Abstract
Background:
The aim of this study was to assess the safety and effectiveness of robotic-assisted versus laparoscopic total mesorectal excision (TME) in patients with rectal cancer.
Methods:
We systematically searched PubMed, EMBASE, Cochrane library, Web of science, and Chinese Biomedical Literature Database up to July 2016 to identify case-controlled studies that compared robotic TME (RTME) with laparoscopic TME (LTME) for rectal cancer. GRADE was used to interpret the primary outcomes of this meta-analysis.
Results:
We included 17 case–control studies (3601 participants: 1726 underwent RTME and 1875 LTME for rectal cancer) that compared RTME with LTME for rectal cancer. We found no statistically significant differences between techniques for local recurrence [odds ratio (OR) = 0.68, P = .216] and overall survival at 3 years (OR = 0.71, P = 1.140), complications (OR = 1.02, P = .883), positive circumferential resection margin (PCRM) (OR = 0.80, P = .256), the first passing flatus [weighted mean difference (WMD) = −0.11, P = .130], reoperation (OR = 0.66, P = .080), estimated blood loss (EBL) (WMD = −12.45, P = .500), and length of stay in hospital (LOS) (WMD = −0.69, P = .089). Compared with LTME, RTME was associated with lower rate of conversion (OR = 0.35, P < .001), urinary retention (OR = 0.41, P = .025), and longer operative time (WMD = 57.43, P < .001). The overall quality of evidence was poor in all outcomes.
Conclusion:
RTME in patients with rectal cancer was associated with a lower rate of conversion and less incidence of urinary retention. Generally, operative time in RTME was significantly longer than in LTME. The long-term oncological and function outcomes of RTME seem to be equivalent with LTME. Therefore, analysis of current studies to date did not indicate a major benefit of RTME over LTME.
Keywords: laparoscopy, meta-analysis, rectal cancer, robotic, total mesorectal excision
1. Introduction
Colorectal cancer is one of the most common malignancies in the world, including 1.4 million cases and 690,000 deaths in 2012. It is the third and second most common cancer in men and women, respectively.[1] Colorectal cancer is also the leading cause of death and a major public health problem in China, with estimated 191,000 deaths in 2015.[2] The effective treatment option for patients with rectal cancer continues to be surgical resection. In particular, if rectal cancer is appropriate for resection, total mesorectal excision (TME), which is defined as complete and sharp resection of the mesorectal envelope en bloc with the rectums, is considered the standard surgical technique in this patient population,[3] because it is associated with a significant reduction in the risk for local recurrence and has a better overall survival rate in patients with rectal cancer.
Currently, 3 surgical techniques, including open surgery, laparoscopy, and robotic-assisted surgery, are frequently used for TME. As the laparoscopic technique was first introduced in 1988, it has been widely used in various surgical specialties.[4] And the first report of robotic-assisted TME (RTME) was successfully used in patients with rectal cancer published in 2006.[5] Compared with laparoscopy, robotic-assisted surgery has several advantages: high-quality 3-dimensional imaging; free-moving multijoint forceps; avoiding surgeons trembling; a stable platform camera controlled by the surgeon; and better ergonomics. However, it also has several disadvantages, including longer operative time, complex installation process, a steep learning curve, the lack of haptic feedback, and high cost.[6]
The aim of our study was to evaluate the safety and effectiveness of RTME versus laparoscopic TME (LTME) in patients with rectal cancer. Although 3 meta-analyses[7–9] focused on this topic had been published, all these published meta-analyses searched data before 2014, demonstrated short-term outcomes, including conversion and bowel recovery. And several new original studies[10–19] including 2438 patients have been published in recent years that could significantly improve the sample size and statistical power of another meta-analysis. Therefore, on the basis of the previous studies and an extensive search of new studies, we conducted a new meta-analysis to further investigate the safety and effectiveness of RTME versus LTME. In addition, we used Grades of Recommendation, Assessment, Development, and Evaluation Working Group (GRADE)[20] to interpret the primary outcomes of this meta-analysis. GRADE was used as a tool to rate the quality of a body of evidence of meta-analyses and other forms of evidence and received with great enthusiasm in many national and international organizations.
2. Methods
2.1. Literature search
We conducted a comprehensive literature search to identify all relevant trials using the following databases: PubMed, EMBASE, Cochrane Library, Web of Science, and the Chinese Biomedical Literature Database. The following search terms were used: “rectal cancer,” “rectal neoplasms,” “robotics,” “Da Vinci,” “laparoscopy,” and “laparoscopic surgery,” using free text and Mesh searches for keywords. To broaden our search, the surgical approach or study language used in the study was not limited. We limited the search of the key terms listed above to the study titles and abstracts to ensure the accuracy of our search. References were also manually reviewed from selected papers to identify other potentially relevant research papers. The last search was conducted on July 27, 2016.
2.2. Study selection
Studies were eligible for inclusion if they compared RTME with LTME in patients with rectal cancer; and studies reporting on at least one of the outcome measures mentioned below. If the same institution and/or authors reported more than 1 study, the higher quality or the most recent publication was included. Two authors (LXF and HLD) independently examined full articles and determined studies relevance on the basis of the criteria of inclusion. Any disagreements were resolved through discussion and consensus with a third author (YL). The following studies were excluded: letters, editorials and expert opinions, reviews without original data, case reports, and studies lacking control groups; reports on protectomy that did not contain a distinct group of patients with rectal cancer who underwent TME; unclear patient outcomes and parameters; could not extract available data from the published studies; literature with the same author and institution; and Newcastle–Ottawa scale score (NOS) <7.[21]
2.3. Outcomes of interest
Outcomes of interest for the 2 techniques were compared as follows: intraoperative parameters, including operative time, estimated blood loss (EBL), and conversion to open procedure; postoperative parameters, such as length of stay in hospital (LOS), the first passing flatus, reoperation in 30 days, and total complications before discharge; pathological parameters, including the number of lymph nodes harvested, distal resection margin (DRM), and involved positive circumferential resection margin (PCRM); long-term parameters, including local recurrences and overall survival at 3 years. If there was an overlap or duplication in data sets, only the latest information was included.
2.4. Data extraction and quality assessment
Two reviewers (LXF and HLD) independently extracted available data from studies included according to the parameters mentioned above and then compared them. Disagreements were resolved by discussion. The quality of studies was evaluated using NOS.
2.5. Statistical analysis
The meta-analysis was conducted using Stata 12.0 (Stata Corp, College Station, TX). We analyzed dichotomous variables using estimation of odds ratios (ORs) with a 95% confidence interval (CI). Continuous variables were evaluated using weighted mean difference (WMD) with a 95% CI. P values <.05 were considered significant. In studies that only reported medians, the mean and standard deviation were evaluated using the means of the method provided by HOZO.[22] Higgins’ I2 statistic values <25, 25 to 50, and >50 were defined as having low, moderate, and high heterogeneity, respectively. A random effect model was used in data analysis procedure. We conducted a sensitivity analysis to assess the robustness of the major outcomes and investigate reasons of high heterogeneity.
2.6. Quality of evidence
We created a “Summary of findings” table and rated the quality of the primary outcomes, including local recurrences at 3 years, PCRM, conversion to open procedure, operative time, LOS, and complications. The 5 GRADE considerations (risk for bias, consistency of effect, imprecision, indirectness, and publication bias) were used to assess the quality of the primary outcomes. We concluded our evaluation of the quality of evidence using the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions and using the GRADEpro software.
