Abstract
To investigate the clinical features and perinatal treatment of thrombocytopenia induced by different causes during pregnancy.
Clinical data from 195 pregnant women with thrombocytopenia attending 2 tertiary hospitals from January 2014 to October 2016 were retrospectively studied. The obtained data were analyzed with SPSS 19.0 software.
There were 117 (60.0%), 55 (28.2%), and 23 cases (11.8%) of pregnancy-associated thrombocytopenia (PAT), idiopathic thrombocytopenia (ITP), and hypertensive disorder in pregnancy (PIH), respectively. The percentage of nulliparous women, gestational age at delivery, date of diagnosis of thrombocytopenia, and delivery mode significantly differed between the patients in these 3 groups (P < .05). Patients with PIH had a higher percentage of premature delivery and of lower birth weight infants than patients in the other 2 groups. The 3 groups had similar incidences of postpartum hemorrhage, rates of stillbirth, and neonatal Apgar scores at 5 minutes. PAT and PIH patients had different platelet counts after delivery compared with at diagnosis, whereas the platelet counts of the ITP patients were similar at diagnosis and after delivery. ITP patients in the nontreatment group and the treatment group had significantly different platelet counts (P < .05), and in the treatment group, the maternal platelet count did not differ for treatment with intravenous immunoglobulin (IVIg) versus corticosteroids.
The causes of thrombocytopenia in pregnancy are diverse, and the clinical features vary widely. Timely analysis is needed to determine the primary cause of thrombocytopenia, and appropriate therapy should then be selected to effectively improve the prognosis of pregnancies.
Keywords: pregnancy, thrombocytopenia, treatment differential
1. Introduction
Thrombocytopenia is one of the most common hematologic complications of pregnancy and is caused by diverse factors. The most common type is pregnancy-associated thrombocytopenia (PAT), which accounts for 65% to 80% of the cases,[1] followed by idiopathic thrombocytopenia (ITP) and hypertensive disorder in pregnancy (PIH). Other less common causes of thrombocytopenia in pregnancy include coagulopathy related to sepsis/disseminated intravascular coagulation (DIC), microangiopathic hemolytic anemia with thrombotic thrombocytopenic purpura and kidney injury; however, these cases were not detected in our study population. Severe thrombocytopenia in pregnancy increases the risk of postpartum hemorrhaging, neonatal asphyxia, and neonatal thrombocytopenia. PAT is currently considered a benign disease,[2] but its diagnosis remains primarily a matter of exclusion; therefore, a differential diagnosis is of particular importance. In contrast, mothers with typical ITP often have more severe thrombocytopenia, and the treatment options for ITP in pregnancy remain limited. Both corticosteroids and intravenous immunoglobulin (IVIg) are acceptable treatments for ITP in pregnancy, but studies that compare the efficacy of these 2 common treatments to select the initial treatment are rare. Therefore, guidance regarding the selection of an appropriate treatment based on the different causes of thrombocytopenia in pregnancy is urgently needed; however, the differentiation between PAT and ITP remains a diagnostic challenge. Previous studies have discovered that the time of onset of thrombocytopenia and patients’ platelet counts are the strongest predictors of ITP for pregnant women.[3] In this study, we examined the early clinical predictors of ITP and methods to distinguish it from PAT and PIH. Additionally, regarding PIH, which is one of the most common causes of thrombocytopenia in pregnancy, moderate or severe thrombocytopenia can also be observed in partial PIH patients. The clinical features that distinguish PIH from other causes of thrombocytopenia during pregnancy and the associated therapies for thrombocytopenia during pregnancy are limited and rarely reported.
Based on the findings above, a protocol for the clinical prediction and early diagnosis of the potential causes of thrombocytopenia during pregnancy is urgently needed. In this retrospective study, the clinical features of the different causes of thrombocytopenia in pregnancy were investigated. The treatment and prognosis of these different causes were also analyzed among pregnancies complicated with thrombocytopenia.
