Abstract
Background:
To date, literature has emerged that shows contradictory results about the prognostic role of microvessel density (MVD) in esophageal squamous cell cancer (ESCC). The aim of the study set out to evaluate the correlation between MVD and the prognosis of ESCC.
Methods:
Identified publications from various databases were obtained and reviewed. A meta-analysis was performed to evaluate the prognostic role of MVD among ESCC patients.
Results:
A total of 11 eligible studies containing 891 ESCC cases were included in the meta-analysis. The pooled hazard ratio for overall survival was 2.39 (95% confidence interval 1.92–2.96, P < .001). Heterogeneity among the studies was not significant, and publication bias was not found. Subgroup analyses were also performed on different issues, such as districts, antibodies, and median age.
Conclusion:
High MVD is a prognostic factor among ESCC that indicated worse prognosis in these patients. More studies are needed, and through abundant evidence, the topic could be re-evaluated by then.
Keywords: esophageal squamous cell cancer, meta-analysis, microvessel density, prognostic factor
1. Introduction
Esophageal cancer is the eighth most frequent cancer worldwide. It is also the sixth most common cause of cancer death, accounting for over 5.4% of all cancer deaths.[1] The occurrence of the disease varies from geographic regions. The incidence is 4.5 per 100,000 individuals in USA, while some of the highest incidences are found in Asia, with approximately 100 per 100,000 individuals affected in the Linxian district of China.[1,2] It remains one of the most lethal cancers of all malignancies, with a 5-year survival rate of 17% once diagnosed.[3] Esophageal squamous cell cancer (ESCC) comprises the majority cases of esophageal malignancies, followed by adenocarcinomas.[4] Apart from independent prognostic factors such as histological type, tumor size, lymph node metastases,[5,6] several biological factors have been recognized to affect the outcomes of the disease as well. These biomarkers include vascular endothelial growth factor (VEGF), p53, proliferating cell nuclear antigen (PCNA), Her-2, and microvascular density (MVD).[7–10] The correlation between tumor metastasis and angiogenesis was first reported by Weidner et al.[11] Angiogenesis as an intratumoral process to form new blood vessels was later proved to be related with the outcomes of various malignancies, such as lung cancer,[12] colorectal cancer,[13] breast cancer,[14] etc. MVD is the most common pathological approach to assess angiogenesis, involving microscopic estimation and microvessel staining.[11] Currently, routine antibodies for staining endothelial cells of microvessel include those against pan-endothelial marker CD34,[15] homodimer trans-membrane protein CD105,[16] platelet/endothelial cell adhesion molecule CD31,[17] and von Willebrand Factor (vWF).[18] The prognostic role of MVD in ESCC was reported in various studies, and many suggested MVD as a crucial prognostic factor in ESCC and led to adverse outcomes,[17–20] whereas some did not reach to any conclusive result indicating that MVD is associated with the prognosis of ESCC.[21,22]
Due to those inconsistent results above, we herein aimed to perform a systematic review and meta-analysis with summarized evidence to determine the prognostic role of MVD among ESCC patients.
2. Methods
2.1. Literature search
The current study is a meta-analysis; hence, ethical approval was not necessary. Two reviewers (GM and JZ) independently searched PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wanfang Database for eligible studies up till March 25, 2017. The search keywords were as follows: “Microvascular Density” or “Microvessel Density” and “Esophageal Neoplasms” or “Esophageal Cancer” or “Esophageal Carcinoma” and “Survival” or “Prognosis” or “Outcome.”
2.2. Inclusion criteria
Eligible studies should met all the criteria as follows: In studies on esophageal cancer, all included patients should be confirmed with squamous cell carcinoma; MVD was assessed and its association with ESCC prognosis was reported; Data provided within the literatures were feasible for log hazard ratio (log HR) calculation, according to methods by Parmar et al,[23] Williamson et al,[24] and Tierney et al[25]; Eligible study categories include cohort study, case–control study, and randomized controlled trials (RCTs), if any.
