Abstract
Rationale:
Cyclodialysis cleft is a relatively rare but severe condition with persistent ocular hypotony, which can cause morphologic changes and visual loss. Here we report a case of a traumatic cyclodialysis cleft that was successfully managed with direct cyclopexy via anterior chamber perfusion. During the operation, if there is aqueous humor flowing out of the deep scleral incision, the cleft is not closed, and surgery should continue until there is no aqueous outflow.
Patient concerns:
A 66-year-old man was treated for severe blunt ocular trauma of the left eye and a resultant cyclodialysis cleft, lens subluxation, choroidal detachment and a cataract. His intraocular pressure was 6 mm Hg, he presented with a shallow anterior chamber, phacodonesis, iridodonesis, 360° ciliary body detachment, and a suspicious cyclodialysis cleft in the 5 to 8 o’clock position.
Diagnoses:
ocular blunt trauma (left eye), cyclodialysis cleft (left eye), lens subluxation (left eye), choroidal detachment (left eye), cataract (both eyes).
Interventions:
The cataract was extracted by phacoemulsification and a posterior chamber intraocular lens was implanted with 2 capsular tension rings, one in the lens bag and the other in the ciliary sulcus. Throughout the following month, intraocular pressure fluctuated between 4 and 6 mm Hg and the ciliary body failed to reattach. A cyclopexy via anterior chamber perfusion was thus deemed necessary and performed.
Outcomes:
After cyclopexy, intraocular pressure increased to 27 mm Hg and decreased to 16 mm Hg after brinzolamide eye drops treatment twice daily for 4 days. Subsequently intraocular pressure stabilized between 10 to 21mm Hg. Complete closure of the cyclodialysis cleft was confirmed with ultrasound biomicroscopy.
Lessons:
Cyclopexy via anterior chamber perfusion for patients with cyclodialysis cleft is a simple, safe, and efficient technique that ensures a successful surgery.
Keywords: anterior chamber perfusion, cyclodialysis cleft, cyclopexy, ultrasound biomicroscopy
1. Introduction
Cyclodialysis cleft results from the separation of the meridional ciliary muscle fibers from the scleral spur, producing a new pathway of aqueous humor drainage into the suprachoroidal space.[1] The magnitude of the cyclodialysis cleft is often much smaller than ciliary body detachment involving the separation of the ciliary body from the sclera, not including the scleral spur. It is important to improve the determination of the precise location and extent of the cyclodialysis cleft, close the fistula, and stop aqueous humor outflow to the suprachoroidal space.
The newly created drainage pathway results in increased uveoscleral outflow via the suprachoroidal space and a reduction in aqueous humor production due to diminished blood supply to the ciliary body.[2–4] A combination of increased uveoscleral outflow and decreased aqueous secretion by the detached ciliary body usually results in persistent severe ocular hypotony with consecutive corneal edema, aqueous flaring, cataract formation, choroidal detachment, retinochoroidal folding, papilledema, macular edema, and in some cases, permanent visual loss.
The main goal of treatment is to close the cleft, restore the apposition of the ciliary body to the sclera and consequently increase intraocular pressure (IOP), improve visual acuity, and prevent further complications, such as permanent visual loss. We report a case of a cyclodialysis cleft caused by trauma that was successfully managed with direct cyclopexy via anterior chamber perfusion.
2. Case report
A 66-year-old man was treated for severe blunt ocular trauma of the left eye caused by a strike from a wooden block. His best-corrected visual acuity as measured by a Snellen chart was 20/30 in the right eye (OD) and 20/50 in the left eye (OS). Anterior segment examination revealed the presence of mild cataracts in both eyes, a shallow anterior chamber, aqueous flaring, Tyndall (+++), phacodonesis, and iridodonesis in the left eye. Fundus examination revealed no abnormalities in either eye. IOP was 10 mm Hg OD and 6 mm Hg OS. Gonioscopy revealed inferonasal angle recession OS. Ultrasonography revealed peripheral choroidal detachment and thickening (Fig. 1). Ultrasound biomicroscopy (UBM) revealed an anterior chamber depth of 2.29 mm and an infiltrate of inflammatory cells, a suspected cyclodialysis cleft at the 6 o’clock position (Fig. 2), and a 360°ciliary body detachment forming a cavernous pattern in the suprachoroidal space.
Figure 1.
