Abstract
Kawasaki disease (KD) may be an acute systemic immune-mediated disease which occurs after infection of unknown KD pathogen(s). The aim of this study is to evaluate the changes in platelet count and immunoglobulin (Ig) levels (IgG, IgM, IgA, and IgE) during hospitalization.
Forty-three patients with complete KD who received intravenous Ig at 2 g/kg were enrolled in South Korea. The platelet count and Ig levels of the patients were examined twice at presentation and around discharge (mean 6.2 ± 2.4 days apart) and the relationships between platelet level and Ig levels were evaluated.
The mean patient age was 31 ± 18 months; 28 patients were male and 15 were female. The values of all parameters measured, with the exception of IgE, were significantly increased at the second examination compared with their values at presentation. These values gradually increased over time after fever onset, over periods ranging from 2 to 16 days. The extent by which platelet levels increased over these 2 time points was correlated with the extents by which IgG (P < .01), IgM (P < .01), and IgA levels (P = .01) increased.
Both the platelet count and the Ig (IgG, IgM, and IgA) levels increased with a correlation each other during the early convalescent stage of KD. This finding suggests that all Ig subtypes except IgE and platelets may be involved in the recovery from KD and that the extent of increased parameters may reflect the degree of systemic inflammation in acute KD.
Keywords: immunoglobulin A, immunoglobulin G, immunoglobulin M, Kawasaki disease, platelet
1. Introduction
Kawasaki disease (KD) is a self-limiting systemic immune-mediated disease with a fever duration of 1 to 2 weeks (mean 10–11 days), and some severely affected patients can experience prolonged fever and severe complications such as giant coronary artery aneurysms.[1] Etiologic agent(s) and immunopathogenesis of the disease remain unknown, despite of extensive studies for a half-century. However, epidemiological and clinical characteristics of KD such as emerging of KD in East Asian countries in order of Japan, South Korea, Taiwan, and China with time-gaps of 5 to 10 years, same age predilection of <5 years of age from emergence to present time in these countries, and marked different incidence across the populations have suggested that the immunopathogenesis of KD may be associated with an infection-related immune-mediated disease such as acute rheumatic fever rather than an infectious disease such as scarlet fever.[2,3]
During the self-limiting clinical course of KD, the intensity of systemic inflammation in the acute febrile stage of KD gradually increases and reaches a peak, after which the inflammation gradually decreases and the disease progresses to the convalescent stage.[3,4] Therefore, it is postulated that the host immune reactions preceding this peak of inflammation process may mediate tissue cell injury, whereas the immune reactions following the peak may be involved in preventing from tissue cell injury.[3] During this process, the state of systemic inflammation is potentially reflected in laboratory parameters, including C-reactive protein (CRP), white blood cell (WBC) count, platelet count, albumin, and other inflammatory indices. Both platelet count and immunoglobulin (Ig) levels, including immunoglobulin G (IgG), IgM, and IgA, have been shown to be increased in the convalescent stage of KD.[5–7] However, the precise reason underlying this phenomenon remains to be elucidated.
In the present study, we wanted to evaluate the changes in platelet count and Ig level (IgG, IgM, IgA, and IgE) during hospitalization. Also, we found that elevated platelet count and Ig (IgG, IgM, and IgA) levels were correlated with each other in the early convalescent stage of KD. We discuss on this finding as a part of characteristics of systemic inflammation in KD.
