Abstract
Background:
The prognostic value of the neutrophil-to-lymphocyte ratio (NLR) for nasopharyngeal carcinoma (NPC) remains controversial. This study was designed to provide a more accurate assessment of the prognostic value, based on a meta-analysis.
Methods:
A comprehensive search for relevant studies published before June 2016 was performed using the PubMed, Cochrane Library, and Web of Science databases. The correlations of NLR with overall survival (OS) and progression-free survival (PFS) were evaluated for NPC. Hazard ratios (HRs) and associated 95% confidence intervals (CIs) were calculated to estimate the effects.
Results:
Six studies with a total of 4359 NPC patients were included in this meta-analysis. The pooled results showed that, among patients with NPC, elevated pretreatment NLR was associated with poorer OS (HR = 1.74, 95% CI = 1.45–2.10) and PFS (HR = 1.48, 95% CI = 1.30–1.69). Subgroup analyses indicated that the use of different cut-off values for NLR (<3 or ≥3) did not affect the consistent prognostic value of NLR for OS or PFS. No significant heterogeneity or publication bias was observed among the included studies for OS or PFS (P > .05).
Conclusions:
This meta-analysis indicates that elevated pretreatment NLR might be a valuable predicative biomarker of poor prognosis for patients with NPC.
Keywords: meta-analysis, nasopharyngeal carcinoma, neutrophil-to-lymphocyte ratio, prognosis
1. Introduction
Nasopharyngeal carcinoma (NPC) is one of the common tumors of the head and neck. NPC is rare in most parts of world, except in southern China, where the incidence is as high as 80 per 100,000 person-years.[1] Radiotherapy is the conventional method of treatment for NPC, with 5-year overall survival (OS) rates ranging from 66% to 70%.[2] However, the long-term survival of most patients remains poor because of high rates of local recurrences and distant metastasis after radiotherapy. At present, the commonly used biomarkers for NPC prognosis are tumor stage and metastasis; however, it has been reported that these parameters do not provide sufficiently precise predictions of prognosis. To date, several biomarkers for the prognosis of NPC have been identified, such as C-X-C chemokine receptor type 7 (CXCR-7),[3] lysyl oxidase,[4] and HS1-associated protein X-1.[5] However, few of these markers are currently available in the clinical setting, and some of them were identified based on immunohistochemical stains of tumor tissue, which cannot be applied easily in clinical practice. Therefore, to provide better prognostic assessments in patients with NPC, it remains important to identify biomarkers that are accurate and easy to use.
Systemic inflammatory response has been shown to promote cancer progression and metastasis by facilitating angiogenesis, inhibiting apoptosis, and damaging DNA.[6] The neutrophil-to-lymphocyte ratio (NLR) is an important biomarker that reflects systemic inflammation. NLR is reported to be a useful biomarker for the prognosis of many kinds of malignancies, including breast cancer,[7] gastric neuroendocrine neoplasms,[8] esophageal squamous cell carcinoma,[9] and nonsmall cell lung cancer.[10] Moreover, NLR is easy to obtain from the results of complete blood counts. Thus, NLR seems to be a promising prognostic biomarker for NPC. To date, several studies have investigated the associations of pretreatment NLR with NPC characteristics and the prognostic value of pretreatment NLR for patients with NPC. However, the results of these studies have been inconsistent. Given the potential prognostic value of NLR, we performed a comprehensive meta-analysis to provide a more reliable assessment of pretreatment NLR as a prognostic biomarker for patients with NPC.
2. Materials and methods
2.1. Search strategy and selection criteria
This meta-analysis was carried out in accordance with the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analyses statement (PRISMA). Using the following electronic databases, comprehensive searches were performed to identify eligible articles that had been listed before June 2016: PubMed, Cochrane Library, Web of Science, Google Scholar, and Chinese National Knowledge Infrastructure (CNKI). The following search terms were employed: “neutrophil lymphocyte ratio” or “NLR,” “nasopharyngeal carcinoma” or “NPC,” “prognosis” or “predict,” and “survival.” Included articles were limited to studies of humans. Conference abstracts were not selected because this form of publication generally contains insufficient data. Relevant articles were also searched using the related articles function in PubMed. References within the identified articles were also searched manually. The study was approved by the Review Boards of the Sichuan Cancer Hospital & Institute.