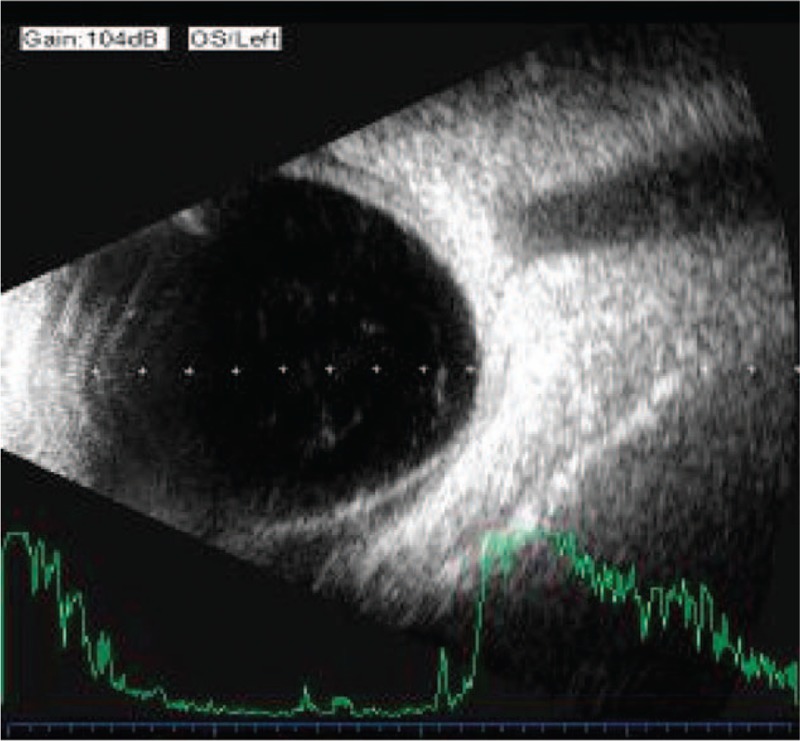
Ultrasonography revealing peripheral choroidal detachment and thickening.
Figure 2.
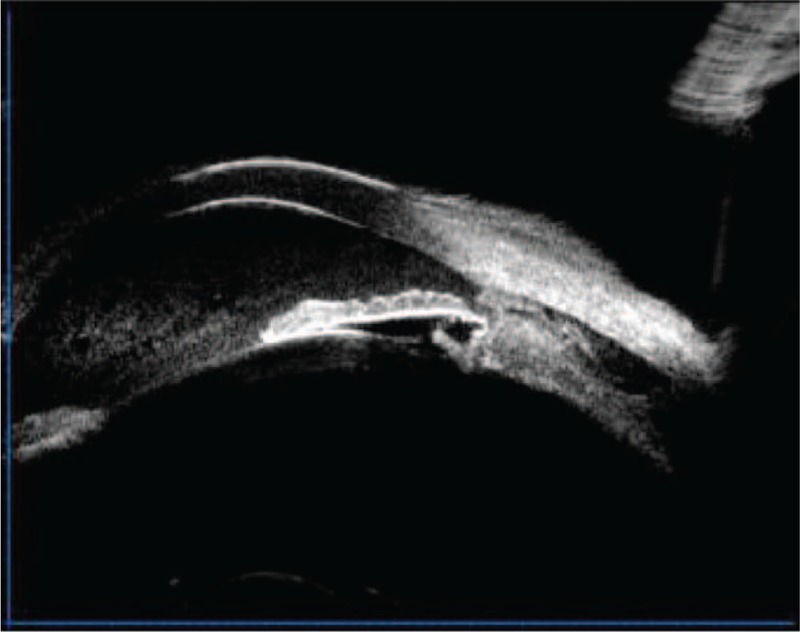
Ultrasound biomicroscopy revealing a suspected cyclodialysis cleft at the 6 o’clock position.
The patient was initiated on conservative treatment with topical 1% atropine twice daily, tobramycin–dexamethasone eye drops 4 times daily, and 40 mg/d of intravenous methylprednisolone. On the 6th day of trauma, IOP was 4 mm Hg OS.
