Supplemental Digital Content is available in the text
Keywords: antiphospholipid antibodies, antiphospholipid syndrome, Coxiella burnetii, infection, Q fever, thrombosis
Abstract
Q fever is a neglected and potentially fatal disease. During acute Q fever, antiphospholipid antibodies are very prevalent and have been associated with fever, thrombocytopenia, acquired heart valve disease, and progression to chronic endocarditis. However, thrombosis, the main clinical criterion of the 2006 updated classification of the antiphospholipid syndrome, has not been assessed in this context. To test whether thrombosis is associated with antiphospholipid antibodies and whether the criteria for antiphospholipid syndrome can be met in patients with acute Q fever, we conducted a cross-sectional study at the French National Referral Center for Q fever.
Patients included were diagnosed with acute Q fever in our Center between January 2007 and December 2015. Each patient's history and clinical characteristics were recorded with a standardized questionnaire. Predictive factors associated with thrombosis were assessed using a rare events logistic regression model. IgG anticardiolipin antibodies (IgG aCL) assessed by an enzyme-linked immunosorbent assay were tested on the Q fever diagnostic serum. A dose-dependent relationship between IgG aCL levels and thrombosis was tested using a receiver operating characteristic (ROC) analysis.
Of the 664 patients identified for inclusion in the study, 313 (47.1%) had positive IgG aCL and 13 (1.9%) were diagnosed with thrombosis. Three patients fulfilled the antiphospholipid syndrome criteria. After multiple adjustments, only positive IgG aCL (relative risk, 14.46 [1.85–113.14], P = .011) were independently associated with thrombosis. ROC analysis identified a dose-dependent relationship between IgG aCL levels and occurrence of thrombosis (area under curve, 0.83, 95%CI [0.73–0.93], P < .001).
During acute Q fever, antiphospholipid antibodies are associated with thrombosis, thrombocytopenia, and acquired valvular heart disease. Antiphospholipid antibodies should be systematically assessed in acute Q fever patients. Hydroxychloroquine, which has been previously shown to antagonize IgG aCL pathogenic properties, should be tested in acute Q fever patients with anticardiolipin antibodies to prevent antiphospholipid-associated complications.
Key Point: In addition to fever, thrombocytopenia and acquired valvular heart disease, antiphospholipid antibodies are associated with thrombosis during acute Q fever.
1. Introduction
Q fever is a worldwide ubiquitous infection caused by the intracellular bacterium Coxiella burnetii and has a high incidence in epidemic and endemic areas.[1] Acute Q fever, the symptomatic primary infection, principally manifests as fever, pneumonia, and/or hepatitis.[1] Cardiovascular complications are the main risk of C burnetii infection, including acute and chronic endocarditis and vascular infections.[1,2] During primary infection, antiphospholipid antibodies and specifically IgG anticardiolipin antibodies (IgG aCL) are highly prevalent (57 to 80%) and are associated with fever, thrombocytopenia, valvular heart disease, and progression toward chronic endocarditis.[3–5] This prevalence is characteristic of acute Q fever since prevalence is only 1% to 5% in the general population and 10% to 30% in other infections including HCV and parvovirus B19.[6,7] Only older studies on HIV reported prevalence of up to 59%.[7]
The classification criteria for definite antiphospholipid syndrome include 1 or more clinical episodes of arterial, venous, or small vessel thrombosis and at least 1 of the following 3 laboratory criteria: lupus anticoagulant, anticardiolipin antibodies, or anti-β2glycoprotein I antibodies of IgG or IgM isotype on 2 or more occasions, at least 12 weeks apart.[8] During acute Q fever, IgG aCL are more frequent than lupus anticoagulant and IgM anticardiolipin antibodies, whereas anti-β2glycoprotein I antibodies are very rare.[3,9] However, “infectious” aCL, which are generally β2-glycoproetin I independent, were believed to be found in conditions not involving thrombotic complications,[7] whereas antiphospholipid-associated thrombosis during infections has been reported with Mycoplasma pneumoniae,[10–13] Bacteroides,[14] EBV,[15] CMV,[16,17] HIV,[18,19] HBV,[20] and HCV.[21]
In this context, we treated an acute Q fever patient presenting a severe pulmonary embolism during acute Q fever. IgG aCL were increased to 13,000 immunoglobulin G-type phospholipid units (GPLU), or 590 times the normal value (<22 GPLU). In addition, several cases with a similar association have been reported in the literature (see Table 1 and Table, Supplemental Content 1). Strikingly, although antiphospholipid antibodies are increasingly recognized as pathogenic per se,[22] and considering that thrombosis is the main clinical classification criterion of the antiphospholipid syndrome,[8] no study has systematically tested the link between antiphospholipid antibodies and thrombosis during acute Q fever.
Table 1.
Summary of the characteristics of thrombosis in acute Q fever patients.
Consequently, the purpose of this cross-sectional study of the French National Referral Center for Q fever was to determine whether IgG aCL were associated with thrombosis in acute Q fever patients. We also sought to find out whether the criteria for antiphospholipid syndrome can be met in acute Q fever patients.