2.2. Inclusion and exclusion criteria
Studies that meet the requirement of the criteria was included in the meta-analysis: the NPC was diagnosis using histopathology methods on the tissue samples; the data of OS or progression-free survival (PFS) in NPC patients was reported before the treatment; the data of hazard ratio (HR) and its 95% confidence interval (CI) were provided, or they could rebuild by P values and other data reported. A study was excluded with any of the following characteristics: abstracts, letters, review articles, case reports, or nonclinical studies. For duplicate or overlapping studies, we selected the most recent ones. Two reviewers (JY and YQ) evaluated all the candidate articles independently. Disagreements were resolved by discussion or upon consensus from a third reviewer (YYL).
2.3. Data extraction and quality assessment
The following items of each study were extracted: the first author's name, the year when the article published, the country where the study conducted, total number of NPC patients, the NLR cut-off, the type of study design, duration of follow-up time, and survival data (OS and PFS). The Newcastle-Ottawa Quality Assessment Scale (NOS)[11] was used to evaluate and score the quality of included study by 2 independent reviewers (JY and YQ). The NOS consists of three parts: selection (4 scores), comparability (2 scores), and outcome assessment (3 scores). Discrepancy between reviewers was settled by repeating the study review and discussion or upon consensus from a third reviewer (YYL). Studies that labeled with scores of 6 or higher were considered to be high quality.
2.4. Statistical analysis
The pooled HR and associated 95% CI were utilized to quantitatively assess the prognostic value of NLR for patients with NPC. Heterogeneity among studies was assessed using Cochran Q test and the I2 statistic. Cochran Q test is likely to result in the false acceptance of the null hypothesis (Type II error) disproportionately often, and is therefore likely to be less powerful than evaluations based on I2. Therefore, we also used the I2 test to assess heterogeneity. The I2 test was documented for the percentage of the observed variation between studies which was caused by heterogeneity rather by chance. Generally, I2 values <25% can be interpreted as an indicator of mild heterogeneity, I2 values between 25% and 50% correspond to moderate heterogeneity, and I2 values >50% correspond to considerable heterogeneity. Both a fixed-effects model (Mantel–Haenszel method) and a random-effects model (DerSimonian–Laird method) were used to calculate pooled HRs and associated 95% CIs. Publication bias was assessed using Egger test and Begg test. All statistical tests in this meta-analysis were performed using Stata 11.2 software (Stata Corp, College Station, TX) with 2-tailed P values. P values <.05 were considered statistically significant.
3. Results
3.1. Study selection and characteristics
The initial search strategy retrieved a total of 68 articles. After screening the titles or abstracts, 35 studies were excluded as they were either duplicate reports, laboratory studies, reviews, case reports, or studies that were irrelevant to the current analysis. Next, the 33 remaining articles were evaluated further. Twenty-seven of these articles were discarded for the following reasons: 12 did not provide specific NLR data for OS or PFS, 10 failed to note the cut-off value that had been used to define elevated NLR, and 5 did not provide enough data to calculate the HR and associated 95% CI. Finally, 6 studies[12–17] with 4359 NPC patients were included in our meta-analysis. A flow chart of the articles selection process is shown in Fig. 1.
Figure 1.