3. Results
3.1. Study selection
Our literature search initially yielded 1337 results. After excluding duplicates, 970 results were reviewed. We identified 16 studies that compared RTME with LTME in patients with rectal cancer, which were selected by carefully reviewing titles, abstracts, and their full text. We also identified 1 study during our manual search.[23] Finally, 17 observational studies were included in the meta-analysis (Fig. 1).
Figure 1.
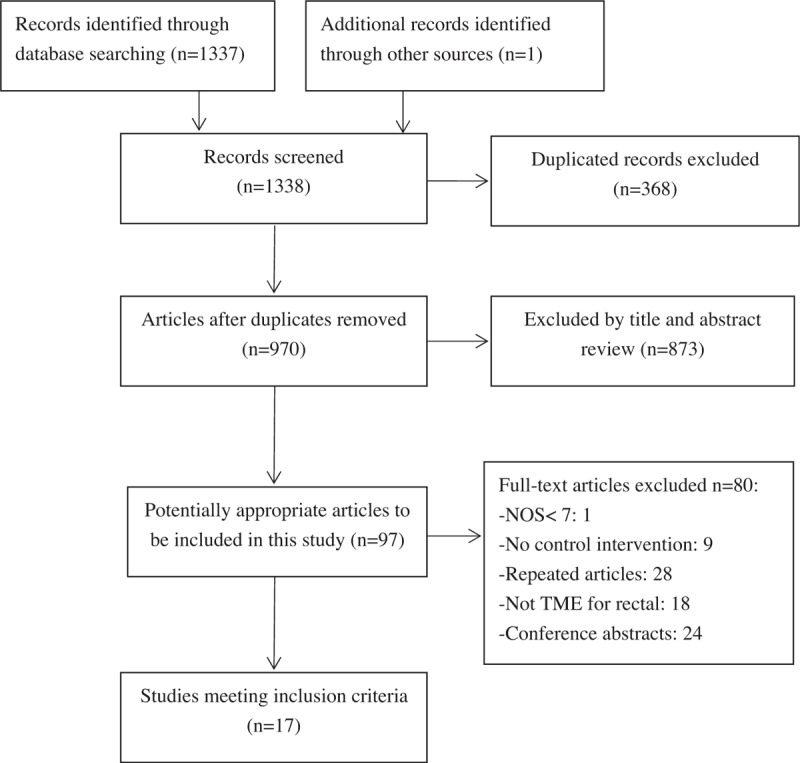
Flow chart of studies identified, included, and excluded.
3.2. Study characteristics
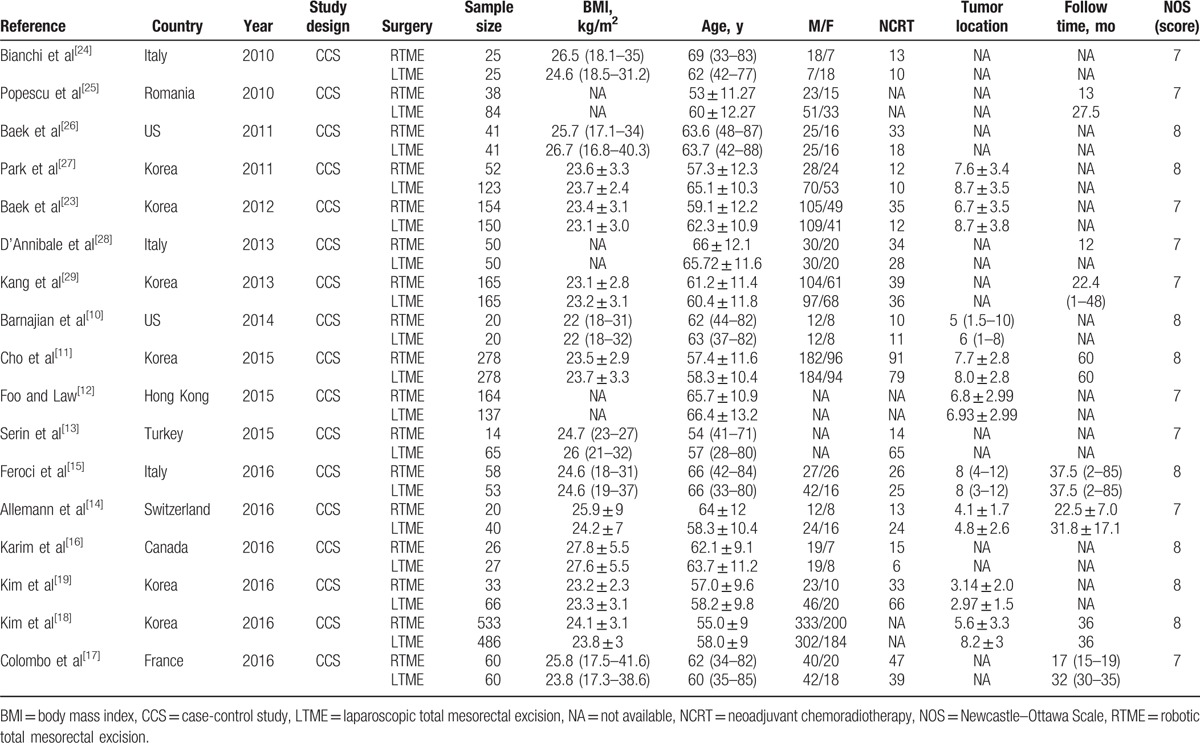
The studies included in the meta-analysis[10–19,23–29] comprised 3601 patients with rectal cancer, among whom 1726 underwent RTME and 1875 underwent LTME. All of the studies had a retrospective design, because randomized controlled trials on this topic are lacking. Fifteen were single-center studies,[10–19,24–26,28,29] 1 was multicenter study,[23] and 1 was unclear.[27] Only 1 study reported that the surgical approach used was based on a joint decision by the patients and surgeons.[27] Whether patients received neoadjuvant chemoradiotherapy (NCRT) before surgery was unclear in 3 studies.[12,18,25] More information about the characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of the selected studies included in the meta-analysis.
3.3. Intraoperative parameters
3.3.1. Operative time
Sixteen studies[10–13,15–19,23–29] reported operative time. In particular, 1 study[28] indicated a shorter operative time with RTME, 3 studies[10,24,26] showed no significant difference between techniques, and the rest of studies suggested that operative time was longer with RTME than LTME. Pooled data analysis demonstrated that the average operative time of 57 minutes was longer in RTME group
[WMD = 57.43, 95% CI (36.70–78.15); P < .001], and there was a high heterogeneity among the studies (I 2 = 96.0%, P < .001) (Fig. 2).
Figure 2.