2. Patients and methods
2.1. Center and patients
This cross-sectional study was performed on acute Q fever patients diagnosed at the French National Referral Center for Q fever between January 2007 and December 2015. Clinical and echocardiographic data were prospectively obtained for all positive samples and collected in a computerized database. Patients with a past infection, with missing age or sex data, or insufficient clinical data were not eligible. All patients with acute Q fever and for whom age and sex data were available were included. Patients with persistent C burnetii focalized infection without acute Q fever diagnosed in our center (chronic endocarditis, vascular infection, osteo-articular infections, persistent lymphadenitis, and other rare forms of persistent infections) were excluded. Pregnant women and patients for whom IgG aCL could not be quantified because of an insufficient amount of serum (IgG anticardiolipin antibodies were assessed on the Q fever diagnostic serum) were also excluded. The main outcome measure was the occurrence of a thrombosis during acute Q fever (acute Q fever thrombosis). Data regarding the history of thrombosis, recent surgery, or other hypercoagulable states were collected in cases (Q fever patients with thrombosis) but not in Q fever patients without thrombosis as these data are not part of our standardized Q fever questionnaire.
2.2. Acute Q fever thrombosis definition
Acute Q fever thrombosis was defined as the presence of an arterial, venous, or small vessel thrombosis diagnosed by ultrasound or computed tomography (CT scan) within 3 months of the onset of symptoms in patients with acute Q fever according to the definition previously reported.[1,2,5] Acute Q fever patients progressing toward chronic Q fever endocarditis were treated by doxycycline and hydroxychloroquine for 18 to 24 months.[1] Thrombosis occurring during chronic endocarditis and/or more than 3 months after the onset of symptoms or estimated date of primary infection (seroconversion) were excluded.
2.3. Detection of anticardiolipin antibodies
IgG anticardiolipin antibodies were assessed on the Q fever diagnostic serum providing an early measure using the reference technique and standardized enzyme-linked immunosorbent assay (ELISA), as previously reported.[5,8] IgG aCL were tested retrospectively before and prospectively after January 2012.
2.4. Antiphospholipid antibody syndrome definition
Antiphospholipid antibody syndrome was defined according to the international classification updated in 2006.[8] Antiphospholipid antibody syndrome (APS) was considered present if 1 or more clinical episodes of arterial, venous, or small vessel thrombosis in any tissue or organ was diagnosed by unequivocal findings of appropriate imaging studies and if IgG aCL in serum or plasma were present in medium or high titers (i.e., > the 99th percentile), on 2 or more occasions, at least 12 weeks apart, measured by a standardized ELISA.
2.5. Statistical analysis
This cross-sectional study was reported following the STROBE statement. Receiver operating characteristic (ROC) analysis was used to test a dose-dependent relationship between IgG aCL levels and thrombosis occurrence. A rare events logistic regression model was used to assess potential predictors of acute Q fever thrombosis as previously reported.[2,23,24]P-values < .05 were considered significant and all hypothesis tests were 2-sided.
2.6. Ethics statement
Patients’ medical data were retrospectively reviewed, and all collected data were anonymized in standardized forms according to the procedures of the Commission Nationale de l’Informatique et des Libertés (the French commission for data protection). Informed consent was obtained from all participating patients. The study was approved by the local ethics committee (Comité de Protection des Personnes Sud Mediterranée 1) under registration number 1355 and by the French National Drugs and Health Products Agency.
3. Results
3.1. Patients with acute Q fever thrombosis
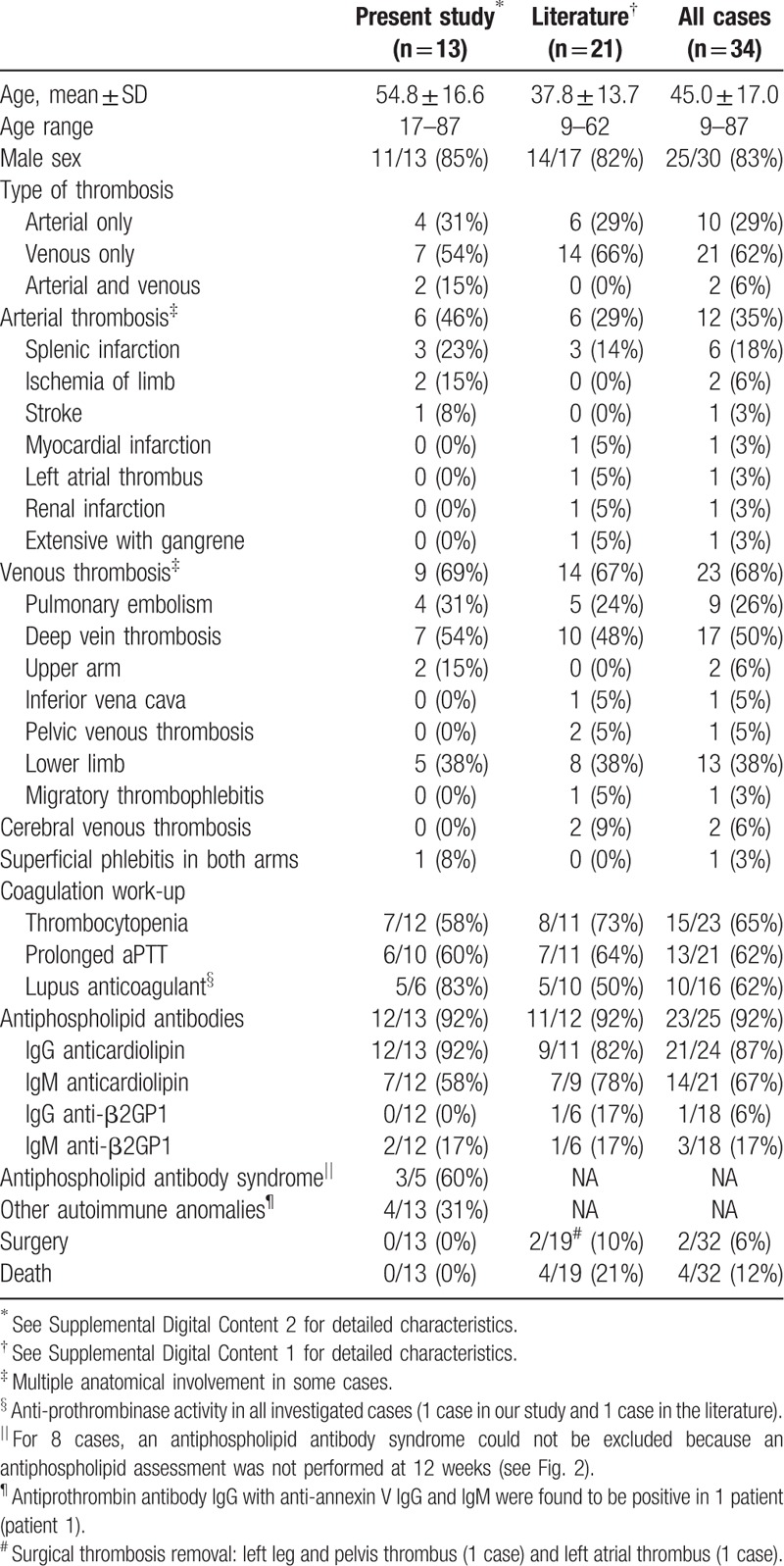