2. Patients and methods
2.1. Study design and patients
This was a retrospective study of pregnant patients with thrombocytopenia attending 2 tertiary hospitals from January 2014 to October 2016. This study was approved by the Institutional Review Board (IRB) of the 2 hospitals and was performed in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent before enrolment in this study. A total of 13,286 pregnant women visited our hospitals during the study period. Women were eligible for the study if they had 2 platelet counts lower than 100 × 109/L during their pregnancy. Patients were excluded from the study if they had any of the following: multiple gestations, missing platelet counts before treatment, at delivery or postpartum, or irregular examinations during pregnancy. Ultimately, a total of 195 pregnant women were enrolled in our study. Data were extracted from patients’ medical records, hospital computerized databases, or clinical charts by means of a questionnaire.
2.2. Study definition
The inclusion criteria for patients included pregnancy with a diagnosis of ITP or pregnancy with a previous history of ITP, defined as ITP in pregnancy.[4] PAT was defined as a healthy pregnant woman who had no history of thrombocytopenia, was first diagnosed with thrombocytopenia during the gestation period, had negative maternal platelet-associated immunoglobulin G and had no other positive clinical targets after assessments, such as bone marrow cytomorphologic examination and coagulation function test; normalization of platelet count within 12 weeks of delivery further confirmed the diagnosis. PIH was diagnosed according to the definition in Obstetrics and Gynaecology,[5] which defines PIH as new-onset hypertension with blood pressure values of 140/90 mm Hg or more and 24 hours proteinuria of 0.3 g or more that occurs after 20 weeks of gestation. Patients with preexisting medical disorders such as hypertension, diabetes mellitus, heart disease, and renal disease and patients with connective tissue disorders were excluded. Patients who had a platelet count less than 50 × 109/L or a tendency to bleed received active treatment. Corticosteroid treatment referred to oral prednisolone (1 mg/kg/day for 5–7 days) or intravenous dexamethasone (20 mg/day for 3–5 days). Intravenous immunoglobulin (IVIg) (400 mg/kg/day for 5 days) was administered when there was an inadequate response to corticosteroid treatment and was also used as an initial therapy in some patients. Patients who received corticosteroids, IVIg, or both treatments during the pregnancy and perinatal period were included in the treatment group, and other patients, including those who received blood transfusions, were included in the nontreatment group.
2.3. Statistical analysis
All data were statistically analyzed using commercially available statistical software (SPSS 19.0; IBM Corp., Armonk, NY). Continuous variables were compared using Student t test, 1-way ANOVA, or the Brown–Forsythe test for independent samples. Categorical variables were evaluated using Pearson Chi-squared test or Fisher exact test as appropriate. The correlation between maternal platelet count at delivery and the nadir neonatal platelet count was evaluated by Pearson correlation analysis. All tests were bilateral, and a P-value < .05 was considered statistically significant. The results were expressed as the mean ± standard deviation (SD) for continuously distributed variables and percentages and frequencies for categorical variables.