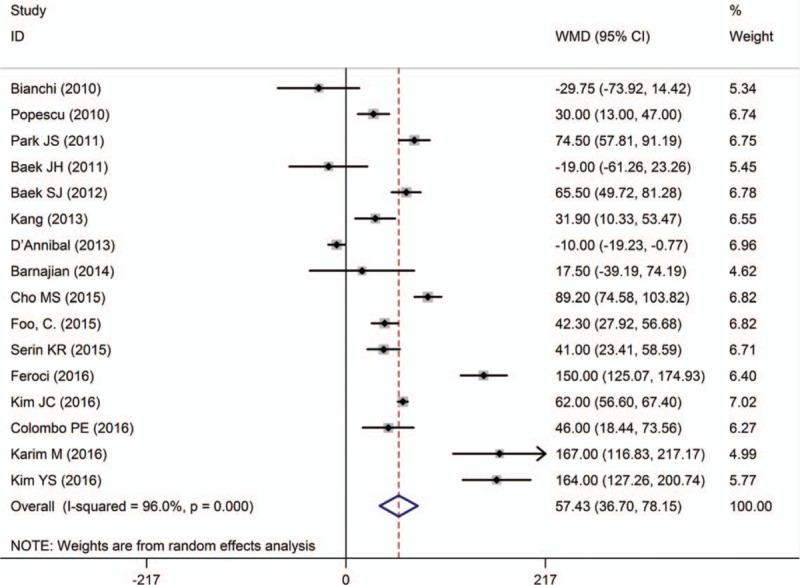
A meta-analysis of operative time for RTME versus LTME.
3.3.2. EBL
EBL was described in 11 studies.[10,11,14–17,19,23,25,26,29] Three studies[14,16,25] showed that EBL was lower for RTME than LTME, although no significant difference was found in the rest of studies. Pooled data analysis revealed that there was no significant difference in EBL between the techniques [WMD = −12.45, 95% CI (−48.66 to 23.76), P = .500], with a high heterogeneity (I 2 = 75.9%; P < .001) (Fig. 3).
Figure 3.
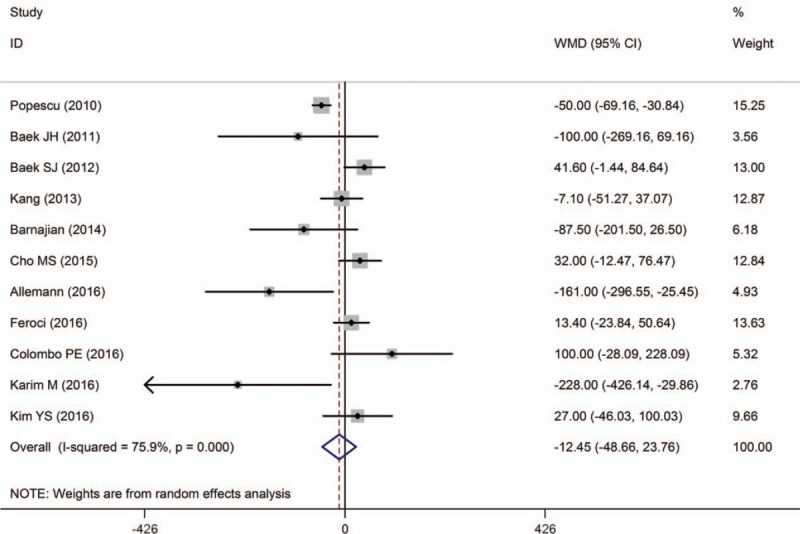
A meta-analysis of EBL for RTME versus LTME.
3.3.3. Conversion to open procedure
Twelve studies[10–14,16,19,24–26,28,29] showed rate of conversion to open procedure. Overall, no significant difference was found between techniques, except in 1 study.[16] Pooled data analysis demonstrated that the rate of conversion to open procedure was lower in RTME than LTME [OR = 0.35, 95% CI (0.19–0.62); P < .001; I 2 = 0.0%] (Fig. 4).
Figure 4.
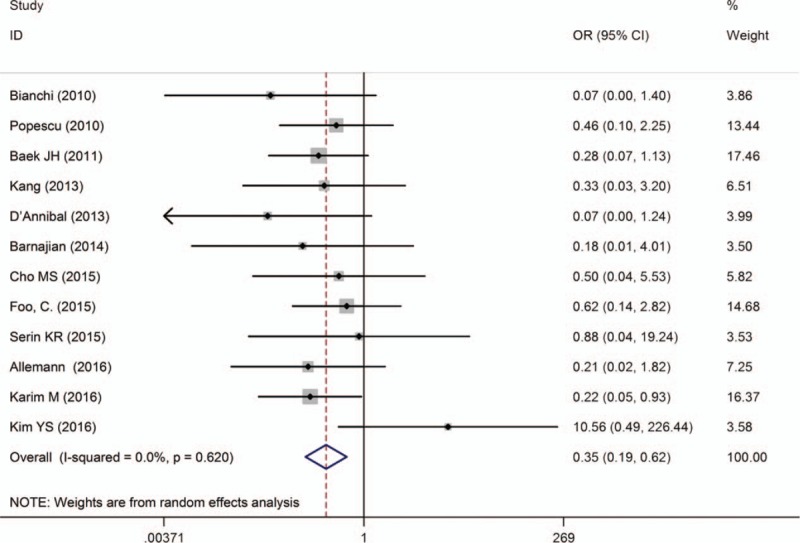
A meta-analysis of conversion rate for RTME versus LTME.
3.4. Postoperative parameters
3.4.1. LOS
LOS was reported in 16 studies.[10–13,15–19,23–29] Two studies[10,28] showed that it was shorter for RTME, 1 study[18] reported that it was a little longer for RTME, and the remaining studies indicated no significant difference between techniques. Pooled data analysis showed that there was no significant difference between the techniques [WMD = −0.69, 95% CI (−1.48 to 0.10); P = .089], with high heterogeneity (I 2 = 81.8%; P < .001) (Fig. 5).
Figure 5.
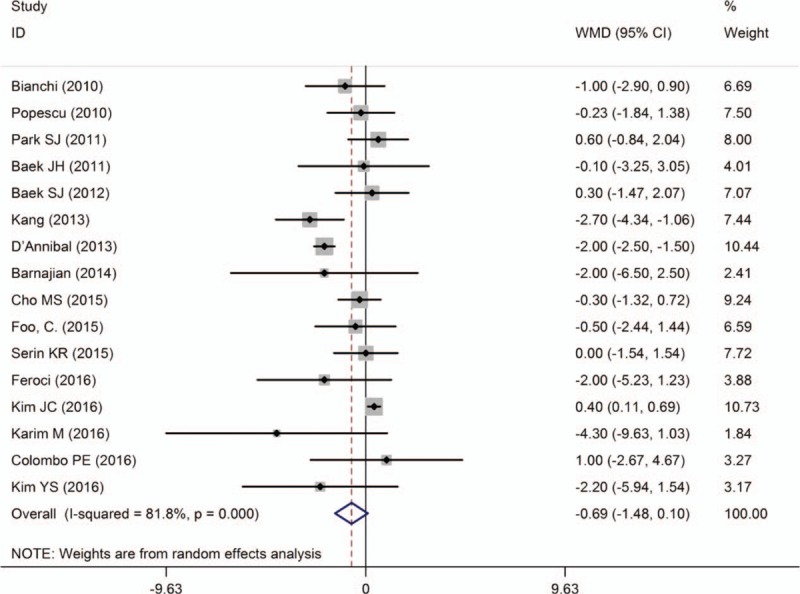
A meta-analysis of LOS for RTME versus LTME.