Of the 664 assessable patients (Fig. 1), 13 (1.9%) presented thrombosis (see Table 1 and Table, Supplemental Digital Content 2, which reports the characteristics of the patients with acute Q fever and thrombosis reported in this study). Eleven of these 13 patients were male, and the mean age (± standard deviation) was 56 ± 17 years. None had a previously known valvular heart disease or immunodeficiency. Five had a cardiovascular risk factor (smoking (n = 5), high blood pressure (3), diabetes (2), dyslipidemia (2), obesity (1), and overweight (1)). The 8 other individuals had no cardiovascular risk factors. Three were alcoholics. Only 2 had a history of thrombosis (1 with deep vein thrombosis and pulmonary embolism 10 years ago and treated with long-term anticoagulation, 1 with 3 deep vein thromboses 12, 10, and 8 years ago without long-term anticoagulation) but none had a previously identified known risk factor for thrombosis.
Figure 1.
Study flowchart. ∗Different persistent infections can co-occur.
Thrombosis was arterial in 4 cases, venous in 7, and arterial and venous in 2 cases. The 6 arterial thrombosis included a stroke, an ischemia of the left upper arm, a lower limb ischemia, and 3 splenic infarctions. The 9 venous thromboses included 4 pulmonary embolisms, bilateral in 2 patients, associated with lung infarction in 2 cases, and with lower limb deep vein thrombosis in 2 cases. In 5 cases, deep vein thrombosis was not associated with pulmonary embolism and involved the upper arm (2) or lower limb (3 cases). The 2 patients with both arterial and venous thrombosis had a splenic infarction followed by pulmonary embolism and a lower limb arterial thrombosis with superficial phlebitis in both arms, respectively. In all 13 cases, transthoracic echocardiography was performed and no valvular heart disease was found, although pericarditis was noted in 1 case. In 2 cases, transesophageal echocardiography discovered a patent foramen ovale associated with atrial septal aneurysm 1 and 3 months after acute Q fever diagnosis, respectively. A right-left shunt was found in 1 of these 2 cases.
Antiphospholipid antibodies were positive in 12/13 cases. The IgG aCL median was 310 GPLU (IQR 55–757) (range 9–13,000) or 14 times the normal value for our laboratory (< 22 GPLU). Coagulation work-up revealed thrombocytopenia in 4 patients and prolonged activated partial thromboplastin time (aPTT) was found in half of the cases (4/8) ranging from 1.3 to 1.98. The Rosner indice was increased in 2/3 of the tested patients (24 and 28, N < 15). Dilute Russell Viper Venom Time (DRVVT) was increased in all 3 tested patients. In 5 cases, a lupus anticoagulant was found with antiprothrombinase activity. In 3 patients, a more exhaustive coagulation check-up was performed and was negative (no cancer was found and antithrombin III, protein C, protein S, Factor II and V Leyden, activated C protein resistance, HPN clone, Jak2 mutation, and homocysteine were normal).
3.2. Patient outcomes
Median follow-up of cases was of 17 months (range 0–48 months). In addition to anticoagulants, the treatment included doxycycline for 2 to 3 weeks in all documented cases. Four patients progressed to persistent focalized infections including 3 cases of endocarditis (1 definite and 2 possible according to recently updated criteria[1]) and 1 new form, a focalized periduodenal arteritis without any valvular heart disease, and were treated accordingly. This previously unknown arterial involvement, which was only diagnosed only as the result of [18]F-fluorodeoxyglucose positron emission tomography, suggests the cardiovascular pathogenic role of IgG aCL in Q fever. None of these patients died.
All 9 patients with more than 6 months’ follow-up were considered to be cured of C burnetii and none of the patients had a thrombosis recurrence. Long-term sequelae included grade II persistent dyspnea with long-term oxygen requirements. One patient presented uveitis during follow-up, 1 patient with an initial stroke presented a persistent right thermoalgic hypoesthesia which was cured after 3 years of treatment with only slight memory disorders (sequelae on magnetic resonance imaging), and 1 patient with upper arm ischemia presented persistent right hand paresthesia.