2.3. Exclusion criteria
Literatures should be excluded if any of the following was matched: review or systematic review; case reports; studies on animals, in vitro studies, or any other types of laboratory studies; and studies that lack credible or extractable data.
2.4. Data extraction
Basic information was extracted as follows: names of first author, publication year, country, median age, number of patients involved and gender, clinical stage, tumor stages, antibodies applied for immunohistochemical staining, and evaluation of high MVD.
The primary data for calculation were multivariate/univariate Cox hazard regression analysis, the Kaplan–Meier survival curves with P values, or HR with 95% confidence interval (95% CI) for overall survival (OS). The literature selection and data extraction were performed by 2 reviewers (GM and JZ) independently, with any discrepancies being discussed and reassessed.
2.5. Methodological assessment
Quality of each study was assessed according to Newcastle–Ottawa Scale (NOS) criteria.[26] Three aspects of each study were evaluated as follows: subject selection: 0 to 4; comparability of subject: 0 to 2; and clinical outcome: 0 to 3. The total score ranged from 0 to 9; study that scored 6 or more was eligible for data-pooling and any literature that scored 7 or more was considered of good quality. The whole evaluation process was conducted by 2 reviewers independently.
2.6. Statistical analysis
The STATA (version 11; Stata Corporation, College Station, TX) was applied for data analysis. LogHRs and variances were extracted for pooling the survival results. If not directly given among the literatures, the HR with 95% CI or Kaplan–Meier curves with P values were applied for calculation. Multivariate analyses were prior used if univariate and multivariate survival analyses were both provided. Adjusted HR was first applied if adjusted and unadjusted HRs all existed. Heterogeneity assumption of pooled HRs was assessed by I2 statistic test and Chi-square based Q-test.[27] The fixed-effect model (the Mantel–Haenszel method)[28] was applied if the heterogeneity between studies was not statistically significant (P > .10 or I2 < 50%). If else, to reduce the impact of heterogeneity, HR should be evaluated by the random-effect model. Publication bias was assessed through methods of Begg and Mazumdar[29]; if P value was no more than .05, then publication bias was considered statistically significant.
3. Results
3.1. Study selection
A total of 248 studies were retrieved from initial search for eligible studies. Abstracts were carefully screened of each identified literatures. Studies were excluded for reasons as follows: duplicate literatures (n = 25), laboratory studies (n = 113), reviews (n = 48), and case reports (n = 34). Full texts of 28 potential studies were retrieved, and then 16 studies were further excluded: 7 studies aimed on irrelevant topics, 5 focused on biological technics such as immunostaining, 3 studies lack available data for quantitative synthesis, 1 study[30] scored no more than 5 according to quality assessment, and 1 literature[31] reported the association between MVD and survival of esophageal adenocarcinoma. In all, 11 studies eventually met our criteria of inclusion for the final analyses.
The process to obtain eligible publication is displayed in Fig. 1.
Figure 1.
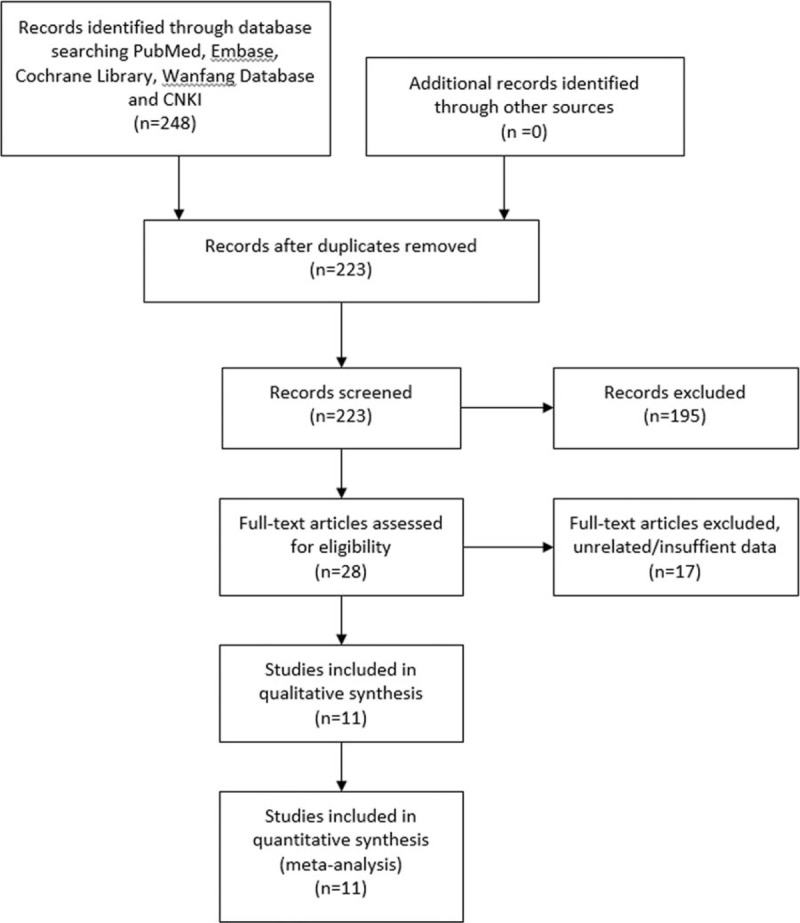
The selection process for eligible studies.