In view of the poor response to conservative management, phacoemulsification cataract extraction was performed and a posterior chamber intraocular lens with 2 capsular tension rings was implanted; one ring in the lens bag and the other in the ciliary sulcus: Tropicamide phenylephrine eye drops were administered as a mydriatic agent 3 times at 15-min intervals before surgery. After retrobulbar anesthesia, a 1.8 mm clear corneal incision was made at the 10 o’clock position, and a 0.5 mm auxiliary clear corneal incision was made at the 3 o’clock position. Subsequently, the anterior chamber was gently filled with viscoelastic agent. A continuous curvilinear capsulorhexis (about 5.5 mm) was performed with forceps. When phacoemulsification was performed, we found that the lens was subluxated about 90 ° (inferonasal quadrant) with a small amount of vitreous present in the anterior chamber. We proceeded to use a modified stop-and-chop technique, taking into consideration its safety and a relatively low flow rate. Once the cortex had been removed, a polymethylmethacrylate injectable capsular tension ring followed by a foldable heparin surface-modified hydrophilic acrylic intraocular lens (+19.5 D) was implanted in the capsular bag. The haptics were placed in the meridian of the zonular dialysis. After removing residual vitreous from the anterior chamber, the other capsular tension ring was inserted into the ciliary sulcus. Finally, the viscoelastic material was removed and the corneal incisions were closed.
After the operation, the patient continued treatment with topical 1% atropine twice daily, tobramycin–dexamethasone eye drops 4 times daily, and 40 mg/d of intravenous methylprednisolone. Four days after surgery, the best-corrected visual acuity of the left eye was still 20/50, and the IOP was 6 mm Hg. The patient insisted to be discharged and was followed as an outpatient for the following month.
During this time period, the IOP of the left eye fluctuated between 4 and 6 mm Hg; UBM revealed that the ciliary body failed to reattach (Fig. 3), while ultrasonography revealed that the peripheral choroidal detachment worsened (Fig. 4). The patient was readmitted to the hospital and treated with direct cyclopexy via anterior chamber perfusion, as follows (Fig. 5): A miotic agent (0.5% pilocarpine) was applied prior to the surgery 3 times at 5-min intervals. After retrobulbar anesthesia, a fornix-based conjunctival flap was created, extending from the 1 o’clock position to each side of the cleft, according to the cleft's location as noted on gonioscopy and UBM (approximately at the 5 to 9 o’clock position). A 0.5 mm clear corneal incision was made at the 4 o’clock position, accompanied with anterior irrigation. The height of the perfusion liquid bottle remained 30 cm above the patient's eye. Following conjunctival flap creation, a limbus-based partial-thickness scleral flap was made 4 mm from the limbus. The scleral flap was about 7 mm long. A deep scleral incision about 3 mm long was made 1.5 mm posterior and parallel to the limbus. The detached ciliary muscle was exposed, and aqueous humor was able to flow out. The deep scleral incision was then closed with a running 10-0 nylon suture. A needle was passed through the anterior scleral lip, the detached ciliary body, and finally the posterior scleral lip. The same procedure was performed for other clefts. The distance between the 2 deep scleral incisions was about 1 mm. The circumferential width of the scleral flap was extended if necessary, and the distance between the 2 scleral flaps was also approximately 1 mm. The cleft was closed, and there was no further aqueous humor outflow. Finally, the partial-thickness scleral flap and conjunctiva were closed with interrupted sutures, and anterior chamber perfusion was interrupted. The corneal incision was closed to aqueous leakage.
Figure 3.
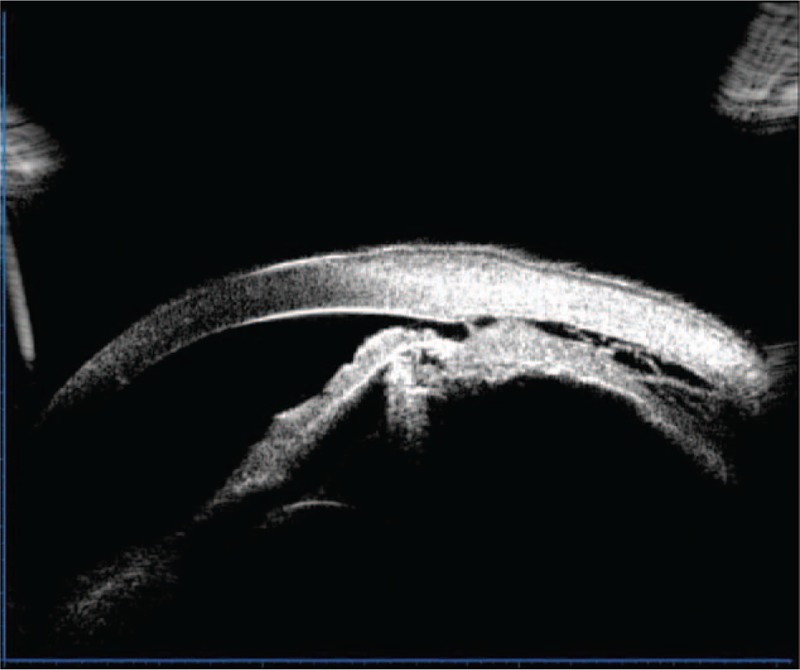
Ultrasound biomicroscopy revealing the ciliary body to have failed reattachment after the first operation.