3.4.2. The first passing flatus
Eight studies[10,11,16,18,19,23,27,29] reported the first passing flatus and none showed a significant difference between the techniques except 1 study.[10] Overall, mean time to first passing flatus seemed to be shorter for RTMR, although the difference was not statistically different [WMD = −0.11; 95% CI (−0.26 to 0.03); P = .130] and heterogeneity was moderate among studies (I 2 = 46.0%, P = .073). It should be noted that the studies used different parameters to assess bowel function recovery, including time-to-bowel moment, time-to-solid diet, time-to-first bowel function recovery, time-to-liquid diet, time-to-oral feeding, time-to-first soft diet, and time-to-first passing flatus. We chose the most frequent parameters to evaluate bowel function recovery (Fig. 6).
Figure 6.
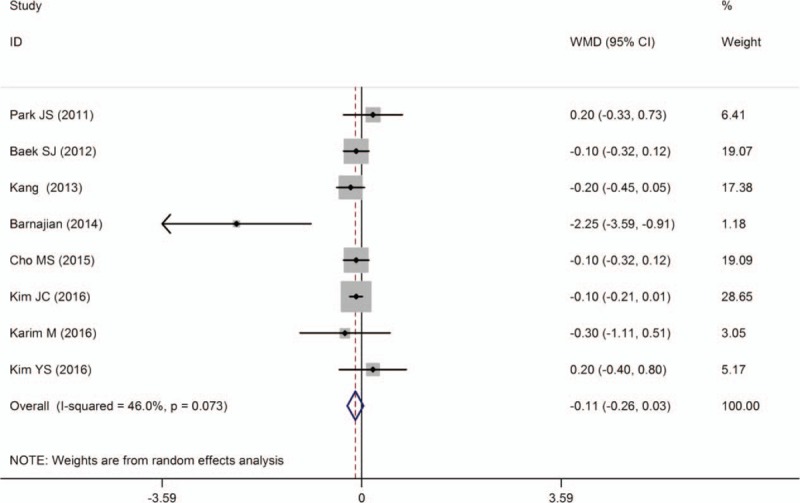
A meta-analysis of the first passing flatus for RTME versus LTME.
3.4.3. Reoperation in 30 days
Data on 30-day reoperation were described in 8 studies[10,14,15,17,19,24,25,28] and there was no significant difference between the techniques. Pooled data analysis further confirmed these results [OR = 0.66, 95% CI (0.41–1.05); P = .080; I 2 = 0.0%] (Fig. 7).
Figure 7.
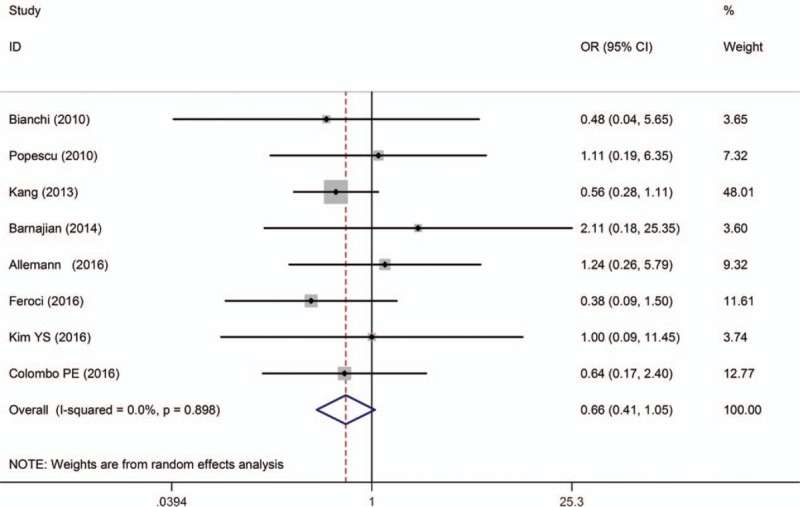
A meta-analysis of reoperation in 30 days for RTME versus LTME.
3.4.4. Total complications
Total complications were reported in 16 studies, which showed that no significant difference was observed between techniques. Pooled data analysis indicated that there was no significant difference in total complications between 2 groups [OR = 1.02, 95% CI (0.82–1.25); P = .883], with low heterogeneity (I2 = 23.9%, P = .184).
We found that the rate of bowel obstruction was higher for RTME than LTME [OR = 1.48, 95% CI (1.02–2.15); P = .040; I2 = 3.2%], whereas the rate of urinary retention was lower for RTME than LTME (OR = 0.41, 95% CI (0.18– 0.89); P = .025; I2 = 0.0%]. No significant difference was observed between the techniques in the rate of anastomotic leaks [OR = 0.80, 95% CI (0.61–1.06); P = .125; I2 = 0.0%], postoperative bleeding [OR = 1.58, 95% CI (0.77–3.26); P = .212; I2 = 0.0%], or wound infection [OR = 0.91, 95% CI (0.41–2.02); P = .813; I2 = 0.0%] (Table 2).
Table 2.
Summary of complications for RTME versus LTME.
3.5. Pathological parameters
3.5.1. Lymph nodes harvested
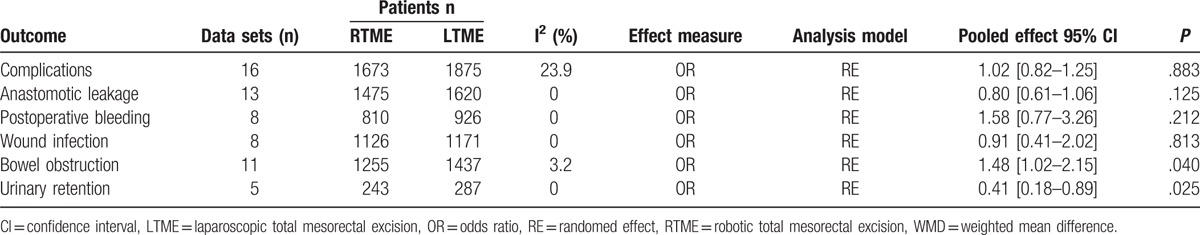
The number of lymph nodes harvested was reported in 12 studies.[11,13–17,19,24–27,29] All studies found no difference between the 2 techniques except 1 study.[13] Pooled data showed that the number of lymph nodes harvested was not significantly different between the techniques [WMD = 0.49, 95% CI (−0.98 to 1.96); P = .515]. There was high heterogeneity among studies (I2 = 64.2%, P < .001) (Fig. 8).
Figure 8.
A meta-analysis of lymph nodes harvested for RTME versus LTME.
3.5.2. DRM
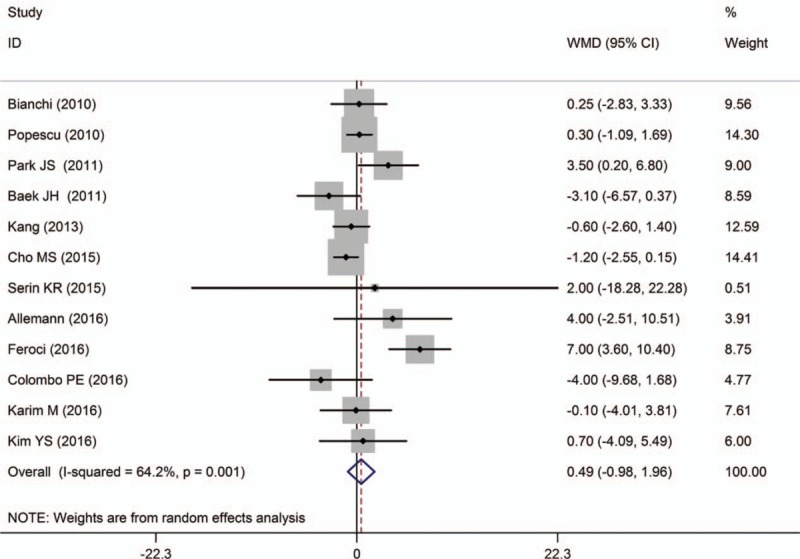
DRM was reported in 8 studies.[13,16–19,26,28,29] Among which, 6 studies showed that there was no significant difference between the 2 groups, while the rest 2 studies found a longer DRM in RTME. And the pooled data indicated a negative result [WMD = 1.98, 95% CI (−1.25 to 5.22); P = .229], with high heterogeneity (I2 = 67.8%; P = .003) (Fig. 9).