3.2. Study characteristics
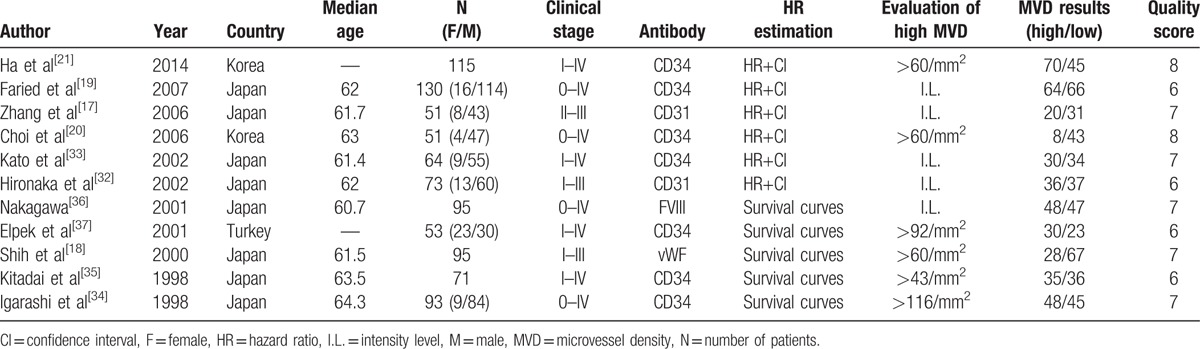
Among the 11 eligible studies, 10 were from Asia, including 8 from Japan[17–19,32–36] and 2 from Korea.[20,21] The study from Turkey[37] was the only one conducted on Caucasian. Altogether, 891 patients were included, with mast majority of male patients. All cases included were ESCC, and tumor stages varied from 0 to IV. Antibodies applied for immunohistochemical staining were against CD34, CD31, Factor VIII, or vWF. HRs were directly given in 6 studies,[17,19–21,32,33] and the rest were extracted from survival curves.[18,34–37] All eligible studies scored no less than 6. High MVDs were assessed quantitatively or defined through intensity levels of staining.
To conclude, basic information for all included studies is summarized in Table 1.
Table 1.
Characteristics of the included literatures.
3.3. Meta-analysis results
The prognostic role of high MVD was valued by survival time OS. All 11 studies were eligible to examine OS, and the pooled HR was 2.39 (95% CI 1.92–2.96, P < .001), indicating that high intratumoral MVD was associated with inferior outcomes on OS (Fig. 2). The heterogeneity was statistically insignificant (I2 = 0%, P = .625); therefore, fixed-effect model was applied for calculation.
Figure 2.