Figure 4.
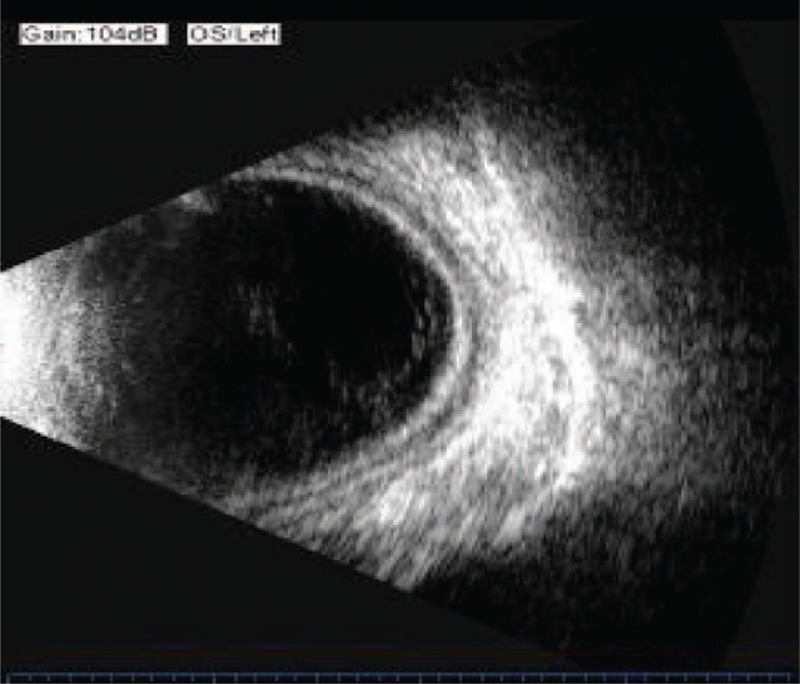
Ultrasonography revealing the peripheral choroidal detachment to become worsened after the first operation.
Figure 5.
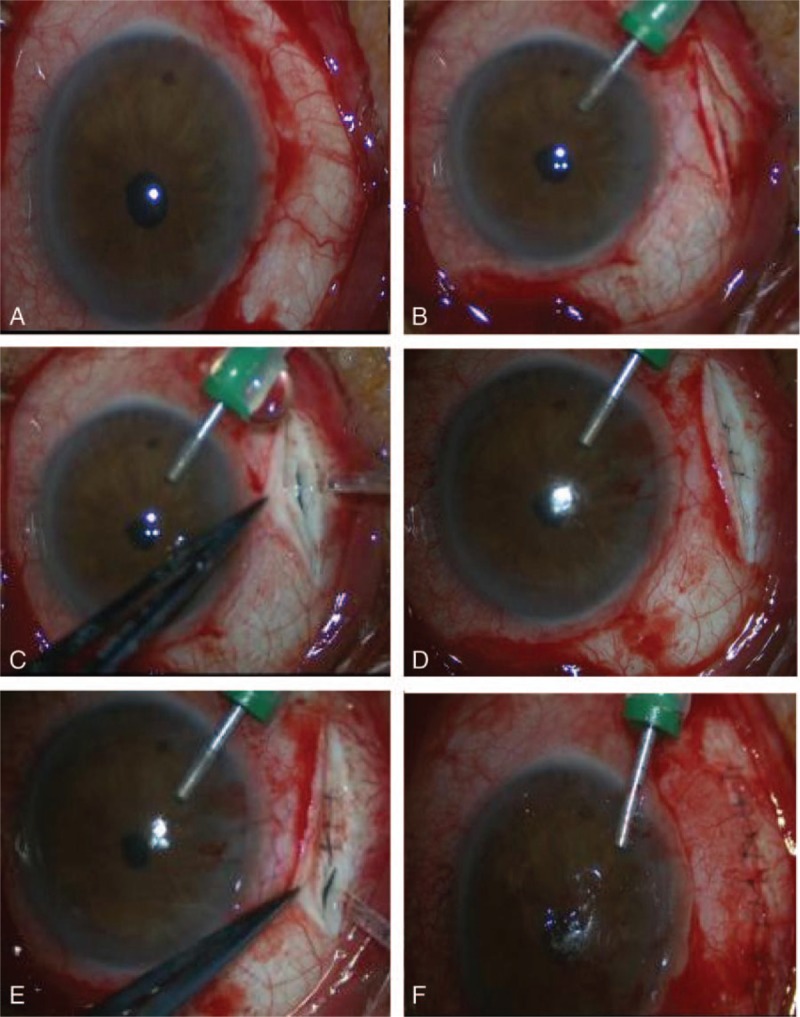
Surgical procedure to close the cyclodialysis cleft. (A) A fornix-based conjunctival flap was created guided by gonioscopy and ultrasound biomicroscopy. (B) A 0.5 mm clear corneal incision was made with anterior irrigation. (C) A limbus-based, partial-thickness scleral flap was made at 4 mm from the limbus; a deep scleral incision was then made 1.5 mm posterior and parallel to the limbus. (D) The deep scleral incision was closed with a running 10-0 nylon suture for scleral-ciliary body-scleral suturing. (E) The same procedure was performed for other clefts, until no aqueous humor was noted to be flowing out of the suprachoroidal space. (F) The partial-thickness scleral flap and conjunctiva were closed with interrupted sutures, while anterior chamber perfusion was interrupted.