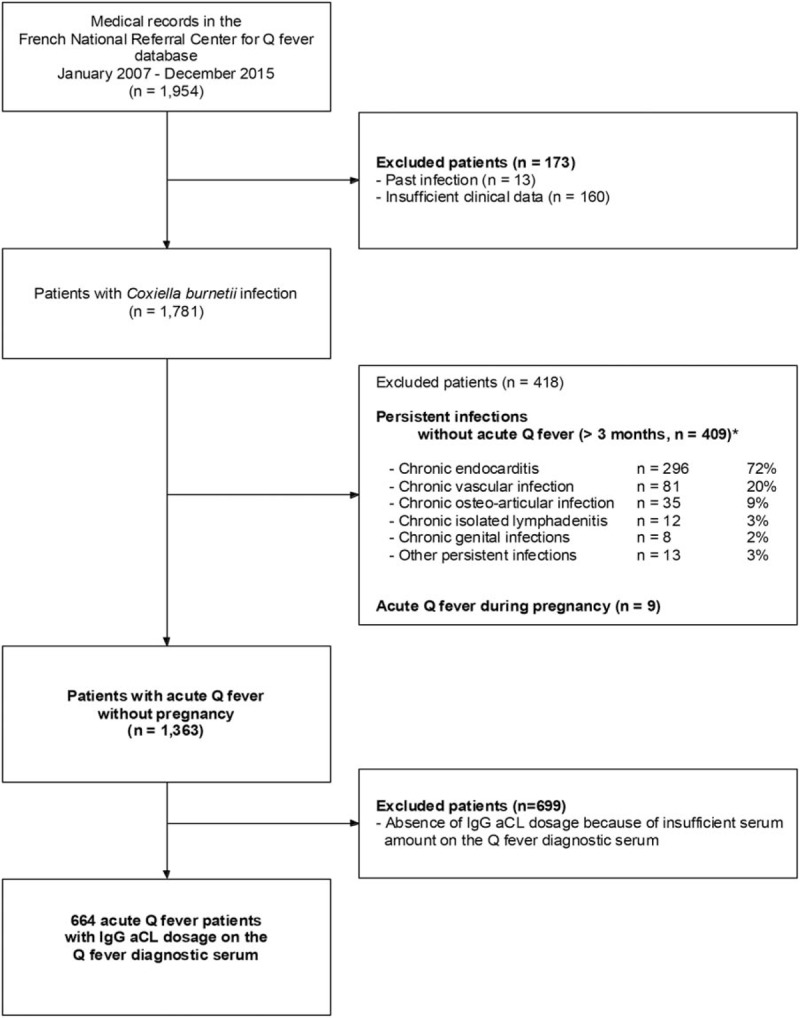
IgG aCL levels decreased gradually in all patients (Fig. 2). Of the 8 patients with acute Q fever thrombosis and at least 12 weeks of follow-up, 3 had persistent IgG aCL (≥ 12 weeks) and IgG aCL normalized at follow-up in only 1 of them (25.4 GPLU at 24 months and 25.2 at 46 months in the 2 other patients, respectively). IgG aCL persistence ≥ 12 weeks was associated with higher initial IgG aCL levels (median IgG aCL levels [GPLU] 1020 versus 98, 2-sided Mann–Whitney test, P = .036). Indeed, 3/3 patients with initial IgG aCL>500 GPLU had IgG aCL persistence over 12 weeks compared to 0/5 with initial IgG aCL<500 GPLU (2-sided Fisher exact test, P < .007).
Figure 2.
Evolution of IgG anticardiolipin levels during and after acute Q fever. Only patients for whom at least 2 IgG anticardiolipin dosages were available are shown. As previously reported, an explosive secretion of IgG aCL during acute Q fever preceded a gradual decrease over several months.[2,5] Three acute Q fever patients had thrombosis and persistently positive IgG aCL (≥ 12 weeks) and consequently fulfill the criteria for antiphospholipid syndrome.[8] IgG aCL = Immunoglobulin G anticardiolipin antibodies.
3.3. Comparison with patients without thrombosis
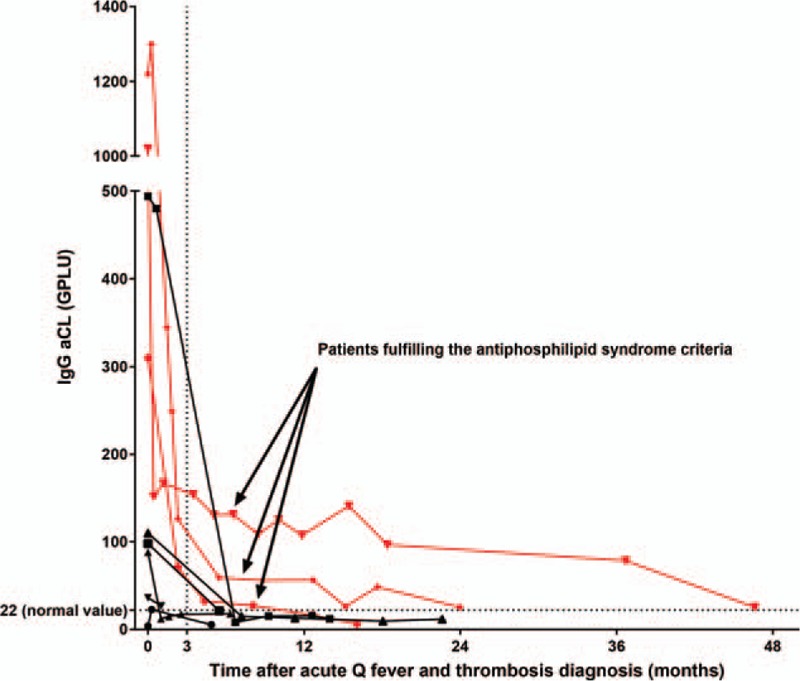
Age, sex, history of valvular heart disease, immunodeficiency, and phase I IgG at diagnosis were not significantly different between acute Q fever patients with or without thrombosis (Table 2). IgG aCL were more frequently positive in acute Q fever patients with thrombosis (12/13) than in acute Q fever patients without thrombosis (301/651, 2-sided Fisher exact test, P < .001) (Table 2). IgG aCL levels were very significantly higher in the acute Q fever thrombosis patients (median 310, interquartile range [IQR] 55–757 GPLU) versus acute Q fever patients without thrombosis (17.7, [5.6–74.8], 2-sided Mann–Whitney, P < .001, Fig. 3).
