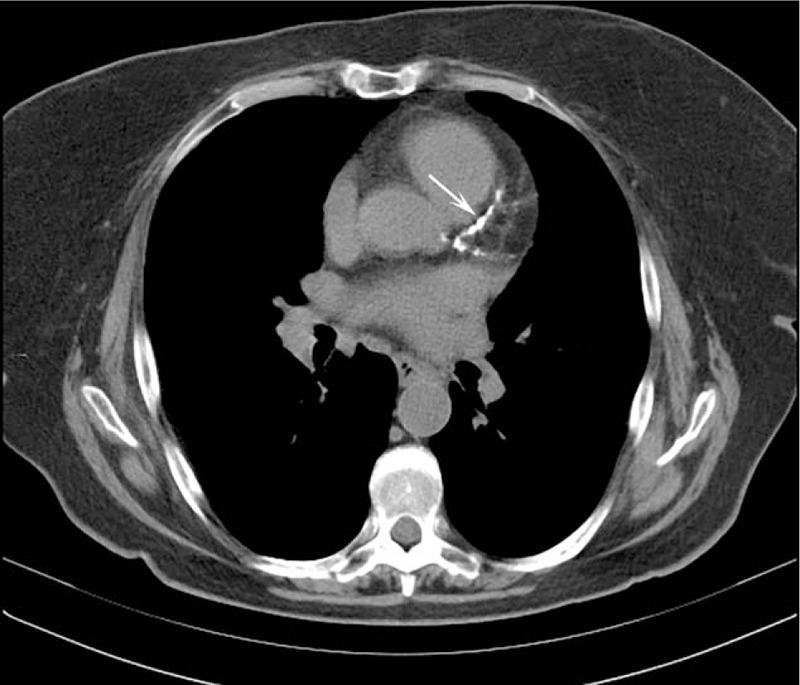
Figure 4.
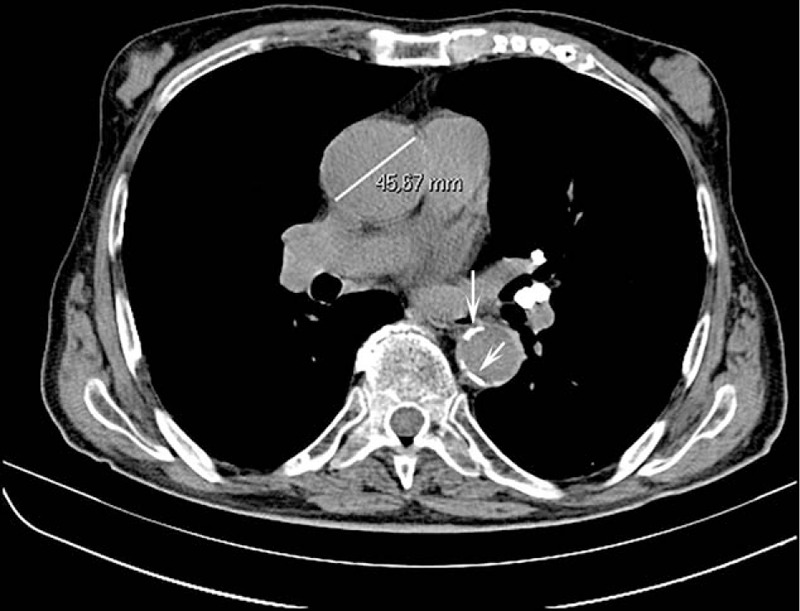
Axial chest computed tomography showing an ascending aorta dilation (45.7 mm). This incidental finding was not mentioned in the original report. Arrows indicate a thoracic aorta atherosclerosis that, conversely, was mentioned in the original report.
Figure 5.
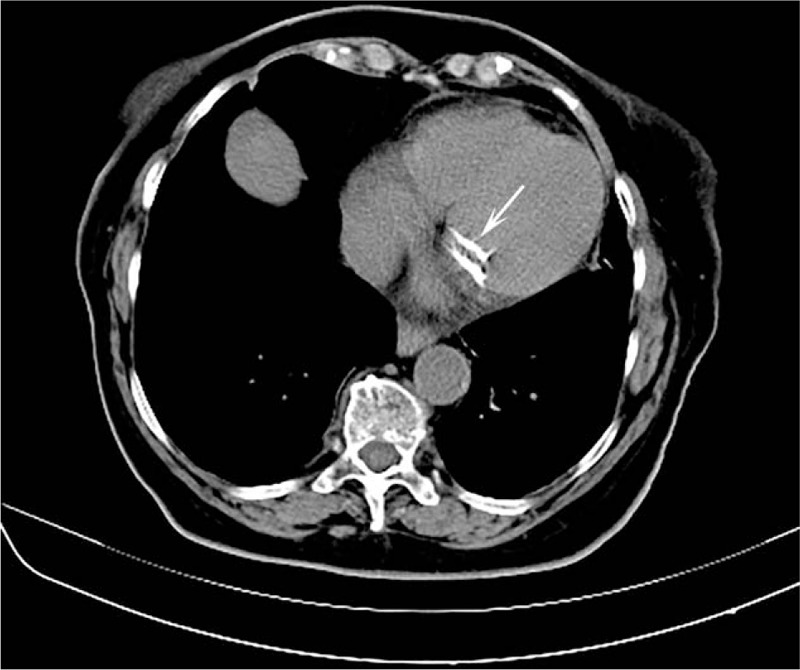
Axial chest computed tomography showing mitral annular calcification. This incidental finding was not mentioned in the original report.
