Abstract
To decrease the gastric cancer related mortality rate, endoscopic screening is widely performed in Korea. However, a precise method for identifying those at a high risk of gastric neoplasms has not been established. This study aims to evaluate serum pepsinogen (PG) levels for risk assessment of gastric neoplasms. Between August 2014 and March 2016, a total of 398 subjects, including 87 with gastric neoplasms, were enrolled in this study. On the basis of the serum PG I/II ratio, the enrolled subjects were classified into 4 groups: group A, PG I/II ratio >4; group B, >3 and ≤4; group C, >2 and ≤3; group D, ≤2. Compared with group A, a stepwise increase in the risk of gastric neoplasm was observed from group B [odds ratio (OR) = 9.9, 95% confidence interval (95% CI) = 4.0–24.4] to group C (OR = 20.9, 95% CI = 8.7–50.5) to group D (OR = 37.3, 95% CI = 14.3–97.4). The optimal cutoff value of the serum PG I/II ratio for predicting gastric neoplasms was 4.5, with a sensitivity of 97.7% and a specificity of 57.6%. A decrease in the serum PG I/II ratio was strongly associated with an increased risk of gastric neoplasms. The serum PG I/II ratio can be used to identify those at a high risk of gastric neoplasms in Korean population.
Keywords: atrophy, gastric neoplasm, Helicobacter pylori, pepsinogen, risk
1. Introduction
Gastric cancer is highly prevalent in Asian countries.[1] In Korea, gastric cancer is the most common cancer in males and the fourth most common in females.[2] The age-standardized mortality rate for gastric cancer was 10.5 per 100,000 in 2013, ranking third after lung and liver cancers. In Korea, the National Cancer Screening Program was introduced to decrease the gastric cancer related mortality rate.[3] An upper gastrointestinal series or endoscopy is provided biennially to all populations aged 40 years or older.
Helicobacter pylori has been recognized as a major pathogen in gastric carcinogenesis.[4] In the H pylori infected stomach, chronic active inflammation becomes persistent, leading to mucosal atrophy with destruction of gastric glands.[5] Gastric atrophic changes are related to secretion of pepsinogen (PG), a proenzyme of pepsin, by chief and mucous neck cells in the gastric mucosa.[6,7] On the basis of the source of secretion, PGs are subdivided into 2 types: PG I and II. PG I is only secreted from the fundic glands in the corpus of the stomach, whereas PG II is secreted from the corpus, as well as the pyloric glands in the antrum and proximal duodenum. PG is excreted mainly into the stomach lumen, but approximately 1% diffuses into the blood stream.[8] A previous study reported that serum PG was significantly related to extensive chronic gastritis.[9] For this reason, measurement of the serum PG level was introduced in gastric cancer screening programs in Japan.[10]
Atrophic gastritis and intestinal metaplasia are well-known risk factors for gastric neoplasms including dysplasia.[11] To identify these premalignant gastric conditions, histological biopsy or image-enhanced endoscopy is performed. However, a precise method for determining the risk of gastric neoplasms has not been proposed in Korea. Serum PG measurements could provide a simple and noninvasive method for screening gastric neoplasms. In this study, we aimed to evaluate serum PG levels for risk assessment of gastric neoplasms and to determine the optimal cutoff value for mass screening.
2. Methods
2.1. Study population
Between August 2014 and March 2016, subjects were enrolled in a single academic hospital. All subjects underwent gastroscopy for gastric cancer screening or further evaluation of biopsy-proven gastric neoplasms. Exclusion criteria were as follows: age <20 or >80 years, anemia (serum hemoglobin level <10 g/dL), severe systemic disease or advanced chronic liver disease, a history of H pylori eradication or gastric surgery, and recent use of certain medications, including proton pump inhibitors, H2-receptor blockers, or antibiotics. This study protocol was approved by the institutional review board of our hospital. Written informed consent was obtained from all subjects.