Figure 9.
A meta-analysis of DRM for RTME versus LTME.
3.5.3. PCRM
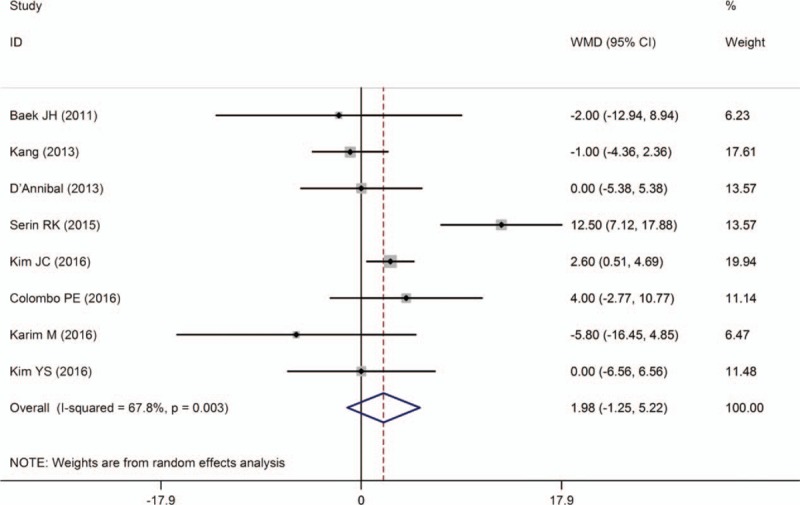
The rate of PCRM was described in 13 studies,[11–15,17–19,24,26–29] and all of them reported there was no difference in PCRM between the 2 groups. No statistical difference was found by the combined data between the 2 groups [OR = 0.80, 95% CI (0.55–1.17); P = .256] (Fig. 10).
Figure 10.
A meta-analysis of PCRM for RTME versus LTME.
3.6. Long-term parameters
3.6.1. Local recurrences
Four studies reported local recurrence rates at 3 years after surgery.[14,15,18,25] All studies suggested that local recurrence rates was not significantly different between the 2 groups, and no significant difference was found by the pooled data analysis [OR = 0.68; 95% CI (0.36–1.26); P = .216]. Cho et al [11] described the rate of local recurrence at 5 years and reported no significant difference between RTME and LTME (5.9% vs 3.9%, respectively) (Fig. 11).
Figure 11.
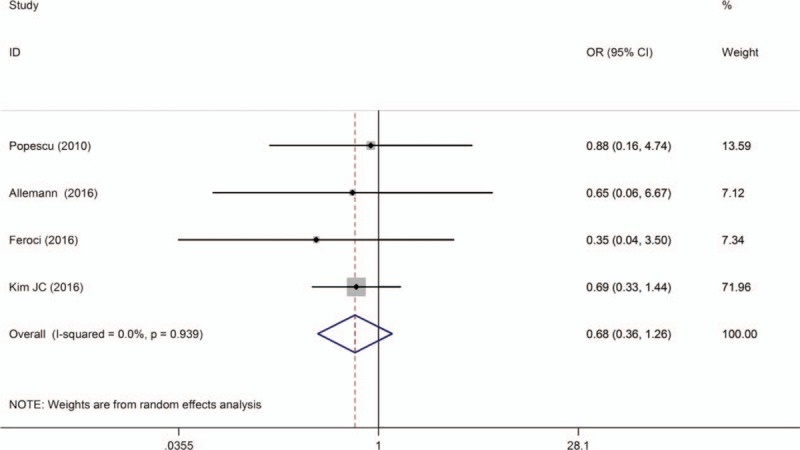
A meta-analysis of local recurrences at 3 years for RTME versus LTME.
3.6.2. Overall survival
Three studies described overall 3-year survival,[14,15,18] and they concluded no significant difference between the 2 techniques. Pooled data analysis showed that there was no statistical difference between techniques [OR = 0.71; 95% CI (0.44–1.12); P = 1.140; I2 = 0.0%]. Cho et al[11] reported overall survival rate at 5 years for RTME and LTME (92.2% vs 93.1%, respectively) and no difference was found between techniques (Fig. 12).
Figure 12.
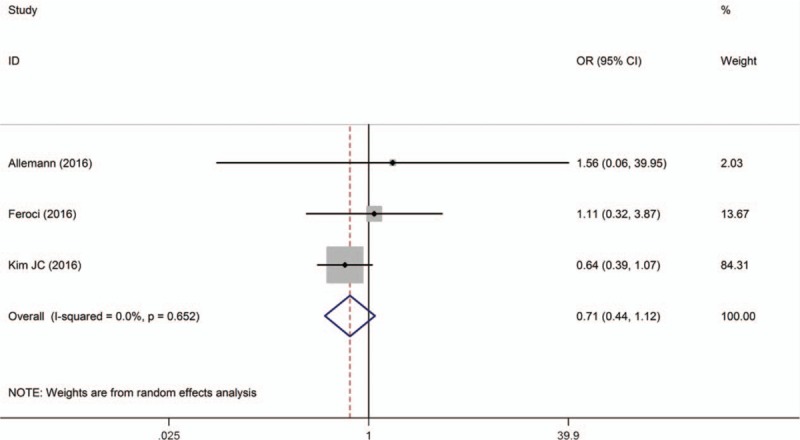
A meta-analysis of overall survival at 3 years for RTME versus LTME.
3.7. Sensitivity analysis
We performed a sensitivity analysis on high heterogeneity outcomes (i.e., postoperative stay, lymph nodes harvested, operative time, and EBL) to investigate their potential sources and assess the robustness of these outcomes. After omitting each of the included studies one by one to each outcomes, we found that D’Annibal et al[28] might be the source of heterogeneity for the postoperative stay, and heterogeneity of the pooled data analysis visibly decreased after the study was excluded (I 2 = 43.2%, P = .038); study exclusion also affected the pooled analysis (WMD = 0.37; 95% CI, [−0.96 to −0.21]; P = .207). Similarly, the study by Feroci et al[15] contributed to high heterogeneity for lymph nodes harvested. Heterogeneity was low after the study was excluded (I 2 = 27.4%; P = .183). However, potential sources of heterogeneity for operative time and EBL could not be identified.