2. Materials and methods
The retrospective study was conducted in 2 secondary general hospitals in South Korea. We have used same treatment protocol for treating KD patients since September 2013. In this series, the subjects were 43 complete KD patients admitted between September 2013 and December 2014 (23 cases in Daejeon St. Mary's Hospital and 20 cases in Eujungbu St. Mary's Hospital). All children satisfied with the diagnostic criteria for KD as defined by the American Heart Association or the Japanese Kawasaki Disease Research Committee; complete KD is defined as having fever of ≥5 days with at least 4 of the 5 principal clinical signs: bilateral conjunctival injection, changes in the lips and oral cavity, polymorphous skin rash, changes in the peripheral extremities, and acute nonpurulent cervical lymphadenopathy. In this series, however, 24 patients admitted before fever duration of 5 days (2–4 days), and 8 patients received intravenous immunoglobulin (IVIg) treatment before 5 days of fever duration. All patients received IVIg (2 g/kg) over 12 hours and medium-dose aspirin (30–40 mg/kg) during febrile period. Patients with initial IVIg nonresponsiveness or those with incomplete KD were excluded in this series (Fig. 1). However, 6 patients presented without all inclusion criteria at admission and satisfied with all clinical and laboratory criteria during hospitalization were included in the study. Five patients had mild coronary artery lesions (CALs, ectasia), but no patients had aneurysms. According to our treatment protocol which was obtained parental consent, serial laboratory examinations were performed at least 3 times during the admission period: before IVIg administration, around 24 hours after IVIg administration, and around discharge (before or soon after discharge, median 6 days apart, range 3–12 days in this study). Laboratory parameters evaluated included WBC with differential, hemoglobin, platelet count, erythrocyte sedimentation rate (ESR), CRP, total protein, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), IgG, IgM, IgA, and IgE. Ig levels were examined twice, once at admission and then again around discharge. The values of laboratory parameters and Ig levels were obtained by automatic analyzers at both institutions, and there were no significant differences in the values between the institutions.
Figure 1.
Flow diagram of the patients selected in the study.
We analyzed the changes of platelet count and Ig levels during hospitalization. To compare the changes over time from admission, patients were divided into 3 groups: 2 to 4 days (24 cases), 5 to 10 days (22 cases), and 11 to 16 days (21 cases). Data for the 2 to 4 days group were obtained from the first examinations for 24 out of the 43 cases, whereas data for the other 2 groups were obtained from the second examinations of the 43 subjects.
Written informed consent was obtained from the parents/guardians of all children for use of their medical records. This study was approved by the Institutional Review Board of The Catholic University of Korea, Daejeon St. Mary's Hospital (XC140IMI0079D).
2.1. Statistical methods
Values are expressed as means (±SDs) or medians (ranges) for skewed data. Paired t tests were used (SPSS, version 14.0, SPSS, Inc., Chicago, IL) to analyze the changes of each parameter from the first examination (at admission) to the second examination (around discharge). Statistical analyses were carried out using the nonparametric Kruskal–Wallis test and Dunn method for each post hoc pair-wise test. P values for the differences between the 2 and 4 days and the 7 and 10 days groups and the 2 and 4 days and the 11 and 16 days groups were determined by post hoc analysis. To determine the significance of the relationships between platelet count and Ig levels, regression analysis was performed and Spearman correlation coefficients were calculated. A P-value of <.05 was considered statistically significant.
3. Results
3.1. Changes in laboratory parameters during hospitalization
The mean patient age was 31 ± 18 months; 28 patients were male and 15 were female. The preadmission and total fever durations were 4.6 ± 1.7 days and 6.6 ± 1.7 days, respectively. The mean laboratory values at presentation and at discharge are shown in Table 1. As expected, indices associated with acute inflammation such as WBC, neutrophil differential, CRP, AST, and ALT were significantly reduced to their near normal values. No significant changes in ESR were observed. In contrast, the levels of total protein, platelet count, IgG, IgM, and IgA increased significantly during hospitalization, particularly total protein and IgG. On the other hand, the albumin value decreased, and the hemoglobin value did not change presumably because of early IVIg's effect on these parameters (Table 1).[7] All patients showed increased platelet counts and elevated Ig levels, with the exception of one patient who did not exhibit an elevated IgA level at the second examination. We also performed a subgroup analysis in which we classified platelet and Ig levels according to the duration of illness starting from fever onset. As described in the Materials and Methods Section, when we divided patients into the 3 groups: <5 days (2–4 days, 24 cases), 7 to 10 days (22 cases), and 11 to 16 days (21 cases), examined parameters gradually increased during the acute and early convalescent stage of KD (Table 2).
Table 1.
Laboratory findings at admission and at discharge of patients with KD (n = 43).

Table 2.
Platelet counts, IgG, IgA, IgM, and IgE levels according to examination time after fever onset.