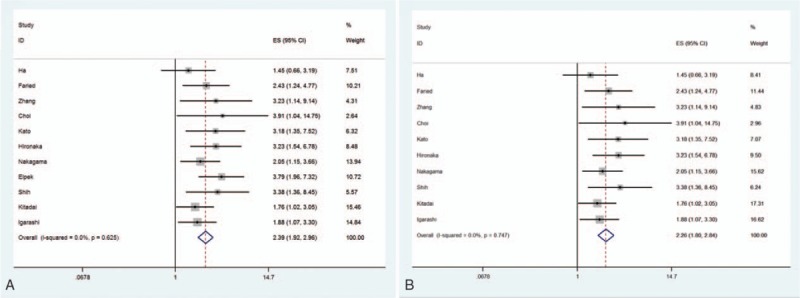
The pooled hazard ratio (HR) for OS in ESCC patients (A) and Asian patients (B) with high intratumoral MVD.
3.4. Subgroup analysis
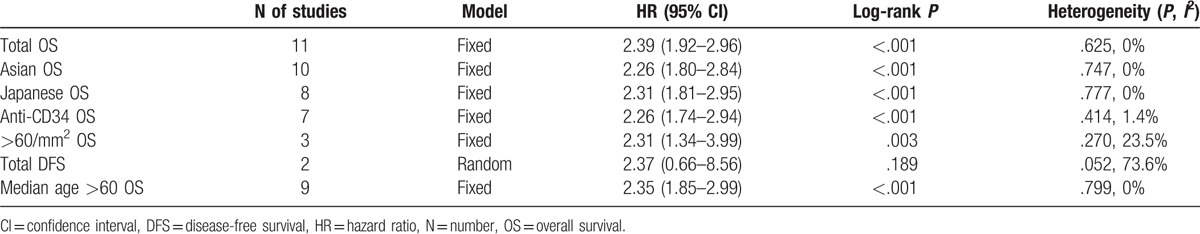
In accordance with basic information and extracted data from all eligible literatures, subgroups were sorted due to varied districts (Asian/Japanese), antibodies for staining (CD34), median age (>60 years), and specific definition of high MVD (>60/mm2). Disease-free survival (DFS) was reported in 2 studies,[20,21] thus the data were also combined for a pooled result.
3.4.1. Asian/Japanese
Altogether, among 10 Asian studies, 8 were from Japan. The combined HR for OS in Asian was 2.26 (95% CI 1.80–2.84, P < .001), heterogeneity was not significant (I2 = 0%, P = .747), and fixed-effect model was applied (Fig. 2). With regard to Japanese patients, heterogeneity was not found and the pooled HR for OS was 2.31 (95% CI 1.81–2.95, P < .001, I2 = 0%).
3.4.2. Antibodies for immunohistochemical staining
Antibodies against CD34 were used within 7 of the included studies for vasculature staining. The combined HR was 2.26 (95% CI 1.74–2.94, P < .001). Heterogeneity was not detected and fixed-effect model was used to perform the analysis (P = .414, I2 = 1.4%).
3.4.3. Definition of high MVD
The quantitative measurement to define high MVD varied between studies, whereas 3 studies were coherent that vessel counts over 60/mm2 be considered as high MVD. The pooled result for OS was also indicative. The HR was 2.31 (95% CI 1.34–3.99, P = .003), and heterogeneity was statistically insignificant (P = .27, I2 = 23.5%).
3.4.4. DFS
The pooled HR for DFS was 2.37 (95% CI: 0.66–8.56, P = .189). Heterogeneity was significant (P = .052, I2 = 73.6%) and random-effect model was used.
3.4.5. Age
Median age was provided in 9 studies that were all over 60 years old. Combined HR for OS in this case was 2.35 (95% CI 1.85–2.99, P < .001). Heterogeneity was not significant, thus fixed-effect model was applied (P = .799, I2 = 0%).
All summarized results are listed in Table 2.
Table 2.
Meta-analyses of high MVD and survival of ESCC patients.
3.5. Publication bias
Publication bias was not found in this meta-analysis, with reference to the plots of publication in Fig. 3 (P = .213).
Figure 3.