4. Discussion
In this meta-analysis, we assessed the safety and effectiveness of RTME versus LTME in patients with rectal cancer. Overall, 17 case–control studies were included (1726 RTME and 1875 LTME). We found no statistically significant differences between techniques for local recurrence and overall survival at 3 years, total complications, lymph nodes harvested, DRM, PCRM, the first passing flatus, reoperation, and LOS. Compared with LTME, RTME was associated with lower rate of conversion and urinary retention; RTME had significantly longer operative time and higher rate of bowel obstruction than LTME.
Conversion rate was one of the important parameters of this minimally invasive techniques feasibility. Our meta-analysis showed that RTME was associated with lower conversion rate than in LTME group. Previous meta-analyses[7–9] demonstrated a similar result. The reasons may be associated with adhesion to adjacent organs, shorter instrumentation, obesity, narrow pelvis, and the tumor invasion.[30] Apart from that, RTME has superior exposure and visualization of the surgical field in the pelvis because of the ability of the fixed arms to grip maneuver organs and 3-dimensional camera to provide a clearer visualization.[24] However, we had checked the 12 studies that described this outcome one by one. Only 1 study[27] reported that a joint decision was taken to choose surgical approach on the basis of the patients and surgeons, while the remaining 11 studies did not report how the patients were selected to RTME and LTME group. Theoretically, there was a possibility that surgeons had performed RTME in “easy cases” or “early stage cancer.” Besides, we also found that the conversion rate was not significantly different in studies that evaluated these techniques in patients with gastric[31] or liver cancer.[32] Usually, converted patients might have higher complication rates[33] and worse oncological outcomes,[34] while outcomes and safety were comparable between the 2 types of surgery in our study. We thought that factors mentioned below had a contribution to the paradox in outcomes. The experience of surgeons performing RTME and LTME was important to the conversion outcome. In general, the technique of LTME was more familiar to the surgeons than RTME because the LTME had been emerged for more than 20 years and had been widely used in many hospitals. What is more, when we checked the experience of surgeons performing RTME in our included studies, we found most of the studies did not report the experience of surgeons performing RTME, except 3 studies.[10,11,18] Besides, the operative time in RTME was longer than LTME group, which could also increase the risk of complications.
Our meta-analysis also indicated that the mean operative time in RTME was significantly longer than LTME, despite heterogeneity was high. The reasons might be associated with that, the robotic system was more complicated and needed more time to install the procedures in general.[35] Furthermore, due to lack of haptic feedback and remote operation, surgeons had to take more time to complete regular tasks during robotic procedure. The experience of surgeons might be a significant factor contributing to the difference of operative time for RTME.[36] D’Annibale et al[28] and Malak et al[37] found that operative time significantly decreased as the number of cases accumulated for robotic-assistant procedure; the difference between the initial and terminal phase was statistically significant in their experience.
PCRM was considered to be an important index of surgery effectiveness, which relates to surgical quality and had an impact on local recurrence.[38] Several studies[39,40] considered that CRM < 1 mm was an important risk factor for distant metastases and decreased survival, whereas CRM <2 mm was a predictor for local recurrence. Contrary to previous meta-analyses,[7–9] our meta-analysis indicated no significant difference in PCRM between the techniques. We believe that our results are more credible, because more patients were included, which increased the sample size and statistical power of the meta-analysis. Moreover, the completeness of TME was an important parameter to evaluate the surgical quality. Only 1 study in the meta-analysis reported that the quality of TME was better in RTME.[15] Incomplete TME would increase the risk for local recurrence and decrease the overall survival rate. It was necessary for the surgical quality to evaluate the completeness of TME macroscopically. RTME benefited from these aforementioned, which improved the quality and oncologic safety of the surgery.
No significant difference was found among the long-term parameters evaluated, including overall survival and local recurrence at 3 years. Current evidence also indicated that long-term outcomes were similar between the techniques in patients with rectal cancer. Only 4 studies in our study[14,15,18,25] reported follow-up time points, but none reported details about patients lost to follow-up. Future studies should focus on long-term follow-up and evaluate the long-term outcomes of the da Vinci surgical system in patients with rectal cancer.
We found a lower rate of unary retention. The robotic surgeries had better dissection of the avascular plane between the presacral fascia and the fascia propria of the rectum, and preserved the integrity of mesorectum without injuring peripheral tissues.[41] Pelvic nerves and blood vessel damage during the surgical procedure are an important reason for urinary dysfunction.[42] The wristed instruments of robotic devices are small and highly flexible in adequately separating and exposing tissues, which dramatically reduces tissue damage.[43] Robotic surgery may be more minimally invasive and better preserve surrounding rectal tissues, which could explain the low incidence of urinary retention. Two studies[23,29] included in our meta-analysis reported the rate of postoperative erectile dysfunction, but they failed to provide a clear method of measurement for this endpoint and was not included in the meta-analysis. In another study, investigators showed that the incidence of partial or total erectile dysfunction was lower for RTME.[43]
Complications were not found to be significantly different between the techniques, except for bowel obstruction. The rate of bowel obstruction was higher for RTME than LTME (Table 2). Several previous meta-analyses[7–9] demonstrated that complications were not significantly different between the techniques; bowel obstruction incidence was visibly different. Risk factors for postoperative bowel obstruction were associated with male gender, advanced age, significant blood loss, the surgical approach used, intro-abdominal infection, anastomotic leak, emergency surgery, and opioid administration.[44] In 1 study, the investigators highlighted opioids’ role in aggravating the risk for postoperative bowel obstruction.[45] Our meta-analysis demonstrated that operative time was visibility longer for RTME than LTME, suggesting that patients were administered more anesthetics, especially opioids. The increasing dose of opioids had an inhibitory effect on bowel function.[45] We believe that this explains the significantly higher rate of postoperative bowel obstruction for which we believe had a RTME than LTME.
The Da Vinci surgical system has revolutionized laparoscopic surgery and is widely used in abdominal surgery. However, we found that the latest international guidelines for patients with rectal cancer do not include recommendations for this technique.[46–51] The American Society of Colon and Rectal surgeons and National Comprehensive Cancer Network only recommended LTME in patients with rectal cancer if it was performed by experienced laparoscopic surgeons.[46,51] Because it is an emerging technology, clinical trials on this surgical system are inadequate, especially those including larger samples sizes and multicenter randomized controlled studies. Additional research is warranted to evaluate the safety and effectiveness of RTME and comprehensively summarize the current body of evidence on this system.
We found that study heterogeneity was high for several outcomes, and unable to identify its source for operative time and EBL. We considered that the heterogeneity for operative time could potentially be due to the experience of surgeons, different types of Da Vinci robotic systems used, and total or hybrid robotic technique. Larger errors in blood loss measurements may be associated with the fact that it is difficult to measure it during surgery precisely, and it is estimated by surgeons. In addition, it is possible that surgeons have been reporting positive results in terms of EBL, because of the system is population in most countries and regions, and because of its high cost. In addition, surgeons’ experience and proficiency to this system may also have contributed to heterogeneity for EBL.