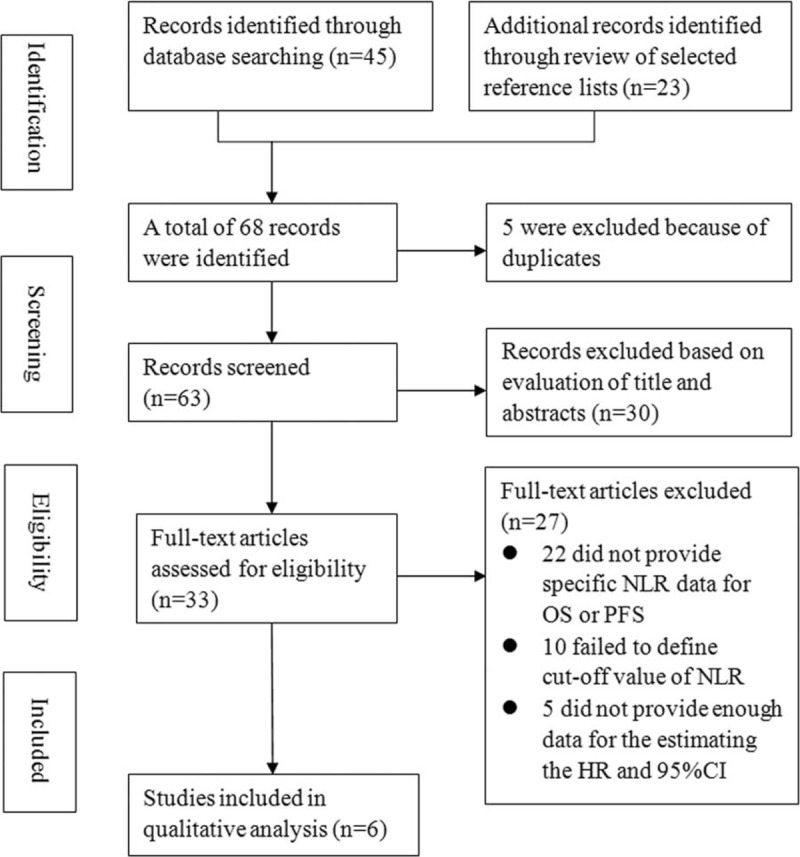
Flow chart of the included studies.
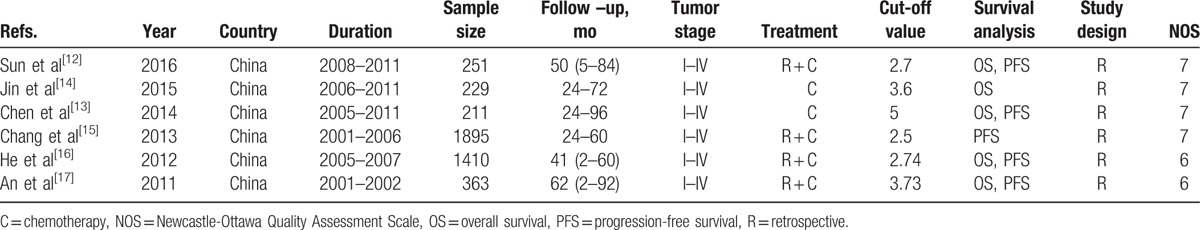
The main characteristics of included studies are shown in Table 1. All 6 studies were conducted in China with a retrospective designs, and provided the data on TMN stages (Union Internationale Contre le Cancer/American Joint Committee on Cancer TNM classification, AJCC/UICC). Five studies with 2464 patients reported results for NLR and OS, and 5 studies with 4130 patients reported results for NLR and PFS. The quality of the studies was high, and their NOS scores ranged from 6 to 8.
Table 1.
Main characteristics of all the studies included in the meta-analysis.
3.2. Prognostic value of NLR for OS in patients with NPC
Five studies provided data on OS according to pretreatment NLR for patients with NPC. The pooled results showed that elevated pretreatment NLR was associated with poorer OS (HR = 1.74, 95% CI = 1.45–2.10, P < .01, Mantel–Haenszel method), and no significant heterogeneity was observed between studies (I2 = 0.0%, Pheterogeneity = .804; Fig. 2). Egger and Begg tests did not reveal any publication bias among these studies (Egger test: P = .301; Begg test: P = .215).
Figure 2.
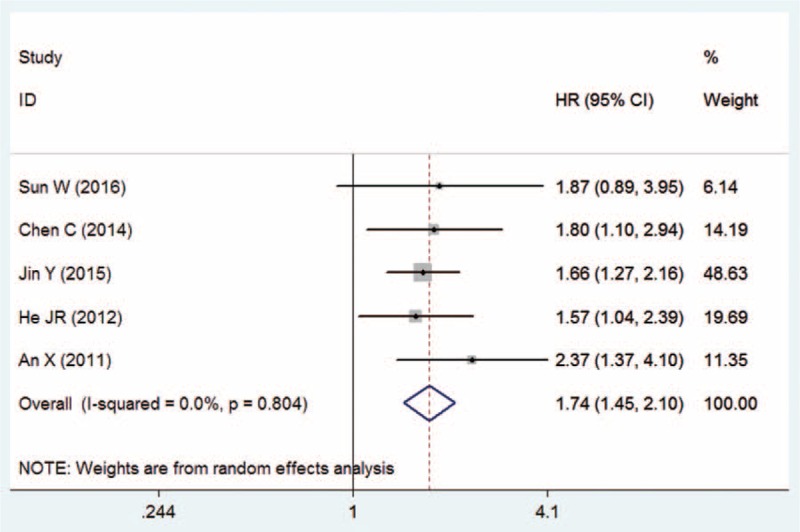
Meta-analysis of the association between NLR and OS in NPC patients.