2.2. Evaluation of H pylori infection, gastric atrophy, and intestinal metaplasia
Two gastric biopsies for the rapid urease test (Pronto Dry; Gastrex Sarl, Gilly les Citeaux, France) were performed in the gastric antrum and body (1 sample each). In addition, 2 biopsy specimens were collected for histological examination from the lesser curvature of the gastric antrum and body (1 sample each). These specimens were fixed in 10% formalin and embedded in paraffin wax, and 5 μm sections were stained with hematoxylin and eosin and modified Giemsa. H pylori infection was confirmed by a positive result on either the rapid urease test or histological analysis. Using the Kimura–Takemoto classification,[12] gastric atrophy was classified endoscopically as closed (C-1, C-2, C-3) or open (O-1, O-2, O-3) type. The degree of gastric atrophy was categorized as mild (C-1, C-2), moderate (C-3, O-1), or severe (O-2, O-3). Intestinal metaplasia was diagnosed on the basis of the biopsy sample by a single expert pathologist.
2.3. Classification of gastric neoplasm risk using serum PG measurements
Before endoscopy, blood samples were collected during a 12-hour fasting period. Serum PG I and PG II levels were measured using a latex turbidimetric immunoassay (HiSens; HBI, Anyang, Korea), and the PG I/II ratios were calculated. According to the serum PG I/II ratio, the enrolled subjects were divided into 4 groups: group A, PG I/II ratio >4; group B, >3 and ≤4; group C, >2 and ≤3; and group D, ≤2.
2.4. Statistical analysis
Continuous variables were presented as means with standard deviation. Student t test or 1-way analysis of variance (ANOVA) was used to compare the continuous variables. When a significant difference was found by 1-way ANOVA, Bonferroni test was performed for post hoc analysis. Categorical variables were presented as sample numbers and proportions. The x2 test or linear-by-linear association was used to analyze the categorical variables. The risk of gastric neoplasms based on the serum PG I/II ratio was expressed as the odds ratio (OR) with 95% confidence interval (CI). To determine the cutoff value, receiver operating characteristic (ROC) curves and the Youden index were used. P values <.05 were considered statistically significant. All statistical analyses were performed using SPSS (version 19.0; SPSS Inc, Chicago, IL).
3. Results
3.1. Characteristics of the study population
Table 1 summarizes the baseline characteristics of the study population. A total of 398 subjects (170 males, 228 females) were eligible for this study, and their mean age was 48.2 (±16.6) years. The mean serum PG I and PG II levels and PG I/II ratio were 55.5 (±29.9), 15.0 (±10.4), and 4.6 (±2.4) ng/mL, respectively. The proportion of subjects with H pylori infection was 52.5%. Atrophic mucosal changes were not observed in the stomach of 184 subjects (46.2%). The remaining 214 subjects had a mild (16.6%), moderate (19.3%), or severe (17.8%) degree of gastric atrophy. Intestinal metaplasia was present in 135 subjects (33.9%) and absent in 263 subjects (66.1%).
Table 1.
Baseline characteristics of the study population according to the presence of gastric neoplasms.