3. Results
3.1. Maternal and neonatal characteristics
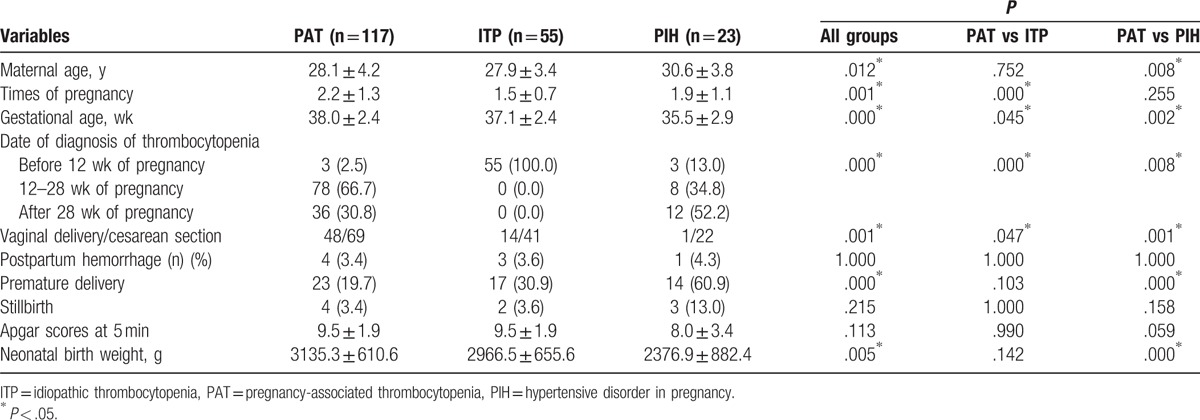
During the study period, 195 pregnancies complicated with thrombocytopenia were identified. Of the patients enrolled, 117 cases (60.0%) were caused by PAT, 55 cases (28.2%) by ITP, and 23 cases (11.8%) by PIH. The mean age of these patients was 28.3 ± 4.2 years (21–40 years). At enrolment, the mean platelet count was 59.6 ± 23.8 × 109/L and ranged from 10 × 109/L to 98 × 109/L. Clinical characteristics of the PAT, ITP, and PIH patients are listed in Table 1. The percentage of nulliparous women, gestational age at delivery, date of diagnosis of thrombocytopenia, and delivery mode significantly differed between the patients in these 3 groups (all P < .05). Thrombocytopenia occurred significantly earlier in women with ITP than in women with PAT and PIH (P = .000). Gestational age at delivery was significantly lower in women with PIH and ITP than in those with PAT (all P < .05). More PAT patients delivered vaginally than ITP and PIH patients (41.0% vs 25.5% and P = .047; 41.0% vs 4.3% and P = .001, respectively). PIH patients had significantly higher percentages of premature and low birth weight infants than women in the other 2 groups (all P < .05). The incidence of postpartum hemorrhaging, rate of stillbirth and neonatal Apgar scores at 5 minutes were similar among the 3 groups (all P > .05). None of the newborns showed any signs of hemorrhagic diathesis, and there were no cases of neonatal death in the 3 groups.
Table 1.
Clinical variables of PAT, ITP, and PIH patients.
The platelet count at initial presentation, delivery day, and postpartum day 2 were significantly lower in women with ITP than in those with PAT and PIH (all P < .05). Additionally, the platelet counts of the PAT and PIH patients were significantly higher 48 hours after delivery than at diagnosis (P = .001 and P = .047, respectively), whereas the platelet counts of ITP patients at diagnosis and after delivery were similar (45.04 ± 20.52 vs 44.87 ± 20.22, P = .830) (Table 2). Patients with PAT, ITP, and PIH were all divided into 3 groups according to platelet counts of 50 × 109 to 100 × 109/ L, 20 × 109 to 50 × 109/L, and <20 × 109/ L. As shown in Fig. 1A, 79.5% of PAT patients and 56.5% of PIH patients had platelet counts more than 50 × 109/ L at diagnosis, whereas 76.4% of ITP patients had counts less than 50 × 109/ L. In addition, nadir platelet counts of less than 20 × 109/L were detected in 67.3% of ITP, 19.7% of PAT, and 39.1% of PIH patients (Fig. 1B). The classification of platelet counts at diagnosis and the nadir platelet counts in ITP patients were significantly different from those in PAT patients (all P < .001), whereas no significant difference was found between the PAT patients and the PIH patients (P = .070 and P = .084, respectively).
Table 2.
Platelet counts at different periods in patients with different disorders (×109/L, mean ± standard deviation).
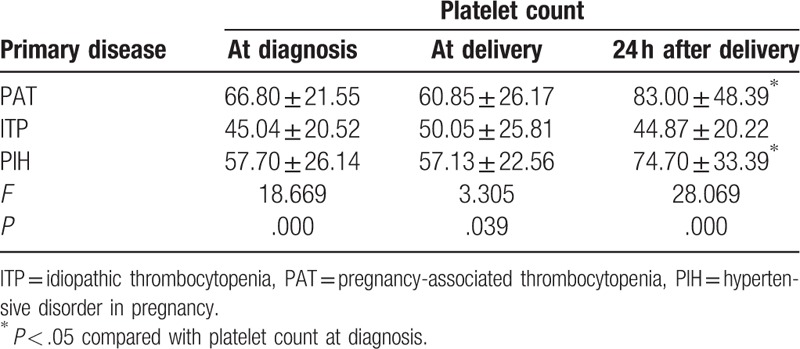
Figure 1.