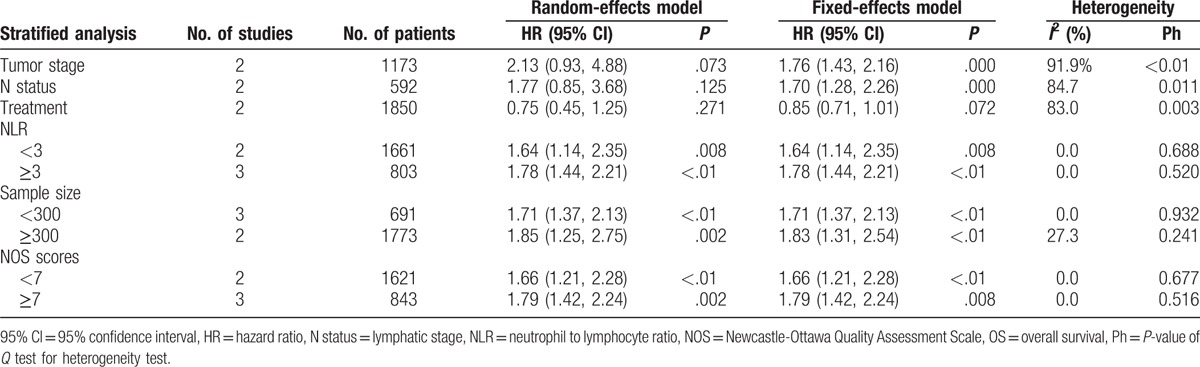
To identify other parameters that were potentially related to OS in patients with NPC, we conducted subgroup analyses based on several confounders, including treatment method, tumor stage, sample size, NLR cut-off values, and NOS score. The stratified results showed that patients who underwent chemotherapy had better OS than patients who did not undergo chemotherapy (HR = 0.75, 95% CI = 0.45–1.25). Additionally, the subgroup analyses did not show any obvious differences between the OS of NPC patients with early and advanced tumor stages (HR = 2.13, 95% CI = 0.93–4.88) or early and advanced lymphatic stages (HR = 1.77, 95% CI = 0.85–3.68). However, significant heterogeneity was observed across the studies (Pheterogeneity < .05).
After stratifying by the NLR cut-off value that had been used (<3 or ≥3), the pooled estimates remained similar (<3: HR = 1.64, 95% CI = 1.14–2.35; ≥3: HR = 1.78, 95% CI = 1.44–2.21), indicating that NLR was a stable prognostic biomarker for OS in patients with NPC. Further, stratifications by sample size (≥300 or <300) and NOS score (≥7 or <7) showed that the differences in sample size and NOS score did not influence the relation of NLR with OS (both P < .05), suggesting that NLR had reliable prognostic value for NPC, regardless of sample size or NOS score (Table 2).
Table 2.
Summary of the meta-analysis results for OS.
3.3. Prognostic value of NLR for PFS in patients with NPC
Five studies provided the data on PFS according to NLR for patients with NPC. The combined data showed that elevated pretreatment NLR was associated with poorer PFS (HR = 1.48, 95% CI = 1.30–1.69, P < .01, Mantel–Haenszel method), and this pooled result was stable in that no significant heterogeneity was observed between studies (I2 = 0.0%, Pheterogeneity = .451; Fig. 3). No evidence of publication bias was observed across the studies (Egger test: P = .309; Begg test: P = .214).
Figure 3.