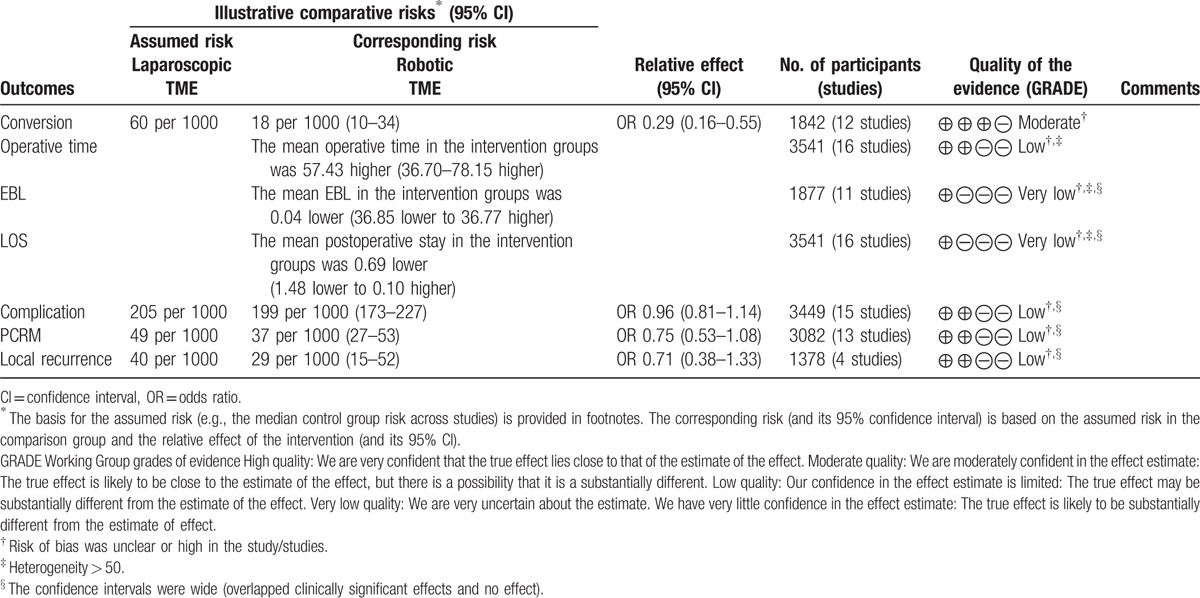
The overall quality of evidence in the meta-analysis was poor (Table 3), particularly for a serious risk of bias and baseline differences between the techniques. Most of the studies did not report the choice for the surgical approach, except for 1 study, which reported that the choice of surgical approaches was based on a joint decision by the patients and physicians. Although patients who underwent NCRT were not statistically significantly different between the techniques, there was a trend that more patients who underwent RTME received NCRT; this dramatically influenced patients’ survival rate of patients. Kang et al[29] showed that NCRT did not affect postoperative outcomes in their study population. In addition, patients with less extensive or low stage cancers were eligible for robotic-assisted surgery. The sample size of studies included was small,[10,13,14,24] which might contribute to a wide 95% CI.
Table 3.
Summary of finding.
This meta-analysis had several limitations. First, studies included in the meta-analysis were observational studies, which could have introduced a biased interpretation of the results. Second, there was high heterogeneity in some outcomes between the techniques, which may have weakened our confidence of the results. Third, although we included more patients in the meta-analysis, the sample size in some outcomes was relatively small, which limited its statistical power. Therefore, we hope that more high-quality studies would be performed comparing RTME with LTME in the future. Fortunately, an international multicenter RCTs is underway.[52]
5. Conclusion
RTME in patients with rectal cancer was associated with a lower rate of conversion and urinary retention. Generally, operative time in RTME was significantly longer than in LTME. The long-term oncological and function outcomes of RTME seem to be equivalent with LTME. Therefore, on the basis of the poor quality of evidence, on the published studies to date, we found no benefit of RTME over LTME. More studies with rigorous study design, and larger sample size, are needed to evaluate the benefit and harm in patients with rectal cancer undergoing RTME
Acknowledgments
The authors thank the Jinhui Tian, Zhenggang Bai, and Kehu Yang, (Evidence Based Medicine Center of Lanzhou University) for their help and support to the methodology and meta-process.
Footnotes
Abbreviations: BMI = body mass index, CCS = case–control study, CI = confidence interval, DRM = distal resection margin, EBL = estimated blood loss, LOS = length of stay in hospital, LTME = laparoscopic total mesorectal excision, NCRT = neoadjuvant chemoradiotherapy, NOS = Newcastle–Ottawa Scale, OR = odds ratio, PCRM = positive circumferential resection margin, RCT = randomized controlled trial, RTME = robotic total mesorectal excision, SD = standard deviation, TME = total mesorectal excision, WMD = weighted mean difference.
XFL and WT contributed equally to this work.
Authorship: TKG and KHY contributed to study concept and design; XFL, TW, LDH, and LY contributed to literature search, review of the studies, and data extractions. PHJ contributed to data analysis and data interpretation. LDH and LY contributed to verify the statistical analysis and scrutinized data. XFL and TW provided expert insight. XFL and LDH contributed to supervision throughout the whole study. All the authors contributed writing the manuscript. All authors approved the final version of the manuscript.
All data and materials in this work were available from publications.
The authors declare that they have no competing interests.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479–82. [DOI] [PubMed] [Google Scholar]
- [4].National Institutes of Health Consensus Development Conference statement gallstones and laparoscopic cholecystectomy. J Laparoendosc Surg 1993;165:77–90. [DOI] [PubMed] [Google Scholar]
- [5].Pigazzi A, Ellenhorn JD, Ballantyne GH, et al. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 2006;20:1521–5. [DOI] [PubMed] [Google Scholar]
- [6].Teljeur C, O’Neill M, Moran PS, et al. Economic evaluation of robot-assisted hysterectomy: a cost-minimisation analysis. BJOG 2014;121:1546–53. [DOI] [PubMed] [Google Scholar]
- [7].Trastulli S, Farinella E, Cirocchi R, et al. Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis 2012;14:e134–56. [DOI] [PubMed] [Google Scholar]
- [8].Xiong B, Ma L, Huang W, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis of eight studies. J Gastrointest Surg 2014;19:516–26. [DOI] [PubMed] [Google Scholar]
- [9].Wang Y, Zhao GH, Yang H, et al. A pooled analysis of robotic versus laparoscopic surgery for total mesorectal excision for rectal cancer. Surg Laparosc Endosc Percutan Tech 2016;26:259–64. [DOI] [PubMed] [Google Scholar]
- [10].Barnajian M, Pettet D3rd, Kazi E, et al. Quality of total mesorectal excision and depth of circumferential resection margin in rectal cancer: a matched comparison of the first 20 robotic cases. Colorectal Dis 2014;16:603–9. [DOI] [PubMed] [Google Scholar]
- [11].Cho MS, Baek SJ, Hur H, et al. Short and long-term outcomes of robotic versus laparoscopic total mesorectal excision for rectal cancer: a case-matched retrospective study. Medicine (Baltimore) 2015;94:e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Foo C, Law W. Comparison of short-term outcome between robotic-assisted and laparoscopic total mesorectal excision for mid to low rectal cancer. Dis Colon Rectum 2015;58:e327. [Google Scholar]
- [13].Serin KR, Gultekin FA, Batman B, et al. Robotic versus laparoscopic surgery for mid or low rectal cancer in male patients after neoadjuvant chemoradiation therapy: comparison of short-term outcomes. J Robot Surg 2015;9:187–94. [DOI] [PubMed] [Google Scholar]
- [14].Allemann P, Duvoisin C, Di ML, et al. Robotic-assisted surgery improves the quality of total mesorectal excision for rectal cancer compared to laparoscopy: results of a case-controlled analysis. World J Surg 2016;40:1010–6. [DOI] [PubMed] [Google Scholar]
- [15].Feroci F, Vannucchi A, Bianchi PP, et al. Mesorectal excision for mid and low rectal cancer: laparoscopic vs robotic surgery. World J Gastroenterol 2016;22:3602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Karim M, Ramji MCC, Jonathan M, et al. Comparison of clinical and economic outcomes between robotic,laparoscopic, and open rectal cancer surgery: early experience at a tertiary care center. Surg Endosc 2016;30:1337–43. [DOI] [PubMed] [Google Scholar]