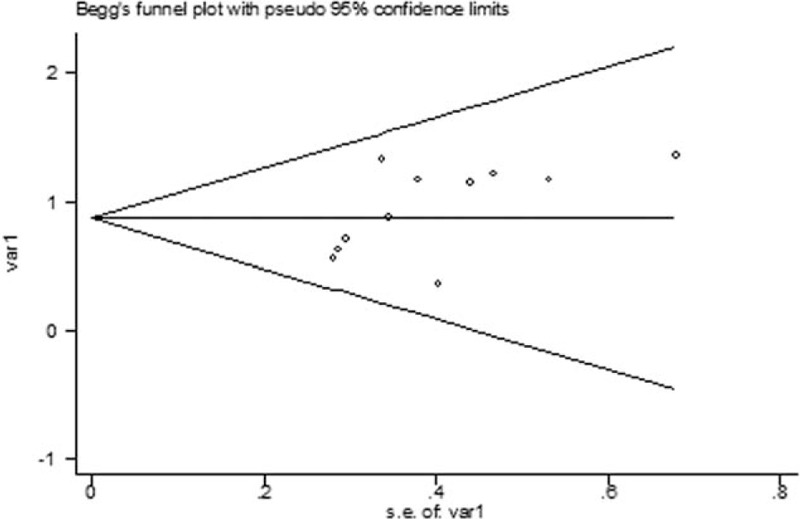
The Begg publication bias plots of the studies that reported the correlation between MVD and ESCC. The publication bias was insignificant (P = .213).
4. Discussion
The present study set out to determine the prognostic role that MVD might have among ESCC patients. Data were pooled and a meta-analysis was performed. As a result, high MVD was a prognostic factor, which indicated poorer outcomes among ESCC patients. Accordingly, the correlation between MVD and Asian/Japanese patients who suffered ESCC was also identified; high MVDs have an adverse impact on these cases. When precisely defined (>60/mm2), the prognostic role of high MVD in ESCC resulted the same. As to ESCC patients whose median ages were above 60 years and intratumoral vessels stained by CD34, high MVD was also a poor prognostic factor among ESCC patients, respectively. As to DFS, the number of included studies is very limited, and heterogeneity was also significant. Therefore, no conclusion could be drawn on the topic of correlation between MVD and DFS of ESCC.
In accordance with the results above, high MVD is related with poorer outcomes among ESCC patients. Such is the case in squamous cell cancer, but when it comes to other histological types of esophageal cancer, little was reported and the correlation remains unclarified. In a cohort study involving 98 adenocarcinomas, no significant association between MVD and survival was found according to Dutta et al.[31] In ESCC, the occurrence of lymph node metastasis is also an independent poor prognostic factor.[38] MVD with lymph node (LMVD) was also reported in several studies. Seemingly, LMVD that indicated lymphatic metastasis should have a negative impact on ESCC survival; interestingly, no correlation between LMVD and OS was detected among any of these studies.[39–41] As to other malignances such as lung adenocarcinoma, LMVD was reported to cause worse prognosis,[42,43] so was the same with colorectal cancer.[44,45] To conclude, although the role of MVD in ESCC has been identified in this study, the prognostic role of MVD in other pathological types and the role of LMVD remains unclear, and they should be revalued when abundant clinical evidence has emerged by then.
Similar to other malignances, ESCC growth is closely associated with vascularization. Folkman[46] firstly revealed the correlation between tumor growth and angiogenesis. Tumor angiogenesis is a complicated process mediated by various angiogenetic factors that were either released from cancer cells or synthesized by host cells.[47] Among these factors, VEGF was considered to be the key factor of most specificity.[48,49] Various prior studies were conducted on the topic to recognize the correlation between VEGF and MVD when ESCC was diagnosed; however, the results were incoherent. Some studies reported a positive relation between VEGF expression and MVD.[35,50] On the contrary, however, no significant result was found on the question of whether VEGF level correlates with MVD.[33,51] Therefore, more studies are needed to further explore the question and MVD results should be referred together with VEGF level to assess the angiogenesis condition of ESCC cases.