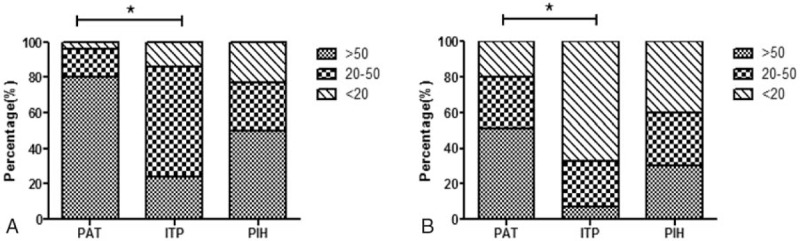
The distribution of platelet counts among PAT, ITP, and PIH patients. (A) Maternal platelet count at diagnosis (∗P < .001, PAT vs ITP). (B) Platelet count at nadir during pregnancy (∗P < .001, PAT vs ITP). ITP = idiopathic thrombocytopenia, PAT = pregnancy-associated thrombocytopenia, PIH = hypertensive disorder in pregnancy.
In total, 195 neonates were live births, and there were no intrauterine deaths. At least 1 postnatal platelet count was available for 105 (53.8%) of the live-born neonates. The mean platelet count was 224.6 ± 62.3 × 109/L and ranged from 12 × 109/L to 305 × 109/L. Nine infants (7.7%) had mild to moderate thrombocytopenia. There was no correlation between maternal platelet count at delivery and the nadir neonatal platelet count in all patients nor in the PAT and ITP groups (r = 0.100 and P = .309, r = 0.187 and P = .067, r = 0.567 and P = .241, respectively).
3.2. Treatment and response
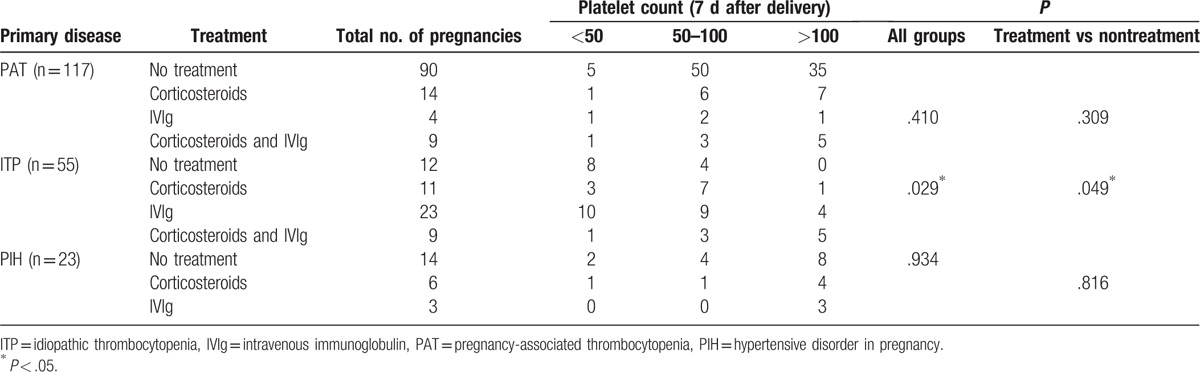
During the study period, 79 (40.5%) women required therapy for thrombocytopenia. Thirty-one were treated with corticosteroids, 30 were treated with IVIg, and 18 were treated with corticosteroids and IVIg. The patients were divided into 3 groups with platelet counts of 50 × 109 to 100 × 109/L, 20 × 109 to 50 × 109/L, and <20 × 109/ L (Table 3). Twenty-seven (23.1%) PAT women and 9 (39.1%) PIH patients required therapy to increase their platelet counts, and the treatment group and nontreatment group of PAT and PIH patients had similar platelet counts 7 days after delivery (P = .309 and P = .816, respectively). Of the ITP patients, 43 (78.2%) received therapy during pregnancy, whereas 15 still required therapy to raise their platelet counts in the first few days after giving birth. There was a significant difference in platelet counts 7 days after delivery between the treatment group and nontreatment group (P = .049). In the treatment group of ITP patients, 11 pregnant women (25.6%) were treated with more than one administration of corticosteroids and 23 cases (53.5%) with IVIg, whereas 9 patients (20.9%) with severe thrombocytopenia received both treatments. Platelet counts of more than 50 × 109/L were more prevalent in patients treated with prednisone as the initial agent than in those treated with IVIg as the initial agent; however, no significant differences were observed between the 2 treatment groups (72.7% vs 56.5%, P = .465). Of the pregnant women treated with IVIg at any point during gestation, including combination therapy using IVIg, adverse events were reported in only 2 (4.2%) and included hemolytic anemia in 1 (2.1%) and headache in 1 (2.1%). In addition, neither patient required specific intervention. Of the pregnant women treated with corticosteroids at any point during gestation, adverse events were reported in 4 (8.2%), including hyperglycemia requiring treatment in 3 (6.1%) and infection in 1 (2.0%).