- [17].Colombo PE, Bertrand MM, Alline M, et al. Robotic versus laparoscopic total mesorectal excision (TME) for sphincter-saving surgery: is there any difference in the transanal TME rectal approach? A single-center series of 120 consecutive patients. Ann Surg Oncol 2016;23:1594–600. [DOI] [PubMed] [Google Scholar]
- [18].Kim JC, Yu CS, Lim SB, et al. Comparative analysis focusing on surgical and early oncological outcomes of open, laparoscopy-assisted, and robot-assisted approaches in rectal cancer patients. Int J Colorectal Dis 2016;31:1179–87. [DOI] [PubMed] [Google Scholar]
- [19].Kim YS, Kim MJ, Park SC, et al. Robotic versus laparoscopic surgery for rectal cancer after preoperative chemoradiotherapy: case-matched study of short-term outcomes. Cancer Res Treat 2016;48:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].The GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Available at: http://www.guidelinedevelopment.org/handbook. Accessed March 20, 2014. [Google Scholar]
- [21].Wells GA, D O’Connell BS, Peterson J, Welch V. The Newcastle-PatentScale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta- analyses. Available at: http://www.ohri.ca/programs/clinical_2014. [Google Scholar]
- [22].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from themedian, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Baek SJ, Kim SH, Cho JS, et al. Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg 2012;36:2722–9. [DOI] [PubMed] [Google Scholar]
- [24].Bianchi PP, Ceriani C, Locatelli A, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc 2010;24:2888–94. [DOI] [PubMed] [Google Scholar]
- [25].Popescu I, Vasilescu C, Tomulescu V, et al. The minimally invasive approach, laparoscopic and robotic, in rectal resection for cancer. A single center experience. Acta Chir Iugosl 2010;57:29–35. [DOI] [PubMed] [Google Scholar]
- [26].Baek JH, Pastor C, Pigazzi A. Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc 2011;25:521–5. [DOI] [PubMed] [Google Scholar]
- [27].Park JS, Choi GS, Lim KH, et al. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc 2011;25:240–8. [DOI] [PubMed] [Google Scholar]
- [28].D’Annibale A, Pernazza G, Monsellato I, et al. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc 2013;27:1887–95. [DOI] [PubMed] [Google Scholar]
- [29].Kang J, Yoon KJ, Min BS, et al. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison: open, laparoscopic, and robotic surgery. Ann Surg Oncol 2013;257:95–101. [DOI] [PubMed] [Google Scholar]
- [30].AI GP. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718–26. [DOI] [PubMed] [Google Scholar]
- [31].Chuan L, Yan S, Pei-Wu Y. Meta-analysis of the short-term outcomes of robotic-assisted compared to laparoscopic gastrectomy. Minim Invasive Ther Allied Technol 2015;24:127–34. [DOI] [PubMed] [Google Scholar]
- [32].Qiu J, Chen S, Chengyou D. A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg Endosc 2016;30:862–75. [DOI] [PubMed] [Google Scholar]
- [33].Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomized controlled trial. Lancet 2005;365:1718–26. [DOI] [PubMed] [Google Scholar]
- [34].Rottoli M, Bona S, Rosati R, et al. Laparoscopic rectal resection for cancer: effects of conversion on short-term outcome and survival. Ann Surg Oncol 2009;16:1279–86. [DOI] [PubMed] [Google Scholar]
- [35].Harr JN, Luka S, Kankaria A, et al. Robotic-assisted colorectal surgery in obese patients: a case-matched series. Surg Endosc 2017;31:2813–9. [DOI] [PubMed] [Google Scholar]
- [36].Memon SHA, Murphy DG, Bressel M, et al. Robotic versus laparoscopic proctectomy for rectal cancer: a meta-analysis. Ann Surg Oncol 2012;19:2095–101. [DOI] [PubMed] [Google Scholar]
- [37].Bokhari MB, Patel CB, Ramos-Valadez DI, et al. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 2011;25:855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jeyarajah S, Sutton CD, Miller AS, et al. Factors that influence the adequacy of total mesorectal excision for rectal cancer. Colorectal Dis 2007;9:808–15. [DOI] [PubMed] [Google Scholar]
- [39].Nagtegaal ID, Marijnen CA, Kranenbarg EK, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 2002;26:350–7. [DOI] [PubMed] [Google Scholar]
- [40].Nagtegaal ID, Krieken van JH. The role of pathologists in the quality control of diagnosis and treatment of rectal cancer-an overview. Eur J Cancer 2002;38:964–72. [DOI] [PubMed] [Google Scholar]
- [41].Baik SH KH, Kim JS. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 2009;16:1480–7. [DOI] [PubMed] [Google Scholar]
- [42].Dowswell G, Ismail T, Greenfield S, et al. Men's experience of erectile dysfunction after treatment for colorectal cancer: qualitative interview study. BMJ 2011;18:d5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang G, Wang Z, Jiang Z, et al. Male urinary and sexual function after robotic pelvic autonomic nerve-preserving surgery for rectal cancer. Int J Med Robot 2017;13:e1725. [DOI] [PubMed] [Google Scholar]
- [44].Venara A, Neunlist M, Slim K, et al. Postoperative ileus: pathophysiology, incidence, and prevention. J Visc Surg 2016;153:439–46. [DOI] [PubMed] [Google Scholar]
- [45].Gan TJ, Robinson SB, Oderda GM, et al. Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Curr Med Res Opin 2016;31:677–86. [DOI] [PubMed] [Google Scholar]
- [46].Monson JR, Weiser MR, Buie WD, et al. Standards Practice Task Force of the American Society of Colon and Rectal Surgeons Practice parameters for the management of rectal cancer (Revised). Dis Colon Rectum 2013;56:535–50. [DOI] [PubMed] [Google Scholar]
- [47].Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015;20:207–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bianco F, Arezzo A, Agresta F, et al. Practice parameters for early rectal cancer management: Italian Society of Colorectal Surgery (Societa ‘ Italiana di Chirurgia Colo-Rettale; SICCR) guidelines. Tech Coloproctol 2015;19:577–85. [DOI] [PubMed] [Google Scholar]
- [49].Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the managementof patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. [DOI] [PubMed] [Google Scholar]
- [50].National Comprehensive Cancer Network (NCCN), NCCN clinical practice guidelines in Oncology:Rectal Cancer(2016.V2). Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines. Accessed April 2016. [Google Scholar]
- [51].Valentini V, Gambacorta MA, Barbaro B, et al. International consensus guidelines on clinical target volume delineation in rectal cancer. Radiother Oncol 2016;120:195–201. [DOI] [PubMed] [Google Scholar]
- [52].Collinson FJ, Jayne DG, Pigazzi A, et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis 2012;27:233–41. [DOI] [PubMed] [Google Scholar]