With regards to MVD, several issues should be considered. Although MVD is closely related with tumor behavior such as invasion and metastasis, the parameter itself has restrictions. First of all, evaluation of MVD value was mostly based on subjective judgments, such as hot-spot selection and vessel-counting.[52] Although software, such as CIAS (computer-aided image analysis system), was designed to mellow these bias, yet its accuracy needs to be further tested.[17,53] Second, the MVD was derived from a tissue section, which means that MVD could not indicate the whole in vivo condition or the dynamic tumoral status. Lastly, to date, debate continues on which antibody was most suitable for immunohistochemical staining in MVD assessment. CD34 was a frequently used marker, but it failed to differentiate normal vessels and newly formed vessels.[15] Some believed that CD105 has superior specificity with newly generated endothelial cells,[53,54] yet few studies measured ESCC MVD through CD105, and evidence remained insufficient to draw a conclusion. Despite the flaws mentioned above, to date, MVD is still the most widely used method, and is considered as the golden standard to assess angiogenesis quantitatively.[11]
To our knowledge, this is the first meta-analysis conducted to demonstrate the prognostic role of MVD among ESCC patients. Yet, there are several limitations in our study. First of all, currently, existing literatures are limited. All the included studies were either cohort study or retrospective study, with no RCTs been found. Second, the basic information of included cases was incoherent. The stages of ESCC ranged from 0 to IV, and the definitions of high MVD were also inconsistent. Despite the subgroups performed on some study characters, we failed to cover them all. For instance, all included patients staged between 0 and IV in each study, respectively; therefore, subgroups could not be performed on tumor stages and our topic. Furthermore, the antibodies applied for microvessel-counting varied between studies. As mentioned earlier, to our knowledge, we cannot define which is the most reliable. However, with detailed protocol, carefully pooled data, neither publication bias nor heterogeneity was found, and the results of the study are guaranteed reliable.
To conclude, high MVD is a prognostic factor among ESCC, and would lead to worse outcomes in these patients. Antibody for histological staining is a crucial issue, and needs to be further compared for liability. More studies are in need to examine the correlation between MVD and clinical outcome of ESCC patients, and through abundant evidence, we may re-evaluate the topic by then.
Acknowledgment
We would like to thank all the reviewers for their constructive comments.
Footnotes
Abbreviations: CI = confidence interval, ESCC = esophageal squamous cell cancer, HR = hazard ratio, MVD = microvessel density, NOS = Newcastle–Ottawa Scale, OS = overall survival, PCNA = proliferating cell nuclear antigen, VEGF = vascular endothelial growth factor, vWF = von Willebrand Factor.
GM, JZ, and HJ contributed equally to this work.
Funding/support: This work was supported by National Natural Science Foundation of China (NSFC81572288).
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [2].Ke L. Mortality and incidence trends from esophagus cancer in selected geographic areas of China circa 1970–90. Int J Cancer 2002;102:271–4. [DOI] [PubMed] [Google Scholar]
- [3].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- [4].Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137–50. [DOI] [PubMed] [Google Scholar]
- [5].Greenstein AJ, Litle VR, Swanson SJ, et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer 2008;112:1239–46. [DOI] [PubMed] [Google Scholar]
- [6].Siewert JR, Stein HJ, Feith M, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg 2001;234:360–7. discussion 368–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shimaya K, Shiozaki H, Inoue M, et al. Significance of p53 expression as a prognostic factor in oesophageal squamous cell carcinoma. Virchows Arch A Pathol Anat Histopathol 1993;422:271–6. [DOI] [PubMed] [Google Scholar]