The day after the operation, IOP of the left eye increased to 10 mm Hg; ultrasonography revealed the choroid still to be thick, but choroidal detachment was ameliorated (Fig. 6). Five days after the operation, UBM revealed the cyclodialysis cleft to be shut, with the distance between the ciliary body and sclera narrowed (Fig. 7). On the 12th postoperative day, IOP increased to 27 mm Hg and then decreased to 16 mm Hg by topical brinzolamide eye drops twice daily for 4 days, eventually intraocular pressure stabilized between 10 to 21 mm Hg. UBM revealed that the suprachoroidal fluid disappeared, and that the ciliary body was reattached.
Figure 6.
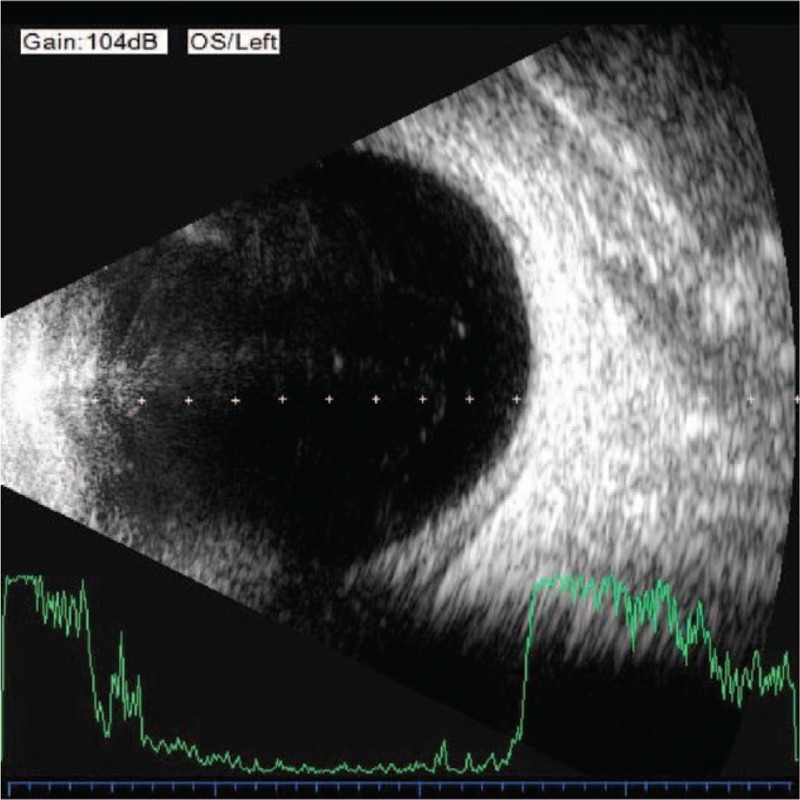
Ultrasonography revealing the choroid still thick, with choroidal detachment ameliorated.