Figure 6.
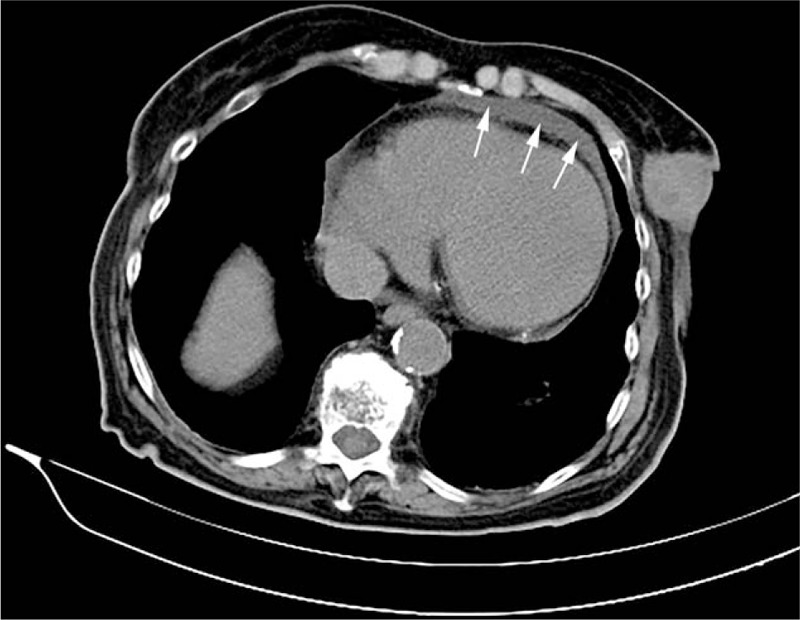
Axial chest computed tomography showing trace of pericardial effusion. This incidental finding was mentioned in the original report.
4. Discussion
The recent improvements in CT technology decreased cardiac motion artifacts in nontriggered chest examinations, permitting a detailed evaluation of some cardiovascular diseases or abnormalities that were not accessible to the old technology.[4] As in many other clinical settings, such an improvement has allowed for a kind of an unwanted collateral screening, already studied in previous studies.[3,11–15]
In this study, 61% of patients had a partially-described original CT report with at least 1 unmentioned ICF. Of course, from our data we cannot exclude the possibility that at least a part of the missing ICFs were actually detected by the original radiologists but considered as not clinically relevant and, as such, not deserving a mention. Consequently, we cannot estimate the frequency of those ICFs that were important in relation to the clinical query.
As noticed by the American College of Radiology, many of such ICFs are of little importance because they are immediately recognized as unrelated to any condition that would threaten the patient's health. In United States, a cost concern is that some radiologists see the identification of incidentalomas as an opportunity to increase referral business for CT.[19,20] Moreover, regardless the cost concerns, we should keep in mind that it is important to avoid overdiagnosis and overtreatment.
As expected, coronary calcification was the most frequent ICF (86/229) although they were not mentioned in the original CT reports in 80% of cases. Presumably, they were minimal calcification in old patients without the need for an immediate follow-up. Although this approach is largely accepted, the presence of even minimal coronary calcifications could lead to high risk of development of symptomatic coronary artery disease and major adverse cardiovascular events.[21] Thus, further diagnostic investigations such as coronary CT angiography may be necessary for the noninvasive morphological assessment of coronary artery disease.[22]
Another important ICF that was clearly visible but not always mentioned in the original reports was the thoracic aorta atherosclerosis, mentioned in 53% (33/62) of cases. Indeed, several studies [23–26] reported that subclinical atherosclerosis could identify individuals at high cardiovascular risk that would benefit from prevention or therapeutic interventions.
Pericardial effusions were always mentioned in original CT reports likely because its iconography is somewhat similar to that of the pleural effusion. As shown by Sun et al,[27] it is important to report a pericardial effusion because the irregular pericardial thickening and mediastinal lymphadenopathy may suggest the presence of a malignant pericardial effusion.