- [8].Takebayashi Y, Natugoe S, Baba M, et al. Angiogenesis in esophageal squamous cell carcinoma. Oncol Rep 1998;5:401–4. [DOI] [PubMed] [Google Scholar]
- [9].al-Kasspooles M, Moore JH, Orringer MB, et al. Amplification and over-expression of the EGFR and erbB-2 genes in human esophageal adenocarcinomas. Int J Cancer 1993;54:213–9. [DOI] [PubMed] [Google Scholar]
- [10].Kimos MC, Wang S, Borkowski A, et al. Esophagin and proliferating cell nuclear antigen (PCNA) are biomarkers of human esophageal neoplastic progression. Int J Cancer 2004;111:415–7. [DOI] [PubMed] [Google Scholar]
- [11].Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- [12].Fontanini G, Bigini D, Vignati S, et al. Microvessel count predicts metastatic disease and survival in non-small cell lung cancer. J Pathol 1995;177:57–63. [DOI] [PubMed] [Google Scholar]
- [13].Des Guetz G, Uzzan B, Nicolas P, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 2006;94:1823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Uzzan B, Nicolas P, Cucherat M, et al. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res 2004;64:2941–55. [DOI] [PubMed] [Google Scholar]
- [15].Kimura H, Nakajima T, Kagawa K, et al. Angiogenesis in hepatocellular carcinoma as evaluated by CD34 immunohistochemistry. Liver 1998;18:14–9. [DOI] [PubMed] [Google Scholar]
- [16].Kubota Y, Kaneko K, Konishi K, et al. The onset of angiogenesis in a multistep process of esophageal squamous cell carcinoma. Front Biosci (Landmark Ed) 2009;14:3872–8. [DOI] [PubMed] [Google Scholar]
- [17].Zhang SC, Hironaka S, Ohtsu A, et al. Computer-assisted analysis of biopsy specimen microvessels predicts the outcome of esophageal cancers treated with chemoradiotherapy. Clin Cancer Res 2006;12:1735–42. [DOI] [PubMed] [Google Scholar]
- [18].Shih CH, Ozawa S, Ando N, et al. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res 2000;6:1161–8. [PubMed] [Google Scholar]
- [19].Faried A, Kimura H, Faried LS, et al. Expression of carbohydrate antigens in human esophageal squamous cell carcinoma: prognostic application and its diagnostic implications. Ann Surg Oncol 2007;14:960–7. [DOI] [PubMed] [Google Scholar]
- [20].Choi JY, Jang KT, Shim YM, et al. Prognostic significance of vascular endothelial growth factor expression and microvessel density in esophageal squamous cell carcinoma: comparison with positron emission tomography. Ann Surg Oncol 2006;13:1054–62. [DOI] [PubMed] [Google Scholar]
- [21].Ha SY, Yeo SY, Xuan YH, et al. The prognostic significance of cancer-associated fibroblasts in esophageal squamous cell carcinoma. PLoS One 2014;9:e99955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sarbia M, Bittinger F, Porschen R, et al. Tumor vascularization and prognosis in squamous cell carcinomas of the esophagus. Anticancer Res 1996;16:2117–21. [PubMed] [Google Scholar]
- [23].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [24].Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337–51. [DOI] [PubMed] [Google Scholar]
- [25].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [27].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [29].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [30].Ikeguchi M, Sakatani T, Ueta T, et al. The expression of thymidine phosphorylase suppresses spontaneous apoptosis of cancer cells in esophageal squamous cell carcinoma. Pathobiology 2001;69:36–43. [DOI] [PubMed] [Google Scholar]
- [31].Dutta S, Going JJ, Crumley AB, et al. The relationship between tumour necrosis, tumour proliferation, local and systemic inflammation, microvessel density and survival in patients undergoing potentially curative resection of oesophageal adenocarcinoma. Br J Cancer 2012;106:702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hironaka S, Hasebe T, Kamijo T, et al. Biopsy specimen microvessel density is a useful prognostic marker in patients with T(2-4)M(0) esophageal cancer treated with chemoradiotherapy. Clin Cancer Res 2002;8:124–30. [PubMed] [Google Scholar]
- [33].Kato H, Yoshikawa M, Miyazaki T, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) in esophageal squamous cell carcinoma. Anticancer Res 2002;22:3977–84. [PubMed] [Google Scholar]