Figure 7.
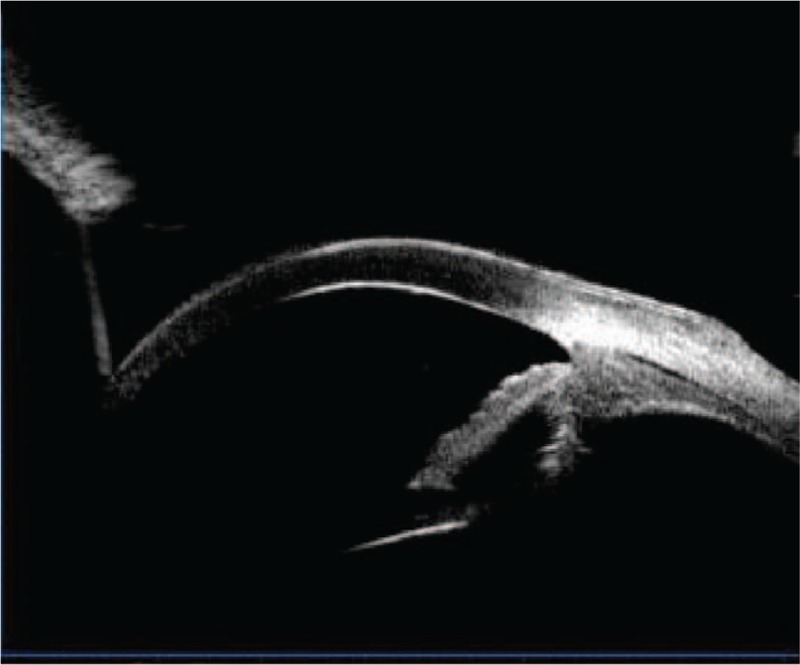
Ultrasound biomicroscopy revealing the cyclodialysis cleft to be shut and the distance between the ciliary body and sclera narrowed.
3. Discussion
Cyclodialysis clefts are a relatively rare condition usually caused by severe ocular trauma; however, it can also be caused iatrogenically during intraocular surgery, such as iridectomy, filtration surgery, intra- or extracapsular cataract removal with or without intraocular lens implantation, and transscleral fixation of secondary intraocular lenses.[5–7]
The prevalence of cyclodialysis clefts following blunt ocular trauma is 2% to 5% or higher,[8,9] based on several studies examining gonioscopic findings in patients with a history of traumatic hyphema.
Many techniques have been proposed to detect clefts either preoperatively or intraoperatively. Slit lamp examination using a gonioscope has been the most common method to confirm the presence of clefts. However, this modality cannot be applied in patients with hyphema, corneal opacity, and excessive hypotonia after trauma or surgery. UBM has become the elective technique of choice used to produce high-resolution ultrasonic images of the anterior segment. This modality has been used to diagnose and identify the location of a cyclodialysis cleft in eyes with ocular hypotony and/or media opacities when a cleft was not visible on gonioscopy.[10,11] UBM is also very helpful in the postoperative interval after surgical closure of the cleft. Anterior segment optical coherence tomography is a noninvasive, painless, noncontact technique that provides accurate and reproducible images.[12,13] Its advantage over UBM is that it is capable of producing higher resolution images without requiring ocular contact. It is easily performed, and while more comfortable for the patient, possesses the disadvantage of poor penetration of opaque tissues. For intraoperative localization of a cleft, gonioscopy is used almost exclusively, but it may also be influenced by certain circumstances, as described above.
A novel method was introduced to repair a cyclodialysis cleft with 2 running sutures from the middle to each end of the cleft under the guidance of a probe.[14] This method is excellent, as the actual localization and range of the cleft may be different from that discovered before the operation. This method also avoids excessive or insufficient sutures, but it may miss hidden clefts, causing the operation to be ultimately unsuccessful.