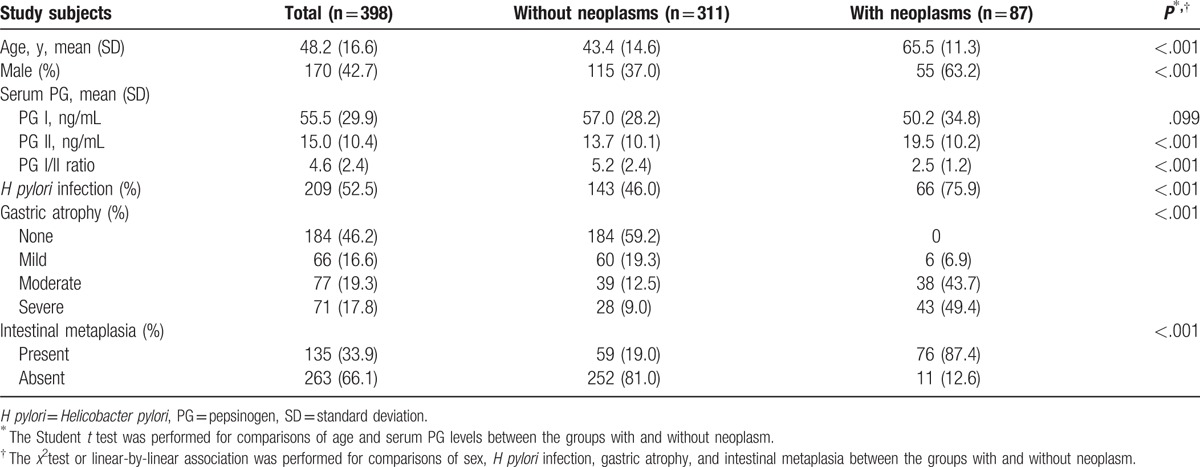
A total of 87 subjects with gastric neoplasms, comprising low-grade dysplasia (n = 19), high-grade dysplasia (n = 16), early gastric cancer (n = 40), and advanced gastric cancer (n = 12), were enrolled in this study. The characteristics of the subjects with gastric neoplasms and those without neoplasms (n = 311) were compared; significant differences in age and proportion of males were found (P < .001). Among the serological markers evaluated, the PG I level was not significantly different between the 2 groups (57.0 ± 28.2 vs 50.2 ± 34.8 ng/mL, P = .099). However, significant differences were found in the serum PG II level (13.7 ± 10.1 vs 19.5 ± 10.2 ng/mL, P < .001) and PG I/II ratio (5.2 ± 2.4 vs 2.5 ± 1.2, P < .001). The rate of H pylori infection was higher in the subjects with neoplasms than in those without neoplasms (75.9% vs 46.0%, P < .001). There were significant differences in the degree of gastric atrophy and intestinal metaplasia between the 2 groups (P < .001).
3.2. Characteristics of the subjects according to serum PG I/II ratio
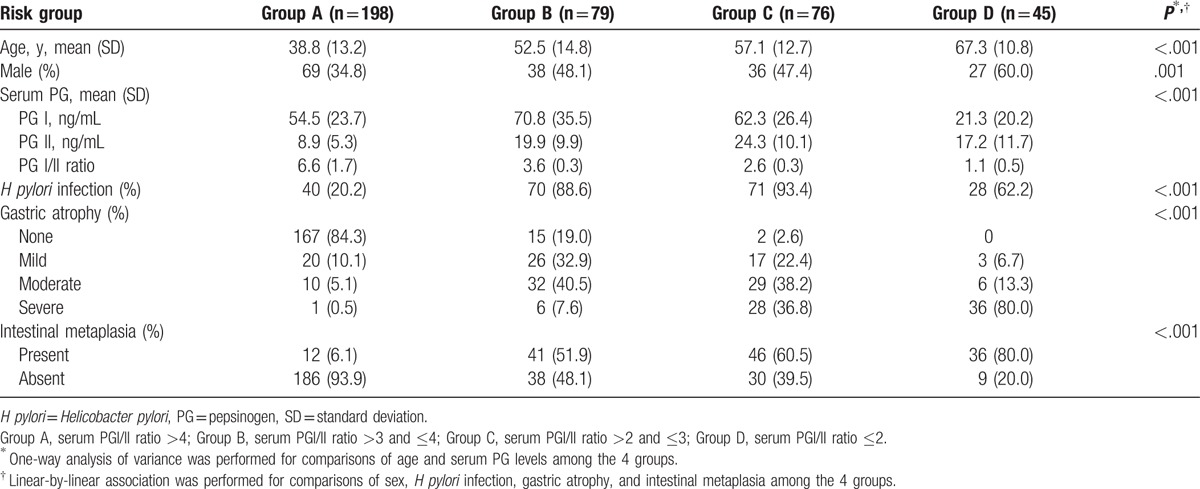
According to the serum PG I/II ratio, 198 (49.7%) subjects were categorized as group A, 79 (19.8%) as group B, 76 (19.1%) as group C, and 45 (11.3%) as group D. In Table 2, the baseline characteristics of the 4 groups were compared. The mean age of the subjects was 38.8 (±13.2) years in group A, 52.5 (±14.8) years in group B, 57.1 (±12.7) years in group C, and 67.3 (±10.8) years in group D (P < .001). The proportion of males was significantly different among all groups (P = .001). The rate of H pylori infection was significantly higher (P < .001) in groups B, C, and D (88.6%, 93.4%, and 62.2%, respectively) than in group A (20.2%). The degree of gastric atrophy was significantly different among all groups (P < .001). Groups C and D had a higher proportion of subjects with moderate to severe gastric atrophy than groups A and B. Significant differences in the presence of intestinal metaplasia were also found among the groups (P < .001).