3.2. Correlations between platelet count and immunoglobulin levels
The mean ratio of platelet count increase from the initial examination to the second examination was 1.5 ± 0.4. Similarly, the mean ratios of Ig increases were 1.6 ± 0.5 (IgM), 1.6 ± 1.0 (IgA), and 3.7 ± 1.4 (IgG), respectively. Correlation analysis revealed that the platelet-increase ratio was significantly correlated with that of IgG (P = .002). Similar correlations were noted between platelet count and IgM (P = .002) and platelet count and IgA (P = .01); however, no such correlation was observed for IgE (Fig. 2).
Figure 2.
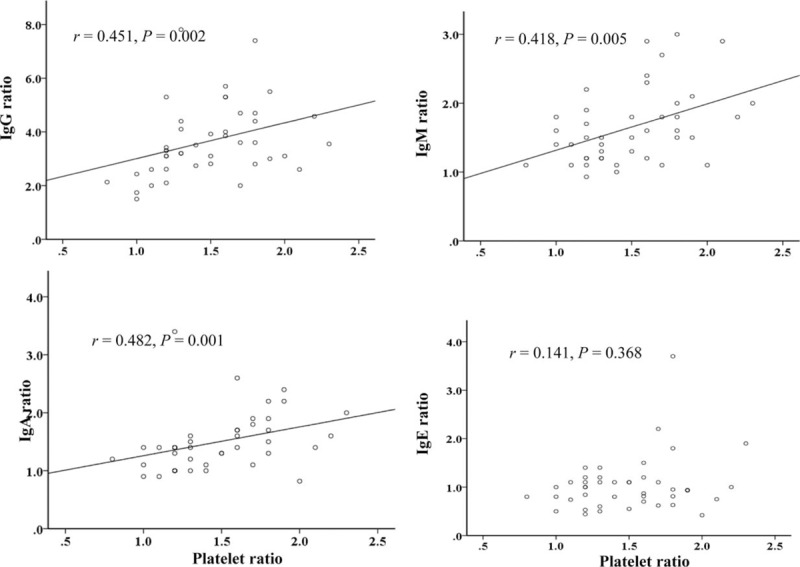
Correlations of platelet increase with IgG, IgA, IgM, and IgE increases in patients with KD (n = 43). The Spearman correlation coefficients (P values) between platelet count and IgG, IgM, IgA, and IgE levels were 0.46 (P < .01), 0.45 (P < .01), 0.39 (P = .01), and 0.11 (P = 0.49), respectively.
4. Discussion
Earlier studies in the pre-IVIg era reported that Ig (IgG, IgA, and IgM) levels increased for 1 to 2 weeks and then declined within 4 weeks after KD onset.[5,6] In addition, an increased platelet count (thrombocytosis, ≥400,000/mm3) has been well-documented in the convalescent stage, that is, the recovery stage from KD.[1,7] In the present study, we confirmed these findings and also found that platelet count and Ig levels increased along with the duration of the disease onset. Moreover, the ratios by which these parameters increased were also correlated with each other in the early convalescent stage. It is quite remarkable that the levels of platelet count, IgM, and IgA all consistently increased by approximately 1.5- to 1.6-fold within a week (median 6 days) after admission. Even though the Ig levels are quite variable from child to child, the IgG increase (mean 3.7-fold) was also correlated with the platelet increase. This finding suggests continuous production of IgG, as well as IgM and IgA, until the second examination, despite the effects of the infused IVIG (2 g/kg) on IgG values.