- [34].Igarashi M, Dhar DK, Kubota H, et al. The prognostic significance of microvessel density and thymidine phosphorylase expression in squamous cell carcinoma of the esophagus. Cancer 1998;82:1225–32. [DOI] [PubMed] [Google Scholar]
- [35].Kitadai Y, Haruma K, Tokutomi T, et al. Significance of vessel count and vascular endothelial growth factor in human esophageal carcinomas. Clin Cancer Res 1998;4:2195–200. [PubMed] [Google Scholar]
- [36].Nakagawa S, Nishimaki T, Suzuki T, et al. Tumor angiogenesis as an independent prognostic factor after extended radical esophagectomy for invasive squamous cell carcinoma of the esophagus. Surgery 2001;129:302–8. [DOI] [PubMed] [Google Scholar]
- [37].Elpek GO, Gelen T, Aksoy NH, et al. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol 2001;54:940–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Millikan KW, Silverstein J, Hart V, et al. A 15-year review of esophagectomy for carcinoma of the esophagus and cardia. Arch Surg 1995;130:617–24. [DOI] [PubMed] [Google Scholar]
- [39].Schiefer AI, Schoppmann SF, Birner P. Lymphovascular invasion of tumor cells in lymph node metastases has a negative impact on survival in esophageal cancer. Surgery 2016;160:331–40. [DOI] [PubMed] [Google Scholar]
- [40].Imamura Y, Watanabe M, Nagai Y, et al. Lymphatic vessel invasion detected by the D2-40 monoclonal antibody is an independent prognostic factor in node-negative esophageal squamous cell carcinoma. J Surg Oncol 2012;105:277–83. [DOI] [PubMed] [Google Scholar]
- [41].Nakayama Y, Matsumoto K, Nagato M, et al. Significance of lymphangiogenesis as assessed by immunohistochemistry for podoplanin in patients with esophageal carcinoma. Anticancer Res 2007;27:619–25. [PubMed] [Google Scholar]
- [42].Zhang BC, Guan S, Zhang YF, et al. Peritumoral lymphatic microvessel density is related to poor prognosis in lung adenocarcinoma: a retrospective study of 65 cases. Exp Ther Med 2012;3:636–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang BC, Gao J, Wang J, et al. Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med Oncol 2011;28:1447–52. [DOI] [PubMed] [Google Scholar]
- [44].Xue J, Wu XL, Huang XT, et al. Correlation of caveolin-1 expression with microlymphatic vessel density in colorectal adenocarcinoma tissues and its correlation with prognosis. Asian Pac J Trop Med 2015;8:655–7. [DOI] [PubMed] [Google Scholar]
- [45].Schweiger T, Nikolowsky C, Graeter T, et al. Increased lymphangiogenesis in lung metastases from colorectal cancer is associated with early lymph node recurrence and decreased overall survival. Clin Exp Metastasis 2016;33:133–41. [DOI] [PubMed] [Google Scholar]
- [46].Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182–6. [DOI] [PubMed] [Google Scholar]
- [47].Folkman J, Klagsbrun M. Angiogenic factors. Science 1987;235:442–7. [DOI] [PubMed] [Google Scholar]
- [48].Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983–5. [DOI] [PubMed] [Google Scholar]
- [49].Bouck N. Tumor angiogenesis: the role of oncogenes and tumor suppressor genes. Cancer Cells 1990;2:179–85. [PubMed] [Google Scholar]
- [50].Koide N, Nishio A, Kono T, et al. Histochemical study of vascular endothelial growth factor in squamous cell carcinoma of the esophagus. Hepatogastroenterology 1999;46:952–8. [PubMed] [Google Scholar]
- [51].Uchida S, Shimada Y, Watanabe G, et al. In oesophageal squamous cell carcinoma vascular endothelial growth factor is associated with p53 mutation, advanced stage and poor prognosis. Br J Cancer 1998;77:1704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Weidner N, Carroll PR, Flax J, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 1993;143:401–9. [PMC free article] [PubMed] [Google Scholar]
- [53].Chen B, Fang WK, Wu ZY, et al. The prognostic implications of microvascular density and lymphatic vessel density in esophageal squamous cell carcinoma: comparative analysis between the traditional whole sections and the tissue microarray. Acta Histochem 2014;116:646–53. [DOI] [PubMed] [Google Scholar]
- [54].Dallas NA, Samuel S, Xia L, et al. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res 2008;14:1931–7. [DOI] [PubMed] [Google Scholar]