Table 2.
Characteristics of the subjects according to the serum pepsinogen I/II ratio.
3.3. Serum PG I and II levels according to serum PG I/II ratio
Serum PG I levels were 54.5 (±23.7) ng/mL in group A, 70.8 (±35.5) ng/mL in group B, and 62.3 (±26.4) ng/mL in group C (Fig. 1). Group B had a significantly higher serum PG I level versus group A (P < .001). In group D, the serum PG I level was 21.3 ± 20.2 ng/mL, which was significantly lower than those of groups A, B, and C (P < .001). The serum PG II level was 8.9 ± 5.3 ng/mL in group A, 19.9 (±9.9) ng/mL in group B, 24.3 (±10.1) ng/mL in group C, and 17.2 (±11.7) ng/mL in group D (Fig. 2). The serum PG II level was significantly higher in groups B, C, and D than in group A (P < .001) and in group C compared with groups B and D (P = .005 and P < .001, respectively).
Figure 1.
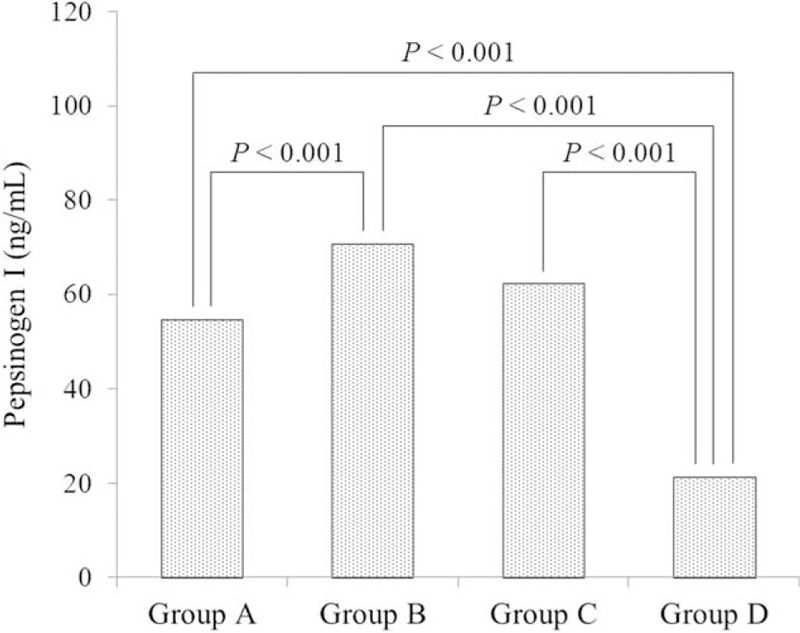
Relationship between the serum PG I level and serum PG I/II ratio. Bonferroni test was performed for comparisons of the PG I levels among the 4 groups. PG = pepsinogen.
Figure 2.