The reason of concurrent increase of 3 Ig isotypes with platelet is unknown, but it may reflect a nonspecific phenomenon of the host immune reaction against insults from systemic infections or acute infection-related immune-mediated disorders such as KD, acute rheumatic fever, or acute postreptococcal glomerulonephritis (APSGN).[8] In systemic infectious diseases, pathogen-specific IgM appear first and are followed by pathogen-specific IgG and IgA antibodies. Thus, the peak levels of pathogen-specific Ig subtypes may differ during the early convalescent stage, suggesting that almost of increased Igs in KD may not be from pathogen specific immune response. Human serum Igs are composed of antibodies not only against external pathogens (specific antibodies) but also those with immunomodulatory function (natural antibodies). These natural antibodies can bind external antigens from pathogens and internal antigens such as polysaccharides, RNAs, and DNAs from the host cells, as a part of innate immune system in the host.[9] The host immune cells react with not only the pathogen-derived substances, including pathogen-associated molecular patterns (PAMPs), but also the substances from the host cells, including damage (danger)-associated molecular patterns (DAMPs) such as heat shock proteins.[10] Because same kinds of immune cells and immune proteins, including Igs and complements, are seen pathologic lesions in nearly all infectious or infection-related immune-mediated diseases, it is possible that the main function of the host immune cells and immune proteins may be same on the molecular level. Thus, it was proposed that immune cells and immune proteins control toxic substances against the host cells, based on their size and biochemical characteristics of the substances.[11] This control system of the host may be important for recovery from the diseases including KD. Therefore, it is possible that severely affected KD patients with giant aneurysms may have improper immune cell repertoire for control against the substances from KD agents or injured coronary artery cells.[3,11]
KD patients in this series showed an improvement of acute inflammation-associated parameters, including CRP and WBC with differential, improved near normal levels after IVIg treatment, as shown in our previous study.[7] The mechanisms of effectiveness of IVIg on immune-mediated diseases, including KD, remain unknown. IVIg has the unique function of systemic protein modulation without influence on electrolytes and osmolality.[7,12,13] It was reported that at 24 hours after IVIg (2 g/kg) infusion, all protein levels, including albumin, hemoglobin, transferrin, and cytokines, were reduced, and lymphocyte differential increased with the reduction of WBC. Whereas, Ig (IgM and IgA) levels were not affected at 24 hours after IVIg infusion, but levels of Igs increased at a week after IVIg infusion in KD, opposed to inflammation-associated proteins.[7] Therefore, the increased Igs in KD as well as in other systemic diseases might be involved in actions of repair against insults of the diseases as one part of host immune/repair system.
Earlier studies have reported that increased serum level of Ig subtypes could be different in infectious diseases. Patients with infectious mononucleosis were found to have higher IgG and IgM levels compared with control patients; however, the IgA levels in this study were comparable.[14] In acute adenovirus conjunctivitis, the serum IgG level was increased, whereas the IgM and IgA levels were not significantly different by the remission stage of the disease.[15] Sela et al[16] observed that serum IgG, IgA, and IgM levels in patients with chronic active pulmonary tuberculosis were elevated, whereas only an elevated IgM level was observed in patients with acute Klebsiella urinary tract infection compared with healthy controls. Youn et al[17] reported that the serum IgG, IgG1, and IgG3 levels in patients with Mycoplasma pneumoniae pneumonia increased slightly during hospitalization (1.1-fold in IgG, mean 6.5 days apart), but some patients had decreased IgG levels. Patients with acute rheumatic fever or APSGN, as infection-related immune-mediated diseases, exhibit increased IgG, IgM, and IgA levels at the early stage of illness compared with controls.[8,18] Kim et al reported that the IgM and IgA levels in APSGN were not higher than those of the age-matched controls. Moreover, the IgG and IgA levels did not change during hospitalization, whereas the IgM level increased during hospitalization (mean 9.5 days apart).[19] These findings suggest that the extent of elevation of Igs and the class of Ig subtypes in the convalescent stage could be different according to disease entities.
Increased platelet count or thrombocytosis has been well-documented in the convalescent stage of infectious diseases, including M pneumoniae and respiratory virus infections.[20,21] However, only a fraction of these patients show thrombocytosis, in contrast to KD in which nearly all patients exhibit thrombocytosis during their clinical course. The peak of thrombocytosis in KD has been reported to occur 2 to 3 weeks after disease onset, similar to the Ig peaks.[22] A high maximum platelet level during the clinical course of KD has been reported to be associated with IVIG nonresponsiveness.[23] In contrast, a lower platelet count at presentation has also been associated with a higher risk of CALs.[24] These discrepant findings could be explained in part by the observations that the platelet count may begin to increase at the peak of inflammation and that platelets may participate in tissue cell repair.[3,4] It is important to note, however, that platelet count in children is affected by their age, and it also appears in KD. The platelet count at 1 week after IVIg treatment has been found to be significantly higher in young infants (<6 months old) than in older children.[25] Platelet production is controlled by a variety of hormones and cytokines, including thrombopoietin, interleukin (IL)-6, and IL-11.[26] Megakaryocytes and platelets may have a much wider role in healthy and disease states than previously believed, including innate immune functions and tissue cell repair in vivo.[27] Taken together, our data indicate that thrombocytosis accompanied by concurrent increases of all Ig subtypes is a characteristic of systemic inflammation in KD, and the extent of increase of the parameters during natural course of KD may be associated with the degree of systemic immune reaction in the acute stage.