Table 2.
Characteristics of acute Q fever patients with and without thrombosis.
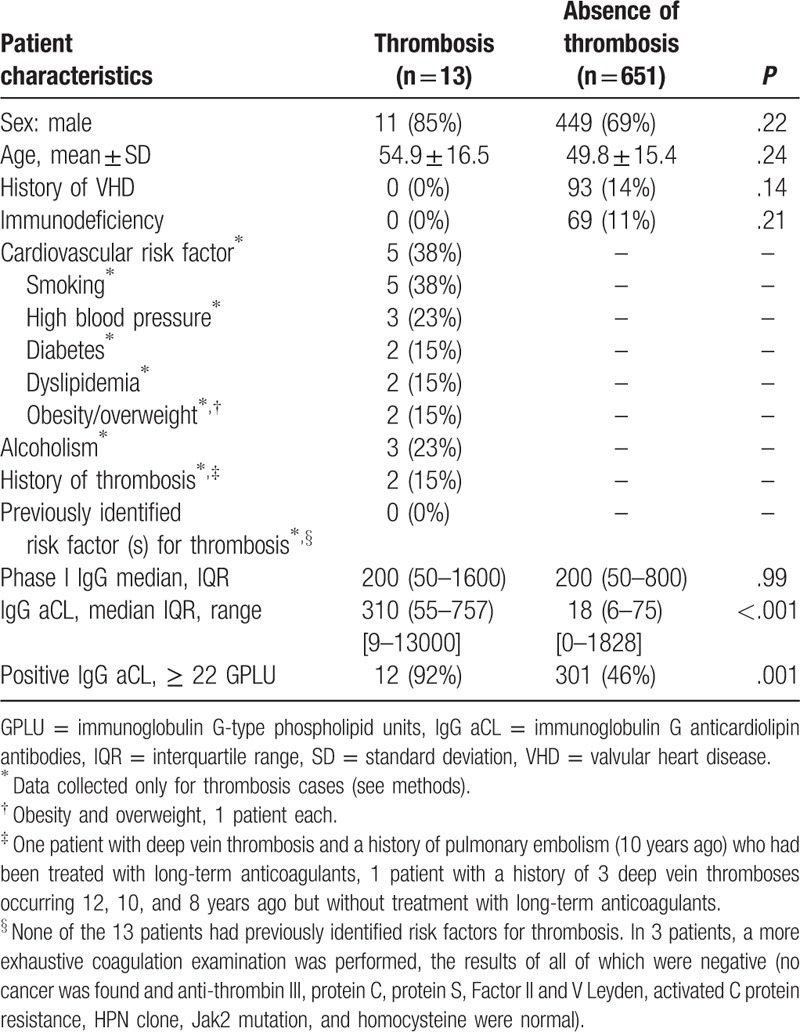
Figure 3.
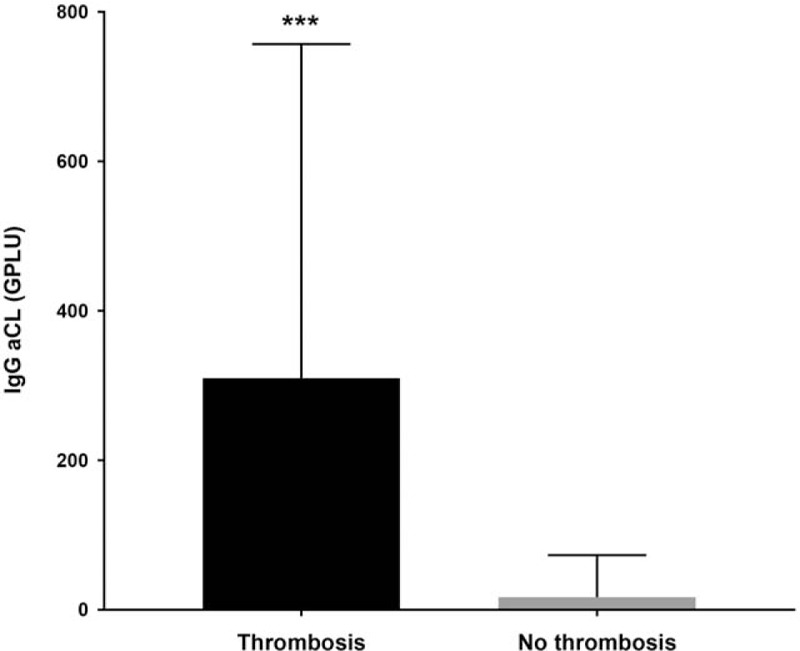
IgG anticardiolipin levels in acute Q fever patients with and without thrombosis. Median and interquartile range. ∗∗∗: P < .0001 (2-sided Mann–Whitney test). IgG aCL = Immunoglobulin G anticardiolipin antibodies.