Table 3.
Platelet counts of patients with different disorders and under different treatments.
4. Discussion
Thrombocytopenia in pregnant women has a number of diverse etiologies. PAT is the most common cause, followed by PIH and ITP, although other medical diseases, such as aplastic anemia, leukemia, systemic lupus erythematosus (SLE), and sicca syndrome, can also cause thrombocytopenia during pregnancy.[2,6] In this retrospective study, 60.0% of the 195 cases had PAT, 28.2% had ITP, and 11.8% had PIH; no other etiologies were identified. Four SLE patients were excluded due to abortion, and 2 women with aplastic anemia were not enrolled due to receiving irregular antenatal care.
The diversity of factors causing thrombocytopenia indicates a variety of pathogeneses. To date, PAT has clearly been shown to be benign based on clinical experience.[2–4] However, the exact mechanism of its pathogenesis is not fully understood. Most scholars believe that PAT may be related to hemodilution or accelerated platelet clearance[7–9]; in this case, there is no destruction of platelets but rather a relative reduction in platelet counts. As a result, the clinical feature is mainly mild thrombocytopenia, which often occurs in the second or third trimester of pregnancy. In this study, 94 women (80.3%) in the PAT group had a platelet count greater than 50 × 109/L, and thrombocytopenia was first detected in almost all (97.5%) PAT patients in their second or third trimester of pregnancy; these findings are consistent with the conclusions of other researchers.[8,9] Obstetric patients with ITP usually have a history of prior thrombocytopenia, and most cases are identified in the first trimester of pregnancy.[10–12] ITP is an autoimmune disease, and its pathogenesis is widely accepted to be the action of antiplatelet antibodies, which recognize specific platelet glycoproteins. These antibody-coated platelets are then cleared by the mononuclear phagocyte/phagocytic system, primarily the spleen,[13] and the severity of thrombocytopenia is associated with maternal antiplatelet antibodies. A history of prior thrombocytopenia, underlying autoimmune disease or severe thrombocytopenia improves the likelihood of a diagnosis of ITP. However, it may be difficult to distinguish ITP from PAT in some cases involving patients with mild thrombocytopenia and no prior history of thrombocytopenia. Kwon et al[3] revealed that the time of onset of thrombocytopenia and platelet count at diagnosis remained independent predictors of ITP in pregnant women. In the present study, all obstetric patients were diagnosed with ITP before 12 weeks of pregnancy, including 45 women with ITP before pregnancy. The platelet count at diagnosis was 45.04 ± 20.52 × 109/L. In addition, 42 patients (76.4%) with ITP had a platelet count of less than 50 × 109/L, and this percentage was significantly higher among ITP patients than in PAT patients (20.5%). Thrombocytopenia in PIH is mainly due to vascular endothelial ischemia and hypoxia caused by vascular vasospasm; vascular viscosity increases with damaged endothelial cells, thereby increasing permeability and accelerating platelet aggregation and consumption.[14] The severity of thrombocytopenia is usually closely related to that of the underlying disease. Severe thrombocytopenia in PIH should be distinguished from that due to ITP. Most women with PIH are primigravida and younger than 20 or older than 30 years old[8]; additionally, they account for 17.6% of the maternal deaths in the United States.[15] A majority of these pregnant women require timely termination of their pregnancy according to their obstetric situation, and this situation results in a high proportion of preterm birth and cesarean sections. Maayan-Metzger et al[16] conducted a retrospective study of 723 pregnant women with thrombocytopenia and verified this conclusion. In the present study, higher percentages of premature delivery and low birth weight infants were observed in the PIH group than in the other 2 groups.