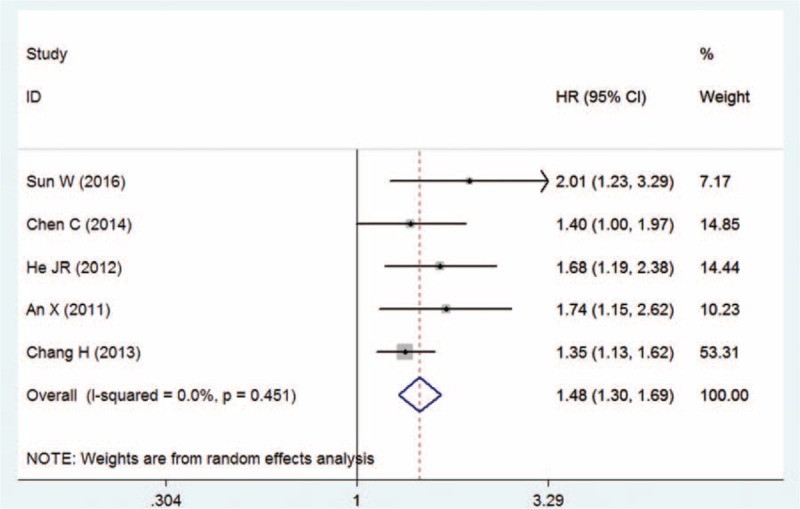
Meta-analysis of the association between NLR and PFS in NPC patients.
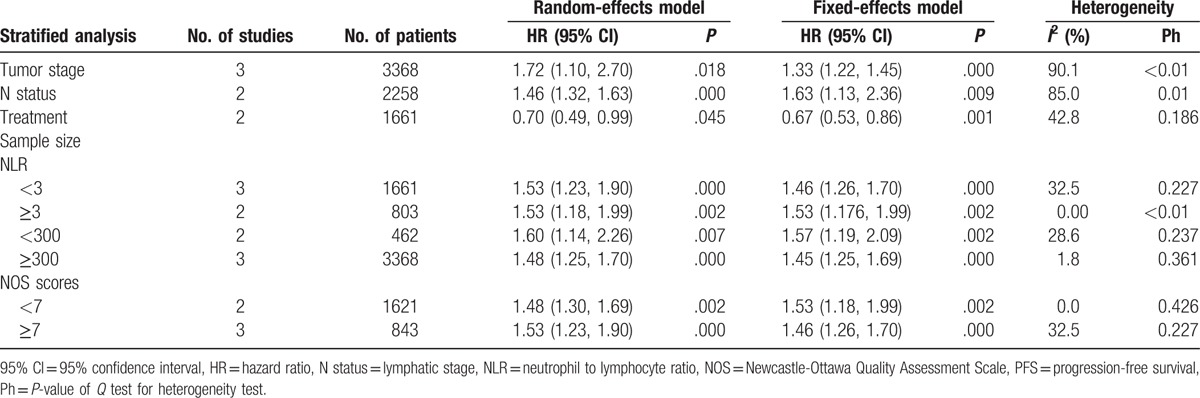
The stratified results showed that patients who received chemotherapy had longer PFS than patients who did not (HR = 0.70, 95% CI = .49–0.99). The subgroup analyses showed significant differences between the OS of NPC patients with early and advanced tumor stages (HR = 1.72, 95% CI = 1.10–2.70) and early and advanced lymphatic stages (HR = 1.46, 95% CI = 1.32–1.63). However, there was substantial heterogeneity across the studies, and these results should therefore be interpreted cautiously.
After stratifying by NLR cut-off values (<3 or ≥3), the pooled estimates remained essentially the same (<3: HR = 1.53, 95% CI = 1.23–1.90; ≥3: HR = 1.53, 95% CI = 1.18–1.99), and no significant heterogeneity was observed across the studies. These results indicate that the different choices of NLR cut-off values did not affect the prognostic value of NLR for PFS. Additionally, the stratifications by sample size and NOS score showed that the different sample sizes and NOS scores did not affect the prognostic value of NLR for PFS (Table 3).
Table 3.
Summary of the meta-analysis results for PFS.
4. Discussion
The present meta-analysis examined the prognostic value of pretreatment NLR for survival in patients with NPC. Our results were obtained by analyzing data on 4359 NPC patients in 6 individual studies, and showed that elevated pretreatment NLR was associated poorer OS and PFS for patients with NPC. Moreover, no significant heterogeneity was observed across the studies, indicating that these results were stable. We also performed subgroup analyses, as stratified by treatment method, tumor stage, lymphatic stage, sample size, NLR cut-off value, and NOS score. The results confirmed that patients with early clinical stages of NPC have better OS and PFS than those with advanced stages. Notably, the results of the subgroup analyses that had been stratified by sample size and NOS score were in agreement with the results of our overall analyses, which further verified the prognostic value of NLR for survival in patients with NPC. In subgroup analyses of NLR, we found that the NLR cut-off values did not have any substantial effects on the associations of NLR with OS and PFS, indicating that NLR was a reliable biomarker for the prognosis of NPC.