ROC analysis identified a dose-dependent relationship between IgG aCL levels and the occurrence of thrombosis (area under curve, 0.83, 95%CI [0.73–0.93], P < .001, Fig. 4). In a logistic regression model including age, sex, history of valvular heart disease, immunodeficiency, phase I IgG at diagnosis, and positive IgG aCL (≥22GPLU), only positive IgG aCL (relative risk, 14.3 (1.85–110.51), P = .011) were independently associated with thrombosis.
Figure 4.
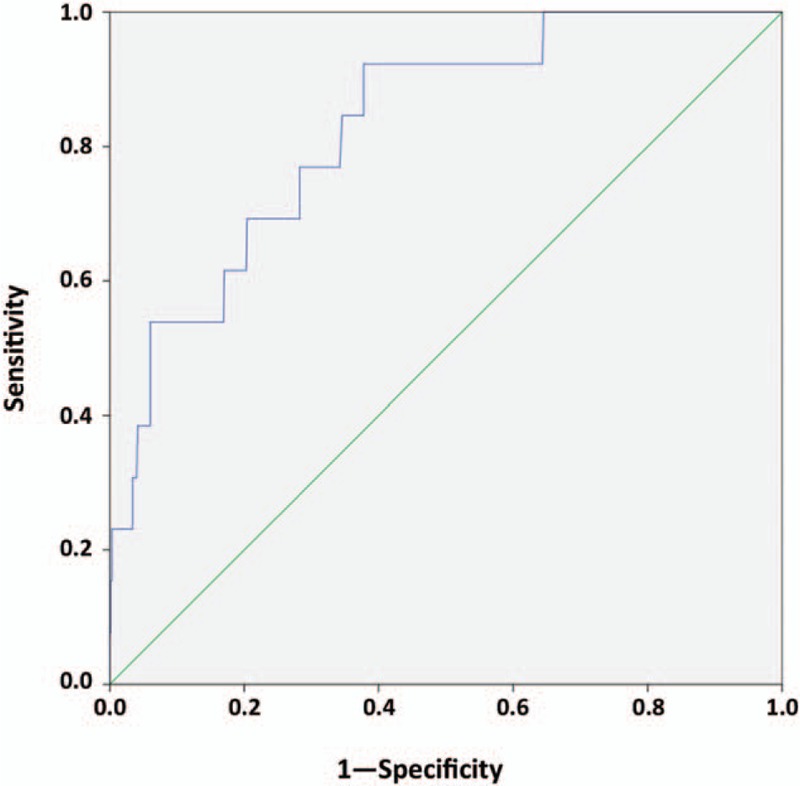
ROC curve between IgG aCL levels and thrombosis during acute Q fever. IgG aCL = Immunoglobulin G anticardiolipin antibodies, ROC = receiver operating characteristic.
4. Discussion
We found that potentially fatal arterial and venous thrombosis may occur during acute Q fever with a dose-dependent relationship with antiphospholipid antibodies (IgG aCL). Cases were diagnosed in 5 different regions of France including French Guiana, supporting a geographical generalizability. All cases were confirmed by adequate diagnostic methods (ultrasound or CT scan) and C burnetii primary infection was confirmed in our national referral center in all cases, based on serology by immunofluorescence, which is the reference method.[1] The associations with high IgG aCL levels and biological gradient were confirmed by several different statistical analyses (ROC analysis, rare event logistic regression model).
Our study confirms preliminary data, including case reports. While reviewing the available literature, we found 21 cases of thrombosis associated with acute Q fever (see Table 1 and Table, Supplemental Digital Content 1, which reports the characteristics of patients with acute Q fever and thrombosis reported in the literature), including 6 pulmonary embolisms, 2 cerebrospinal venous thromboses, and 7 other deep vein thromboses including inferior vena cava, pelvic, bilateral or migratory thrombophlebitis. Six arterial thromboses were also reported including myocardial infarction, humeral artery thrombosis, renal thrombosis, left atrial thrombus, splenic infarction (3 cases) and extensive arterial thrombosis with gangrene. Four patients died. In the investigated cases, antiphospholipid features were frequently found with prolonged aPTT, lupus anticoagulant, antiphospholipid antibodies, anticardiolipin antibodies of IgG or IgM isotype (11/12 cases with available data had positive anticardiolipin antibodies), while anti-β2GPI antibodies were rarely positive.
A causal role of IgG aCL in the occurrence of thrombosis during the symptomatic C burnetii primary infection is suggested by several Bradford-Hill criteria[25]: the strength of the association (relative risk of 14), consistency with the literature (21 case reports), temporal coincidence of IgG aCL secretion and thrombosis, biological gradient (demonstrated by the ROC analysis), plausibility based on extensive experimental data demonstrating the thrombogenic properties of IgG aCL per se,[22] and analogy with other infectious antiphospholipid-associated thromboses.[10–21]
Although Ordi-Ros et al[3] first demonstrated an association between antiphospholipid antibodies and fever or thrombocytopenia during acute Q fever, we previously demonstrated a link with acquired valve heart disease,[4] including valvular vegetation[2] and progression toward chronic endocarditis.[5] However, the main clinical criteria for antiphospholipid syndrome is thrombosis.[8] For the first time, we showed that the antiphospholipid syndrome criteria may be encountered in 3 acute Q fever patients. The persistence of IgG aCL was demonstrated to last up to 46 months after acute Q fever and thrombosis in 1 patient.