At targeted re-evaluation, cardiac valvular calcifications were observed in 23 patients and never mentioned in the original reports. This finding is common in patients with end-stage kidney disease. Moreover, valvular calcifications are shown to be associated to inducible myocardial ischemia in asymptomatic patients. Thus, this ICF may be used to stratify patients at high risk of silent myocardial ischemia.[28] For these reasons, it is important to report cardiac valvular calcifications even in the subclinical condition.
Ascending aorta dilation is another important ICF that was not mentioned in 8/18 (44%) of cases, 2 of them with an aortic diameter exceeding 4.5 cm. Aortic dilatation may lead to aortic dissection or rupture.[29] Although the European Society of Cardiology guidelines recommend not to operate to up 5.0 cm,[30] it is important to monitor the dilatation evolution as, along with other factors such as aortic elasticity, can lead to aortic dissection.[29] Indeed, in the International Registry of Acute Aortic Dissections nearly 60% of patients with dissection had an aortic diameter less than 5.5 cm, whereas 40% had diameters even less than 5.0 cm.[31]
Importantly, the data here presented do not suffer from inter-reader variability. Indeed, the agreement between the 2 readers was very high. Thus, data are robust and reliable. The positive correlation between age and the chance of having an ICF was quite expected, considering that most of ICF are a sign of aging. Although not significantly, we have also observed a higher rate of ICF in male compared to female, that is in line with the evidence.[21]
Our study has limitations. First, it has a retrospective design, that imply the lack of important clinical variables such as systemic factors causing atherosclerosis (e.g., diabetes mellitus, hypertension, dyslipidemia, smoking, etc.). Second, we did not perform patient's follow-up; thus, no conclusion may be drawn on the progression and clinical relevance of ICF. Third, as mentioned above, we cannot exclude that at least a part of the missing ICFs were actually detected by the original radiologists but considered as not clinically relevant. At any rate, our data are in line with those already published by other authors.[16–18]
5. Conclusion
In conclusion, our study showed that about half of patients undergoing a noncardiac chest CT presented with at least 1 ICF, independently from sex but increasing with age. Moreover, 69% of detectable ICFs were not mentioned in the original report. As some of these findings may be of clinical value, noncardiac-dedicated radiologists should pay more attention to the heart.
Footnotes
Abbreviations: CT = computed tomography, ICF = incidental cardiac finding, IQR = interquartile range.
FS and GDL equally contributed to this study.
Funding: FS is on the speaker's bureau of Bracco Imaging SpA (Milan) and received research grants from Bayer AG (Berlin). GDL and FS were sponsored to past congresses by Bracco Imaging SpA (Milan).
The authors have no conflicts of interest to disclose.
References
- [1].The Free Dictionary by Farelex, Inc. Available at: http://medical-dictionary.thefreedictionary.com/_/cite.aspx?url=http%3A%2F%2Fmedical-dictionary.thefreedictionary.com%2Fincidentaloma&word=incidentaloma&sources=wkMed,Segen,MGH_Med,wkHP. Accessed December 27, 2016. [Google Scholar]
- [2].Voigt P, Fahnert J, Schramm D, et al. Clinically relevant incidental cardiovascular findings in CT examinations. Radiologe 2017;57:296–301. [DOI] [PubMed] [Google Scholar]
- [3].Sconfienza LM, Mauri G, Muzzupappa C, et al. Relevant incidental findings at abdominal multi-detector contrast-enhanced computed tomography: A collateral screening? World J Radiol 2015;7:350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bruzzi JF, Rémy-Jardin M, Delhaye D, et al. When, why, and how to examine the heart during thoracic CT: part 1, basic principles. AJR Am J Roentgenol 2006;186:324–32. [DOI] [PubMed] [Google Scholar]
- [5].Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007;357:2277–84. [DOI] [PubMed] [Google Scholar]
- [6].Pannu HK, Flohr TG, Corl FM, et al. Current concepts in multi-detector row CT evaluation of the coronary arteries: principles, techniques, and anatomy. Radiographics 2003;23:S111–25. [DOI] [PubMed] [Google Scholar]
- [7].Hoffmann MH, Lessick J. Multidetector-row computed tomography for noninvasive coronary imaging. Expert Rev Cardiovasc Ther 2006;4:583–94. [DOI] [PubMed] [Google Scholar]