The methods to close cyclodialysis clefts include medical treatment,[15] argon laser cleft photocoagulation,[15] ciliochoroidal diathermy,[1,4,16] anterior scleral buckling,[17,18] transscleral Nd:YAG or diode laser cyclophotocoagulation,[19] direct cyclopexy,[20,21] vitrectomy with cryotherapy with or without gas (silicone oil) endotamponade,[22–24] cyclocryotherapy with gas endotamponade,[25,26] diode laser endophotocoagulation,[27] indirect cyclopexy,[28] and other surgical techniques involving a capsular tension ring or haptics of large intraocular lenses to compress the cleft against the sclera, thus achieving closure.[29–31]
Among various surgical interventions, direct cyclopexy has been widely used by surgeons worldwide. However, unsuccessful cases of cleft repair continue to be reported in clinical practice because small linear ends of a cleft might not easily be detected by UBM or gonioscopy, or due to presence of more than 1 cyclodialysis cleft, not uncommon.[14] Careful preoperative determination of the extent and localization of cyclodialysis clefts is therefore essential for accurate intraoperative detection and successful surgery. We performed direct cyclopexy via anterior chamber perfusion to ensure that the cyclodialysis cleft was closed.
Overall, the novel method described in this paper is accurate and reliable. It is suitable for various causes of cyclodialysis clefts, multiple clefts, and incomplete cyclodialysis. The major advantage of our procedure is that one can be sure surgery is successful during the operation by observing anterior chamber perfusion. If there is aqueous humor flowing out of the deep scleral incision, the cleft is not closed, and surgery should continue until there is no aqueous outflow. Even though this is a single case report, this procedure has been found effective for several patients we have operated on. The procedure is simple, safe, efficient, and certainly worth considering as a management modality for patients with a cyclodialysis cleft.
Footnotes
Abbreviations: IOP = intraocular pressure, OD = right eye, OS = left eye, UBM = ultrasound biomicroscopy.
All procedures performed in this study were in accordance with the ethical standards of the institution, the First Affiliated Hospital of Zhejiang Chinese Medical University.
Informed consent was obtained from the patient included in this study.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Maumenee AE, Stark WJ. Management of persistent hypotony after planned or inadvertent cyclodialysis. Am J Ophthalmol 1971;71:320–7. [DOI] [PubMed] [Google Scholar]
- [2].Brubaker RF, Pederson JE. Ciliochoroidal detachment. Surv Ophthalmol 1983;27:281–9. [DOI] [PubMed] [Google Scholar]
- [3].Ioannidis AS, Barton K. Cyclodialysis cleft: causes and repair. Curr Opin Ophthalmol 2010;21:150–4. [DOI] [PubMed] [Google Scholar]
- [4].Shaffer RN, Weiss DI. Concerning cyclodialysis and hypotony. Arch Ophthalmol 1962;68:25–31. [DOI] [PubMed] [Google Scholar]
- [5].Meislik J, Herschler J. Hypotony due to inadvertent cyclodialysis after intraocular lens implantation. Arch Ophthalmol 1979;97:1297–9. [DOI] [PubMed] [Google Scholar]
- [6].Slusher MM. Pseudophakic choroidal detachment with cyclodialysis cyst. Ophthalmic Surg 1987;18:191–4. [PubMed] [Google Scholar]
- [7].Small EA, Solomon JM, Prince AM. Hypotonus cyclodialysis cleft following suture fixation of a posterior chamber intraocular lens. Ophthalmic Surg 1994;25:107–9. [PubMed] [Google Scholar]
- [8].Best W, Hartwig H. Traumatic cyclodialysis and its treatment (author's transl). Klin Monbl Augenheilkd 1977;170:917–22. [PubMed] [Google Scholar]
- [9].Tonjum AM. Gonioscopy in traumatic hyphema. Acta Ophthalmol (Copenh) 1966;44:650–64. [DOI] [PubMed] [Google Scholar]
- [10].Gentile RC, Pavlin CJ, Liebmann JM, et al. Diagnosis of traumatic cyclodialysis by ultrasound biomicroscopy. Ophthalmic Surg Lasers 1996;27:97–105. [PubMed] [Google Scholar]