One limitation of our study is that only IgG aCL were tested, while a study including 1000 patients with antiphospholipid syndrome found that 25% do not have IgG anticardiolipin: 12% had IgM aCL without IgG and 12% had a lupus anticoagulant without any anticardiolipin antibodies.[26] Indeed, although less frequent than IgG aCL,[3] IgM aCL and lupus anticoagulant are frequently found in acute Q fever patients (see Tables, Supplemental Digital Content 1 and 2, which report the characteristics of the patients with acute Q fever and thrombosis reported in the literature and in this study). For instance, Newcombe et al[27] reported an acute Q fever patient with a splenic infarction and isolated IgM anticardiolipin antibodies. Moreover, further studies should test whether high IgG aCL levels are associated with other antiphospholipid-associated complications found in both antiphospholipid syndrome and acute Q fever, including aortitis, arteritis, obstetrical complications, cholecystitis, hemophagocytic syndrome, disseminated intravascular coagulation, neurological and renal involvement, and arthritis.[1] The advent of positron emission tomography recently led to the diagnosis of large vessel vasculitis in an acute Q fever patient with IgM anticardiolipin and anti β2 GPI IgM antibodies,[28] suggesting that antiphospholipid antibodies during acute Q fever may also play a role in the pathogenesis of chronic Q fever vascular infections.
Another limitation of our study is that IgG aCL could only be quantified for half of the acute Q fever patients in the inclusion period because of insufficient serum for the other patients. Indeed, IgG aCL were assessed on the Q fever diagnostic serum, providing an early measure, but the fact that half of eligible patients could not be analyzed for IgG aCL could lead to a possible bias. Conversely, this identical source and centralized method of measurement of IgG aCL limited several biases mentioned in the STROBE checklist. Furthermore, this limitation cannot explain the dramatic difference in IgG aCL between acute Q fever patients with and without thrombosis and the dose-dependent relationship between IgG aCL levels and the occurrence of thrombosis. Finally, the very large sample size of the control group (651 patients without thrombosis) compared to the number of cases (13 patients) make the risk of bias through selection of the controls very low.
In view of our results, clinicians should be aware of the risk of thrombosis and any antiphospholipid-related complications in acute Q fever patients. This risk increases with IgG aCL levels. Strikingly, hydroxychloroquine, which improves the prevention and treatment of Q fever endocarditis,[29,30] directly reduces the binding of antiphospholipid antibodies to phospholipid bilayers,[31] prevents thrombogenic properties of antiphospholipid antibodies,[32–35] and lowers the odds of having persistently positive antiphospholipid antibodies or lupus anticoagulant.[36,37] Consequently, adding hydroxychloroquine to doxycycline in acute Q fever patients with elevated IgG aCL levels could be useful in preventing antiphospholipid-associated complications, including thrombosis, and should be tested in future studies. Prophylactic anticoagulation should also be discussed in acute Q fever patients with a history of thrombosis and/or a procoagulable state and/or high IgG aCL levels.
Acknowledgments
The authors thank Catherine Brossard and all the members of the Q fever serology department, as well as Stéphanie Micalef and Abdou Beziane of the immunology department for their excellent contributions. They thank the entire Q fever team for collaboration at the French National Referral Center for Q fever. They also thank Magdalen Lardière for English reviewing.
Supplementary Material
Footnotes
Abbreviations: C burnetii = Coxiella burnetii, IgG aCL = immunoglobulin G anticardiolipin antibodies.
Authorship: MM analyzed the data and wrote the manuscript. NB and JLM performed all immunological analyses. SB, NN, CD, SE, and EA contributed significantly to the French National Referral Center for Q fever database. AB, OE, SB, BC, KBL, NFM, ED, FR, JR, and FD contributed to the acquisition of data and cared for acute Q fever thrombosis patients. CP and PC performed the statistical analyses. LC contributed helpful expertise on hemostasis and technical comments. DR designed and conducted the study. All authors revised the manuscript and gave their approval before submission.
MM and DR had full access to all the data in the study and have final responsibility for the decision to submit the manuscript for publication.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Eldin C, Melenotte C, Mediannikov O, et al. From Q fever to C burnetii infection: a paradigm change. Clin Microbiol Rev 2017;30:115–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Million M, Thuny F, Bardin N, et al. Antiphospholipid antibody syndrome with valvular vegetations in acute Q fever. Clin Infect Dis 2016;62:537–44. [DOI] [PubMed] [Google Scholar]
- [3].Ordi-Ros J, Selva-O’Callaghan A, Monegal-Ferran F, et al. Prevalence, significance, and specificity of antibodies to phospholipids in Q fever. Clin Infect Dis 1994;18:213–8. [DOI] [PubMed] [Google Scholar]
- [4].Million M, Raoult D. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013;368:2335. [DOI] [PubMed] [Google Scholar]
- [5].Million M, Walter G, Bardin N, et al. Immunoglobulin G anticardiolipin antibodies and progression to Q fever endocarditis. Clin Infect Dis 2013;57:57–64. [DOI] [PubMed] [Google Scholar]
- [6].Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev 2010;9:A299–304. [DOI] [PubMed] [Google Scholar]