Accumulating evidence has demonstrated that inflammation plays a critical role in the pathogenesis of tumors, and that proinflammatory tumor microenvironments are closely related to cancer development and progression.[18,19] Neutrophils and lymphocytes are 2 important cells that reflect systemic inflammation and immune status. An increased NLR implies an elevated neutrophil count and/or a reduced lymphocyte count. Generally, lymphocytes are considered immune cells and exhibit antitumor function, while neutrophils are viewed as inflammatory cells and influence the cytolytic activities of lymphocytes or natural killer cells. Tumor growth is thought to be affected negatively when large amounts of neutrophils migrate to the tumor microenvironment.[20–22] Therefore, NLR is a biomarker that reflects the imbalance of pro- and antitumor activities in the host, with respect to inflammatory response.
Compared with other prognostic biomarkers, the advantages of NLR lie in its convenience and cost-effectiveness. NLR is a routine test and does not add extra costs to the patients, making it an especially attractive biomarker for NPC prognosis in the clinical setting. Additionally, several meta-analyses have reported that NLR has prognostic value for a variety of cancers, including breast cancer,[7] gastric neuroendocrine neoplasms,[8] esophageal squamous cell carcinoma,[9] and nonsmall cell lung cancer.[10] To the best of our knowledge, this is the first meta-analysis of associations between elevated pretreatment NLR and survival status in patients with NPC. In line with previous reports on other cancer sites, our study indicated that pretreatment NLR is a promising biomarker for the prognosis of NPC, suggesting that elevated pretreatment NLR could serve as a prognostic biomarker for several kinds of cancers.
The present meta-analysis has several advantages. First, by including 6 studies, our meta-analysis involved a larger sample size than had been obtained in any single study, providing greater statistical power to detect significant associations. Second, all of the HRs used in our meta-analyses were extracted from multivariate analyses that had been performed in the original studies, which could reduce the influence of confounders. Third, both fixed-effects and random-effects models were used in our meta-analysis, which helps to provide a more comprehensive understanding of the results. However, there are also several limitations to this study. First, the number of eligible articles was relatively small, especially in the subgroup analyses, which undermined their statistical power. Second, all of the included studies had retrospective designs; therefore, selection bias, recall bias, and other biases should not be neglected. Third, although no significant publication bias was detected in our study, some publication bias is inevitably latent in meta-analyses, because most original studies publish positive results. Fourth, there are several limitations to Cochran Q test. For example, this method only determines the presence of a change, and does not evaluate the extent of change. Further, it shows poor performance for detecting true heterogeneity among studies, as is significant in meta-analysis.[23] Therefore, we also used the I2 test to help to ensure that possible heterogeneity could be detected. Fifth, all patients in the included studies were Chinese, and patients from other regions, such as Southeast Asia, were not included. Therefore, our results should be interpreted with caution when extrapolating to patients from other regions. Sixth, Epstein–Barr virus (which is closely related to NPC) and some other factors were not adjusted for in the included studies. Thus, to provide more precise evaluations, there is a remaining need for a well-designed study that accounts for potential confounders.
5. Conclusion
This meta-analysis reveals that NLR is a valuable prognostic biomarker for NPC, and that elevated pretreatment NLR might suggest an unfavorable prognosis for NPC patients. However, our findings need to be interpreted cautiously because of the limitations that have listed above. A large-scale study with a prospective design is warranted to further validate the prognostic value of NLR for patients with NPC.
Footnotes
Abbreviations: CIs = confidence intervals, CXCR-7 = C-X-C chemokine receptor type 7, HRs = hazard ratios, NLR = neutrophil-to-lymphocyte ratio, NOS = Newcastle-Ottawa Quality Assessment Scale, NPC = nasopharyngeal carcinoma, OS = overall survival, PFS = progression-free survival, PRISMA = Preferred Reporting Items for Systematic Review and Meta-analyses statement.
JY and YQ contributed equally to this study.