The outcomes of pregnancies with thrombocytopenia have mainly been analyzed by considering postpartum hemorrhaging and neonatal thrombocytopenia. Pregnant women with thrombocytopenia, especially those with severe thrombocytopenia, are at a high risk of postpartum hemorrhaging. In the present study, the rate of postpartum hemorrhage was 3.6%, which was lower than previously reported rates[17,18] and might be related to the administration of effective treatment before termination of pregnancy in patients with severe thrombocytopenia or a tendency for bleeding. Another reason is that most patients with severe thrombocytopenia have normal coagulant activity. In our study, postpartum hemorrhaging occurred in 7 women due to obstetric factors, including 4 cases of uterine inertia, 2 of placental abruption, and 1 of placenta previa. This result indicated that we should take good care of pregnant women with thrombocytopenia and search for a specific cause of this condition. The timely treatment of primary disease and complications can effectively reduce adverse pregnancy outcomes. Neonatal thrombocytopenia was identified in 9 cases (8 mothers with ITP and 1 with PIH). None of these neonates showed bleeding symptoms or a tendency for bleeding. No correlation was observed between the maternal platelet count at delivery and the neonatal platelet count, and this lack of association was in accordance with research by Hachisuga et al,[19] who also concluded that the presence of a first-born sibling with neonatal thrombocytopenia was a reliable risk factor for neonatal thrombocytopenia in subsequent pregnancies.
The treatment options for thrombocytopenia in pregnancy are limited and are mainly determined by clinical experience. PAT and PIH patients in the nontreatment group and the treatment group showed no significant difference in platelet counts (P > .05). The platelet count after delivery was greater than that during pregnancy. Careful blood pressure assessments and regular examination of platelet counts are highly important in these patients, as blood transfusions could be effective in elevating patients’ platelet count before termination of the pregnancy. Evidence to guide management decisions for pregnant patients with ITP is lacking. In our study, among ITP patients, the nontreatment group and the treatment group had significantly different platelet counts (P < .05). Sun et al[20] conducted a retrospective study of 235 pregnancies using blood samples to compare the effectiveness of treatment with IVIg to that of treatment with corticosteroids in pregnancy; these authors concluded that the maternal platelet count response and neonatal outcomes of IVIg and corticosteroids did not differ in pregnant women with ITP. Our data were consistent with their report, as no significant difference in maternal platelet count response was observed between patients treated with IVIg and those treated with corticosteroids (P = .465). The choice of corticosteroids or IVIg as the first-line treatment of ITP sometimes depends on their inherent toxicities. In addition, splenectomy has been reported to effectively treat refractory ITP in pregnancy.[21,22] Purushothaman et al[23] reported a case of refractory immune thrombocytopenia in pregnancy managed with eltrombopag, which showed a good curative effect. However, there are no adequate and well-controlled studies on the use of eltrombopag in pregnancy after delivery or during follow-up.
Thus, a working knowledge of the clinical features and management of pregnant women with thrombocytopenia due to different causes is important for hematologists. Specific therapies, if promptly administered, may significantly improve the outcomes of pregnant women and their offspring.
Footnotes
Abbreviations: ITP = idiopathic thrombocytopenia, IVIg = intravenous immunoglobulin, PAT = pregnancy-associated thrombocytopenia, PIH = hypertensive disorder in pregnancy.
Funding: This work was supported by the National Natural Science Foundation of China (81400162) and the Military Medical Scientific and Technological Innovation Foundation (15MS071, 10MA051).
The authors have no conflicts of interest to disclose.