Recently, early diagnosis of KD in Korea become difficult, because KD might have changed into a milder phenotype over time and many KD patients visit the pediatrician early (within 2–4 days of fever onset) as shown in the present study, before the full clinical criteria for complete KD develop.[28,29] Because early IVIg treatment is essential for preventing CAL formation and/or progression, pediatricians in Korea should pay more attention to avoid overdiagnosis or missing diagnosis of KD. This dilemma is present both after IVIg treatment as well as before IVIg treatment. Although our finding, thrombocytosis accompanied by correlative IgM and IgA increases within the first week, may be nonspecific for KD, this finding could be helpful for KD patient selection and for the prospect of severity of KD patients after IVIg treatment.
The present study has some limitations. We did not show a KD-like illness control groups, but we reviewed a variety of diseases that have different elevation of Ig subtypes or those in that only a part of patients show thrombocytosis. Secondly, we did not perform serial examinations for the parameters during the convalescent stage. More detailed examinations during the natural course of KD have the potential to refine these relationships between platelet count and Ig levels.
In conclusion, platelet count and Ig levels (IgG, IgM, and IgA) increased in the early convalescent stage of KD, and the increase extent of the parameters was correlated with each other. This finding suggests that all Ig subtypes except IgE and platelets may be involved in the recovery from KD and that the extent of increased parameters may reflect the degree of systemic inflammation in the acute stage of KD.
Acknowledgment
We thank Dr. Ahn Chi-Whan (statistician) for statistical analysis of the data, and Dr. Hong Yu-Ah for figures.
Footnotes
Abbreviations: ALT = alanine aminotransferase, APSGN = acute postreptococcal glomerulonephritis, AST = aspartate aminotransferase, CALs = coronary artery lesions, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, Ig = immunoglobulin, IVIg = intravenous immunoglobulin, KD = Kawasaki disease, WBC = white blood cell.
The authors have no conflicts of interest to disclose.
References
- [1].Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 2004;114:1708–33. [DOI] [PubMed] [Google Scholar]
- [2].Lee KY, Han JW, Lee JS. Kawasaki disease may be a hyperimmune reaction of genetically susceptible children to variants of normal environmental flora. Med Hypotheses 2007;69:642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee KY, Rhim JW, Kang JH. Kawasaki disease: laboratory findings and an immunopathogenesis on the premise of a “protein homeostasis system”. Yonsei Med J 2012;53:262–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee KY, Han JW, Hong JH, et al. Inflammatory processes in Kawasaki disease reach their peak at the sixth day of fever onset: laboratory profiles according to duration of fever. J Korean Med Sci 2004;19:501–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kuno-Sakai H, Ohta T, Kikuchi T, et al. Polyclonal increases of serum immunoglobulins and increase of immunoglobulin-bearing peripheral blood lymphocytes in children with Kawasaki disease. Tokai J Exp Clin Med 1982;7:661–4. [PubMed] [Google Scholar]
- [6].Lin CY, Hwang B. Serial immunologic studies in patients with mucocutaneous lymph node syndrome (Kawasaki disease). Ann Allergy 1987;59:291–7. [PubMed] [Google Scholar]
- [7].Lee KY, Lee HS, Hong JH, et al. High-dose intravenous immunoglobulin downregulates the activated levels of inflammatory indices except erythrocyte sedimentation rate in acute stage of Kawasaki disease. J Trop Pediatr 2005;51:98–101. [DOI] [PubMed] [Google Scholar]