Funding: This study was supported by Project of Sichuan Provincial Science and Technology (No. 2015SZ0053).
The authors have no conflicts of interest to disclose.
References
- [1].Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 2002;12:421–9. [DOI] [PubMed] [Google Scholar]
- [2].Yi JL, Gao L, Huang XD, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten-year experience of a single institution. Int J Radiat Oncol Biol Phys 2006;65:161–8. [DOI] [PubMed] [Google Scholar]
- [3].Zhu L, Luo K, Gu XH, et al. CXCR7 expression in nasopharyngeal carcinoma tissues correlates with disease severity. Int J Clin Exp Med 2015;8:21257–61. [PMC free article] [PubMed] [Google Scholar]
- [4].Hua YJ, Wang HY, Tang LQ, et al. LOX expression in primary nasopharyngeal carcinoma: correlation with prognostic parameters and outcome. Oncotarget 2016;7:8200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].You B, Cao X, Shao X, et al. Clinical and biological significance of HAX-1 overexpression in nasopharyngeal carcinoma. Oncotarget 2016;7:12505–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223–6. [DOI] [PubMed] [Google Scholar]
- [7].Zhang P, Zong Y, Liu M, et al. Prediction of outcome in breast cancer patients using test parameters from complete blood count. Mol Clin Oncol 2016;4:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cao LL, Lu J, Lin JX, et al. A novel predictive model based on preoperative blood neutrophil-to-lymphocyte ratio for survival prognosis in patients with gastric neuroendocrine neoplasms. Oncotarget 2016;7:42045–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen PC, Feng JF. A novel inflammation-based stage (I Stage) in patients with resectable esophageal squamous cell carcinoma. Mediators Inflamm 2016;2016:5396747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gu XB, Tian T, Tian XJ, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep 2015;5:12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2011: Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10 March 2017. [Google Scholar]
- [12].Sun W, Zhang L, Luo M, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck 2016;38(suppl 1):E1332–40. [DOI] [PubMed] [Google Scholar]
- [13].Chen C, Sun P, Dai QS, et al. The Glasgow Prognostic Score predicts poor survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. PLoS ONE 2014;9:e112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jin Y, Ye X, He C, et al. Pretreatment neutrophil-to-lymphocyte ratio as predictor of survival for patients with metastatic nasopharyngeal carcinoma. Head Neck 2015;37:69–75. [DOI] [PubMed] [Google Scholar]
- [15].Chang H, Gao J, Xu BQ, et al. Haemoglobin, neutrophil to lymphocyte ratio and platelet count improve prognosis prediction of the TNM staging system in nasopharyngeal carcinoma: development and validation in 3,237 patients from a single institution. Clin Oncol (R Coll Radiol) 2013;25:639–46. [DOI] [PubMed] [Google Scholar]
- [16].He JR, Shen GP, Ren ZF, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck 2012;34:1769–76. [DOI] [PubMed] [Google Scholar]
- [17].An X, Ding PR, Wang FH, et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in nasopharyngeal carcinoma. Tumour Biol 2011;32:317–24. [DOI] [PubMed] [Google Scholar]
- [18].Fernandes JV, Cobucci RN, Jatoba CA, et al. The role of the mediators of inflammation in cancer development. Pathol Oncol Res 2015;21:527–34. [DOI] [PubMed] [Google Scholar]
- [19].Oliva N, Carcole M, Beckerman M, et al. Regulation of dendrimer/dextran material performance by altered tissue microenvironment in inflammation and neoplasia. Sci Transl Med 2015;7:272ra211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim HS, Ku JH. Systemic inflammatory response based on neutrophil-to-lymphocyte ratio as a prognostic marker in bladder cancer. Dis Markers 2016;2016:8345286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Leliefeld PH, Koenderman L, Pillay J. How neutrophils shape adaptive immune responses. Front Immunol 2015;6:471.doi: 10.3389/fimmu.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hegmans JP, Aerts JG. Immunomodulation in cancer. Curr Opin Pharmacol 2014;17:17–21. [DOI] [PubMed] [Google Scholar]
- [23].Okeh UM, Oyeka ICA, Igwenagu CM. An alternative approach to Cochran Q test for dichotomous data. MOJ Public Health 2016;4:00086. [Google Scholar]