- [7].Asherson RA, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis 2003;62:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- [9].Sanmarco M, Soler C, Christides C, et al. Prevalence and clinical significance of IgG isotype anti-beta 2-glycoprotein I antibodies in antiphospholipid syndrome: a comparative study with anticardiolipin antibodies. J Lab Clin Med 1997;129:499–506. [DOI] [PubMed] [Google Scholar]
- [10].Bakshi M, Khemani C, Vishwanathan V, et al. Mycoplasma pneumonia with antiphospholipid antibodies and a cardiac thrombus. Lupus 2006;15:105–6. [DOI] [PubMed] [Google Scholar]
- [11].Brown SM, Padley S, Bush A, et al. Mycoplasma pneumonia and pulmonary embolism in a child due to acquired prothrombotic factors. Pediatr Pulmonol 2008;43:200–2. [DOI] [PubMed] [Google Scholar]
- [12].Graw-Panzer KD, Verma S, Rao S, et al. Venous thrombosis and pulmonary embolism in a child with pneumonia due to Mycoplasma pneumoniae. J Natl Med Assoc 2009;101:956–8. [DOI] [PubMed] [Google Scholar]
- [13].Nagashima M, Higaki T, Satoh H, et al. Cardiac thrombus associated with Mycoplasma pneumoniae infection. Interact Cardiovasc Thorac Surg 2010;11:849–51. [DOI] [PubMed] [Google Scholar]
- [14].Liappis AP, Roberts AD, Schwartz AM, et al. Thrombosis and infection: a case of transient anti-cardiolipin antibody associated with pylephlebitis. Am J Med Sci 2003;325:365–8. [DOI] [PubMed] [Google Scholar]
- [15].van Hal S, Senanayake S, Hardiman R. Splenic infarction due to transient antiphospholipid antibodies induced by acute Epstein-Barr virus infection. J Clin Virol 2005;32:245–7. [DOI] [PubMed] [Google Scholar]
- [16].Labarca JA, Rabaggliati RM, Radrigan FJ, et al. Antiphospholipid syndrome associated with cytomegalovirus infection: case report and review. Clin Infect Dis 1997;24:197–200. [DOI] [PubMed] [Google Scholar]
- [17].Delbos V, Abgueguen P, Chennebault JM, et al. Acute cytomegalovirus infection and venous thrombosis: role of antiphospholipid antibodies. J Infect 2007;54:e47–50. [DOI] [PubMed] [Google Scholar]
- [18].Witz M, Lehmann J, Korzets Z. Acute brachial artery thrombosis as the initial manifestation of human immunodeficiency virus infection. Am J Hematol 2000;64:137–9. [DOI] [PubMed] [Google Scholar]
- [19].Leder AN, Flansbaum B, Zandman-Goddard G, et al. Antiphospholipid syndrome induced by HIV. Lupus 2001;10:370–4. [DOI] [PubMed] [Google Scholar]
- [20].Elefsiniotis IS, Diamantis ID, Dourakis SP, et al. Anticardiolipin antibodies in chronic hepatitis B and chronic hepatitis D infection, and hepatitis B-related hepatocellular carcinoma. Relationship with portal vein thrombosis. Eur J Gastroenterol Hepatol 2003;15:721–6. [DOI] [PubMed] [Google Scholar]
- [21].Prieto J, Yuste JR, Beloqui O, et al. Anticardiolipin antibodies in chronic hepatitis C: implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology 1996;23:199–204. [DOI] [PubMed] [Google Scholar]
- [22].Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013;368:1033–44. [DOI] [PubMed] [Google Scholar]
- [23].King G, Zeng L. Estimating risk and rate levels, ratios and differences in case-control studies. Stat Med 2002;21:1409–27. [DOI] [PubMed] [Google Scholar]
- [24].Tomz M, King G, Zeng L. ReLogit: rare events logistic regression. J Stat Softw 2003;8:246–7. [Google Scholar]
- [25].Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cervera R, Piette JC, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002;46:1019–27. [DOI] [PubMed] [Google Scholar]
- [27].Newcombe JP, Gray PE, Palasanthiran P, et al. Q Fever with transient antiphospholipid antibodies associated with cholecystitis and splenic infarction. Pediatr Infect Dis J 2013;32:415–6. [DOI] [PubMed] [Google Scholar]
- [28].Baziaka F, Karaiskos I, Galani L, et al. Large vessel vasculitis in a patient with acute Q-fever: a case report. IDCases 2014;1:56–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Million M, Walter G, Thuny F, et al. Evolution from acute Q fever to endocarditis is associated with underlying valvulopathy and age and can be prevented by prolonged antibiotic treatment. Clin Infect Dis 2013;57:836–44. [DOI] [PubMed] [Google Scholar]
- [30].Million M, Thuny F, Richet H, et al. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis 2010;10:527–35. [DOI] [PubMed] [Google Scholar]
- [31].Rand JH, Wu XX, Quinn AS, et al. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-beta2-glycoprotein I complexes to phospholipid bilayers. Blood 2008;112:1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Espinola RG, Pierangeli SS, Gharavi AE, et al. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb Haemost 2002;87:518–22. [PubMed] [Google Scholar]
- [33].Edwards MH, Pierangeli S, Liu X, et al. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation 1997;96:4380–4. [DOI] [PubMed] [Google Scholar]
- [34].Belizna C. Hydroxychloroquine as an anti-thrombotic in antiphospholipid syndrome. Autoimmun Rev 2015;14:358–62. [DOI] [PubMed] [Google Scholar]
- [35].Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost 2013;11:1927–9. [DOI] [PubMed] [Google Scholar]
- [36].Broder A, Putterman C. Hydroxychloroquine use is associated with lower odds of persistently positive antiphospholipid antibodies and/or lupus anticoagulant in systemic lupus erythematosus. J Rheumatol 2013;40:30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nuri E, Taraborelli M, Andreoli L. Long-term use of hydroxychloroquine reduces antiphospholipid antibodies levels in patients with primary antiphospholipid syndrome. Immunol Res 202017;65:17–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