- [8].Lumbreras B, Donat L, Hernández-Aguado I. Incidental findings in imaging diagnostic tests: a systematic review. Br J Radiol 2010;83:276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bruzzi JF, Rémy-Jardin M, Delhaye D, et al. When, why, and how to examine the heart during thoracic CT: part 2, clinical applications. Am J Roentgenol 2006;186:333–41. [DOI] [PubMed] [Google Scholar]
- [10].Bogaert J, Centonze M, Vanneste R, et al. Cardiac and pericardial abnormalities on chest computed tomography: what can we see? Radiol Med 2010;115:175–90. [DOI] [PubMed] [Google Scholar]
- [11].Veronesi G, Bellomi M, Spaggiari L. The rate of incidental findings in lung cancer screening trials is not negligible. Eur Radiol 2008;18:529. [DOI] [PubMed] [Google Scholar]
- [12].Rinaldi MF, Bartalena T, Giannelli G, et al. Incidental lung nodules on CT examinations of the abdomen: prevalence and reporting rates in the PACS era. Eur J Radiol 2010;74:e84–8. [DOI] [PubMed] [Google Scholar]
- [13].O’Connor SD, Pickhardt PJ, Kim DH, et al. Incidental finding of renal masses at unenhanced CT: prevalence and analysis of features for guiding management. AJR Am J Roentgenol 2011;197:139–45. [DOI] [PubMed] [Google Scholar]
- [14].Lazoura O, Vassiou K, Kanavou T, et al. Incidental non-cardiac findings of a coronary angiography with a 128-slice multi-detector CT scanner: Should we only concentrate on the heart? Korean J Radiol 2010;11:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yee J, Kumar NN, Godara S, et al. Extracolonic abnormalities discovered incidentally at CT colonography in a male population. Radiology 2005;236:519–26. [DOI] [PubMed] [Google Scholar]
- [16].Quentin M, Kröpil P, Steiner S, et al. [Prevalence and clinical significance of incidental cardiac findings in non-ECG-gated chest CT scans]. Radiologe 2011;51:59–64. [DOI] [PubMed] [Google Scholar]
- [17].Foley PW, Hamaad A, El-Gendi H, et al. Incidental cardiac findings on computed tomography imaging of the thorax. BMC Res Notes 2010;3:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Verdini D, Lee AM, Prabhakar AM, et al. Detection of cardiac incidental findings on routine chest CT: the impact of dedicated training in cardiac imaging. J Am Coll Radiol 2016;doi:10.1016/j.jacr.2016.02.011. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [19].Blaivas M, Lyon M. Frequency of radiology self-referral in abdominal computed tomographic scans and the implied cost. Am J Emerg Med 2007;25:396–9. [DOI] [PubMed] [Google Scholar]
- [20].Sistrom CL, Dreyer KJ, Dang PP, et al. Recommendations for additional imaging in radiology reports: multifactorial analysis of 5.9 million examinations. Radiology 2009;253:453–61. [DOI] [PubMed] [Google Scholar]
- [21].Madhavan MV, Tarigopula M, Mintz GS, et al. Coronary artery calcification. J Am Coll Cardiol 2014;63:1703–14. [DOI] [PubMed] [Google Scholar]
- [22].Cannaò PM, Schoepf UJ, Muscogiuri G, et al. Technical prerequisites and imaging protocols for dynamic and dual energy myocardial perfusion imaging. Eur J Radiol 2015;84:2401–10. [DOI] [PubMed] [Google Scholar]
- [23].Toth PP. Subclinical atherosclerosis: what it is, what it means and what we can do about it. Int J Clin Pract 2008;62:1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Salonen R, Nyyssönen K, Porkkala E, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 1995;92:1758–64. [DOI] [PubMed] [Google Scholar]
- [25].Lauer MS. Screening asymptomatic subjects for subclinical atherosclerosis: not so obvious. J Am Coll Cardiol 2010;56:106–8. doi:10.1016/j.jacc.2010.01.059. [DOI] [PubMed] [Google Scholar]
- [26].Fernández-Friera L, Ibáñez B, Fuster V. Imaging subclinical atherosclerosis: is it ready for prime time? A review. J Cardiovasc Transl Res 2014;7:623–34. [DOI] [PubMed] [Google Scholar]
- [27].Sun JS, Park KJ, Kang DK. CT findings in patients with pericardial effusion: differentiation of malignant and benign disease. Am J Roentgenol 2010;194:W489–94. [DOI] [PubMed] [Google Scholar]
- [28].Choi MJ, Kim J-K, Kim SG, et al. Association between cardiac valvular calcification and myocardial ischemia in asymptomatic high-risk patients with end-stage renal disease. Atherosclerosis 2013;229:369–73. [DOI] [PubMed] [Google Scholar]
- [29].Cozijnsen L, Braam RL, Waalewijn RA, et al. What is new in dilatation of the ascending aorta? Review of current literature and practical advice for the cardiologist. Circulation 2011;123:924–8. [DOI] [PubMed] [Google Scholar]
- [30].Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection Task Force on Aortic Dissection, European Society of Cardiology. Eur Heart J 2001;22:1642–81. [DOI] [PubMed] [Google Scholar]
- [31].Pape LA, Tsai TT, Isselbacher EM, et al. EKIR of AAD (IRAD) I. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007;116:1120–7. [DOI] [PubMed] [Google Scholar]