- [11].Karwatowski WS, Weinreb RN. Imaging of cyclodialysis cleft by ultrasound biomicroscope. Am J Ophthalmol 1994;117:541–3. [DOI] [PubMed] [Google Scholar]
- [12].Mateo-Montoya A, Dreifuss S. Anterior segment optical coherence tomography as a diagnostic tool for cyclodialysis clefts. Arch Ophthalmol 2009;127:109–10. [DOI] [PubMed] [Google Scholar]
- [13].Radhakrishnan S, Huang D, Smith SD. Optical coherence tomography imaging of the anterior chamber angle. Ophthalmol Clin North Am 2005;18:375–81. [DOI] [PubMed] [Google Scholar]
- [14].Wang M, Hu S, Zhao Z, et al. A novel method for the localization and management of traumatic cyclodialysis cleft. J Ophthalmol 2014;2014:761851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aminlari A, Callahan CE. Medical laser and surgical management of inadvertent cyclodialysis cleft with hypotony. Arch Ophthalmol 2004;122:399–404. [DOI] [PubMed] [Google Scholar]
- [16].Vannas M, Bjorkenheim B. On hypotony following cyclodialysis and its treatment. Acta Ophthalmol (Copenh) 1952;30:63–4. [PubMed] [Google Scholar]
- [17].Mandava N, Kahook MY, Mackenzie DL, et al. Anterior scleral buckling procedure for cyclodialysis cleft with chronic hypotony. Ophthalmic Surg Lasers Imaging 2006;37:151–3. [PubMed] [Google Scholar]
- [18].Portney GL, Purcell TW. Surgical repair of cyclodialysis induced hypotony. Ophthalmic Surg 1974;5:30–2. [PubMed] [Google Scholar]
- [19].Saha N, MacNaught AI, Gale RP. Closure of cyclodialysis cleft using diode laser. Eye (Lond) 2003;17:527–8. [DOI] [PubMed] [Google Scholar]
- [20].Hwang JM, Ahn K, Kim C, et al. Ultrasonic biomicroscopic evaluation of cyclodialysis before and after direct cyclopexy. Arch Ophthalmol 2008;126:1222–5. [DOI] [PubMed] [Google Scholar]
- [21].Kuchle M, Naumann GO. Direct cyclopexy for traumatic cyclodialysis with persisting hypotony. Report in 29 consecutive patients. Ophthalmology 1995;102:322–33. [DOI] [PubMed] [Google Scholar]
- [22].Helbig H, Foerster MH. Management of hypotonous cyclodialysis with pars plana vitrectomy, gas tamponade, and cryotherapy. Ophthalmic Surg Lasers 1996;27:188–91. [PubMed] [Google Scholar]
- [23].Hoerauf H, Roider J, Laqua H. Treatment of traumatic cyclodialysis with vitrectomy, cryotherapy, and gas endotamponade. J Cataract Refract Surg 1999;25:1299–301. [DOI] [PubMed] [Google Scholar]
- [24].Medeiros MD, Postorino M, Pallas C, et al. Cyclodialysis induced persistent hypotony: surgical management with vitrectomy and endotamponade. Retina 2013;33:1540–6. [DOI] [PubMed] [Google Scholar]
- [25].Ceruti P, Tosi R, Marchini G. Gas tamponade and cyclocryotherapy of a chronic cyclodialysis cleft. Br J Ophthalmol 2009;93:414–6. [DOI] [PubMed] [Google Scholar]
- [26].Pinheiro-Costa J, Melo AB, Carneiro AM, et al. Cyclodialysis cleft treatment using a minimally invasive technique. Case Rep Ophthalmol 2015;6:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Caronia RM, Sturm RT, Marmor MA, et al. Treatment of a cyclodialysis cleft by means of ophthalmic laser microendoscope endophotocoagulation. Am J Ophthalmol 1999;128:760–1. [DOI] [PubMed] [Google Scholar]
- [28].Chadha N, Lamba T, Belyea DA, et al. Indirect cyclopexy for treatment of a chronic traumatic cyclodialysis cleft with hypotony. Clin Ophthalmol 2014;8:591–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Malandrini A, Balestrazzi A, Martone G, et al. Diagnosis and management of traumatic cyclodialysis cleft. J Cataract Refract Surg 2008;34:1213–6. [DOI] [PubMed] [Google Scholar]
- [30].Mardelli PG. Closure of persistent cyclodialysis cleft using the haptics of the intraocular lens. Am J Ophthalmol 2006;142:676–8. [DOI] [PubMed] [Google Scholar]
- [31].Yuen NS, Hui SP, Woo DC. New method of surgical repair for 360-degree cyclodialysis. J Cataract Refract Surg 2006;32:13–7. [DOI] [PubMed] [Google Scholar]