References
- [1].Gernsheimer TB. Thrombocytopenia in pregnancy: is this immune thrombocytopenia or…? Hematology Am Soc Hematol Educ Program 2012;2012:198–202. [DOI] [PubMed] [Google Scholar]
- [2].McCrae K. Thrombocytopenia in pregnancy: differential diagnosis, pathogenesis, and management. Blood Rev 2003;17:7–14. [DOI] [PubMed] [Google Scholar]
- [3].Kwon J, Shin J, Lee J, et al. Predictors of idiopathic thrombocytopenic purpura in pregnant women presenting with thrombocytopenia. Int J Gynaecol Obstet 2007;96:85–8. [DOI] [PubMed] [Google Scholar]
- [4].Moore J, Hayward C, Warkentin T, et al. Decreased von Willebrand factor protease activity associated with thrombocytopenic disorders. Blood 2001;98:1842–6. [DOI] [PubMed] [Google Scholar]
- [5].British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol 2003;120:574–96. [DOI] [PubMed] [Google Scholar]
- [6].Gernsheimer T, James A, Stasi R. How I treat thrombocytopenia in pregnancy. Blood 2013;121:38–47. [DOI] [PubMed] [Google Scholar]
- [7].Schwartz KA. Gestational thrombocytopenia and immune thrombocytopenias in pregnancy. Hematol Oncol Clin North Am 2000;14:1101–16. [DOI] [PubMed] [Google Scholar]
- [8].McCrae KR, Samuels P, Schreiber AD. Pregnancy-associated thrombocytopenia: pathogenesis and management. Blood 1992;80:2697–714. [PubMed] [Google Scholar]
- [9].Shehata N, Burrows R, Kelton JG. Gestational thrombocytopenia. Clin Obstet Gynecol 1999;42:327–34. [DOI] [PubMed] [Google Scholar]
- [10].McCrae K, Bussel J, Mannucci P, et al. Platelets: an update on diagnosis and management of thrombocytopenic disorders. Hematology Am Soc Hematol Educ Program 2001;282–305. [DOI] [PubMed] [Google Scholar]
- [11].Crowther M, Burrows R, Ginsberg J, et al. Thrombocytopenia in pregnancy: diagnosis, pathogenesis and management. Blood Rev 1996;10:8–16. [DOI] [PubMed] [Google Scholar]
- [12].Gill K, Kelton J. Management of idiopathic thrombocytopenic purpura in pregnancy. Semin Hematol 2000;37:275–89. [DOI] [PubMed] [Google Scholar]
- [13].Cines D, Blanchette V. Immune thrombocytopenic purpura. N Engl J Med 2002;346:995–1008. [DOI] [PubMed] [Google Scholar]
- [14].Mehta B, Kumar V, Chawla S, et al. Hypertension in pregnancy: a community-based study. Indian J Community Med 2015;40:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lain K, Roberts J. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA 2002;287:3183–6. [DOI] [PubMed] [Google Scholar]
- [16].Maayan-Metzger A, Leibovitch L, Schushan-Eisen I, et al. Predictors for neonatal thrombocytopenia in infants of thrombocytopenic mothers during pregnancy. Pediatr Blood Cancer 2010;55:145–8. [DOI] [PubMed] [Google Scholar]
- [17].Yassaee F, Eskandari R, Amiri Z. Pregnancy outcomes in women with idiopathic thrombocytopenic purpura. Iran J Reprod Med 2012;10:489–92. [PMC free article] [PubMed] [Google Scholar]
- [18].Borna S, Borna H, Khazardoost S. Maternal and neonatal outcomes in pregnant women with immune thrombocytopenic purpura. Arch Iran Med 2006;9:115–8. [PubMed] [Google Scholar]
- [19].Hachisuga K, Hidaka N, Fujita Y, et al. Can we predict neonatal thrombocytopenia in offspring of women with idiopathic thrombocytopenic purpura? Blood Res 2014;49:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun D, Shehata N, Ye XY, et al. Corticosteroids compared with intravenous immunoglobulin for the treatment of immune thrombocytopenia in pregnancy. Blood 2016;128:1329–35. [DOI] [PubMed] [Google Scholar]
- [21].Griffiths J, Sia W, Shapiro A, et al. Laparoscopic splenectomy for the treatment of refractory immune thrombocytopenia in pregnancy. J Obstet Gynaecol Can 2005;27:771–4. [DOI] [PubMed] [Google Scholar]
- [22].Bleau N, Czuzoj-Shulman N, Spence AR, et al. Safety of splenectomy during pregnancy. J Matern Fetal Neonatal Med 2017;30:1671–5. [DOI] [PubMed] [Google Scholar]
- [23].Purushothaman J, Puthumana KJ, Kumar A, et al. A case of refractory immune thrombocytopenia in pregnancy managed with elthrombopag. Asian J Transfus Sci 2016;10:155–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