- [8].Potter EV, Shaughnessy MA, Poon-King T, et al. Serum immunoglobulin A and antibody to M-associated protein in patients with acute glomerulonephritis or rheumatic fever. Infect Immunity 1982;37:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schwartz-Albiez R, Monteiro RC, Rodriguez M, et al. Natural antibodies, intravenous immunoglobulin and their role in autoimmunity, cancer and inflammation. Clin Exp Immunol 2009;158(suppl 1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Broggi A, Granucci F. Microbe- and danger-induced inflammation. Mol Immunol 2015;63:127–33. [DOI] [PubMed] [Google Scholar]
- [11].Lee KY. A common immunopathogenesis mechanism for infectious diseases: the protein-homeostasis-system hypothesis. Infect Chemother 2015;47:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee KY, Han JW, Lee JS, et al. Alteration of biochemical profiles after high-dose intravenous immunoglobulin administration in Kawasaki disease. Acta Paediatr 2002;91:164–7. [DOI] [PubMed] [Google Scholar]
- [13].Lee KY, Lee JS. Immunoglobulin G has a role for systemic protein modulation in vivo: a new concept of protein homeostasis. Med Hypotheses 2006;67:848–55. [DOI] [PubMed] [Google Scholar]
- [14].Kaschka WP, Hilgers R, Skvaril F. Humoral immune response in Epstein-Barr virus infections. I. Elevated serum concentration of the IgG1 subclass in infectious mononucleosis and nasopharyngeal carcinoma. Clin Exp Immunol 1982;49:149–56. [PMC free article] [PubMed] [Google Scholar]
- [15].Gupta AK, Sarin GS. Serum and tear immunoglobulin levels in acute adenovirus conjunctivitis. Br J Ophthalmol 1983;67:195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sela O, el-Roeiy A, Pick AI, et al. Serum immunoglobulin levels in patients with active pulmonary tuberculosis and patients with Klebsiella infection. Immunol Lett 1987;15:117–20. [DOI] [PubMed] [Google Scholar]
- [17].Youn YS, Lee KY, Hwang JY, et al. Comparison of diagnostic methods and the changes of IgG subclasses in children with Mycoplasma pneumoniae pneumonia. Pediatr Allergy Respir Dis 2009;19:137–45. [Google Scholar]
- [18].Rodrigez-Iturbe B, Carr RI, Garcia R, et al. Circulating immune complexes and serum immunoglobulins in acute poststreptococcal glomerulonephritis. Clin Nephrol 1980;13:1–4. [PubMed] [Google Scholar]
- [19].Kim DH, Lee SW, Lee KY, et al. The change of immunologic parameters in acute poststreptococcal glomerulonephritis. J Korean Soc Pediatr Nephrol 2009;13:138–45. [Google Scholar]
- [20].Youn YS, Lee KY, Hwang JY, et al. Difference of clinical features in childhood Mycoplasma pneumoniae pneumonia. BMC Pediatr 2010;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kubota M, Maeda H, Yoshimoto, et al. Thrombocytosis at an early stage of respiratory tract viral infection. Acta Paediatr 2005;94:364–6. [PubMed] [Google Scholar]
- [22].Ishiguro A, Ishikita T, Shimbo T, et al. Elevation of serum thrombopoietin precedes thrombocytosis in Kawasaki disease. Thromb Haemost 1998;79:1096–100. [PubMed] [Google Scholar]
- [23].Wei M, Huang M, Chen S, et al. A multicenter study of intravenous immunoglobulin non-response in Kawasaki Disease. Pediatr Cardiol 2015;36:1166–72. [DOI] [PubMed] [Google Scholar]
- [24].Nakano H, Ueda K, Saito A, et al. Scoring method for identifying patients with Kawasaki disease at high risk of coronary artery aneurysms. Am J Cardiol 1986;58:739–42. [DOI] [PubMed] [Google Scholar]
- [25].Lee KY, Hong JH, Han JW, et al. Features of Kawasaki disease at the extremes of age. J Paediatr Child Health 2006;42:423–7. [DOI] [PubMed] [Google Scholar]
- [26].Schafer AI. Thrombocytosis. N Engl J Med 2004;350:1211–9. [DOI] [PubMed] [Google Scholar]
- [27].Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost 2011;105(suppl 1):S13–33. [DOI] [PubMed] [Google Scholar]
- [28].Rhim JW, Youn YS, Han JW, et al. Changes in Kawasaki disease during 2 decades at a single institution in Daejeon, Korea. Pediatr Infect Dis J 2014;33:372–5. [DOI] [PubMed] [Google Scholar]
- [29].Kim GB, Park S, Eun LY, et al. Epidemiology and clinical features of Kawasaki disease in South Korea, 2012–2014. Pediatr Infect Dis J 2017;36:482–5. [DOI] [PubMed] [Google Scholar]