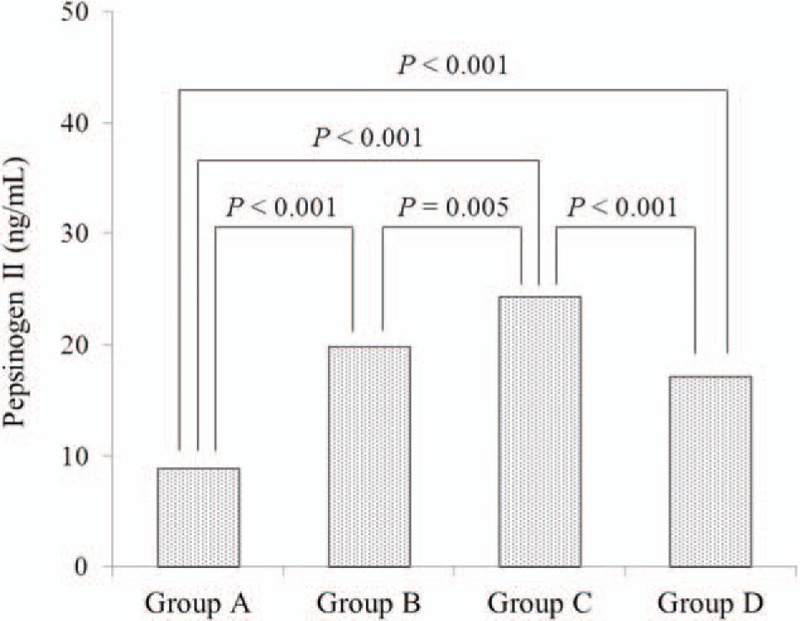
Relationship between the serum PG II level and serum PG I/II ratio. Bonferroni test was performed for comparisons of the PG II levels among the 4 groups. PG = pepsinogen.
3.4. Risk assessment of gastric neoplasms according to serum PG I/II ratio
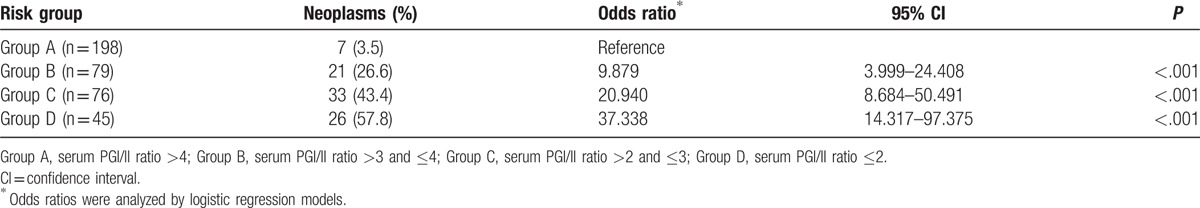
Table 3 summarizes the risk of gastric neoplasms in subjects grouped by serum PG I/II ratio. Gastric neoplasms were detected in 3.5% (n = 7/198) of those in group A, 26.6% (n = 21/79) in group B, 43.4% (n = 33/76) in group C, and 57.8% (n = 26/45) in group D. Compared with group A, significantly higher risks of gastric neoplasm were seen in group B (OR) = 9.879; 95% CI = 3.999–24.408, P < .001) and group C (OR = 20.940; 95% CI = 8.684–50.491, P < .001), with the highest risk observed in group D (OR = 37.338; 95% CI = 14.317–97.375, P < .001).
Table 3.
Risk assessment of gastric neoplasms according to the serum pepsinogen I/II ratio.
3.5. Optimal cutoff value of the serum PG I/II ratio for predicting gastric neoplasms
A ROC curve for the serum PG I/II ratio, used to predict gastric neoplasms, is shown in Fig. 3. The area under the curve was 0.840 (CI = 0.800–0.881). The optimal cutoff value for the serum PG I/II ratio was 4.5. The sensitivity, specificity, and positive and negative predictive values were 97.7% (CI = 91.5–99.6), 57.6% (CI = 55.8–58.1), 39.2% (CI = 36.7–39.9), and 98.9% (CI = 95.9–99.8), respectively.
Figure 3.
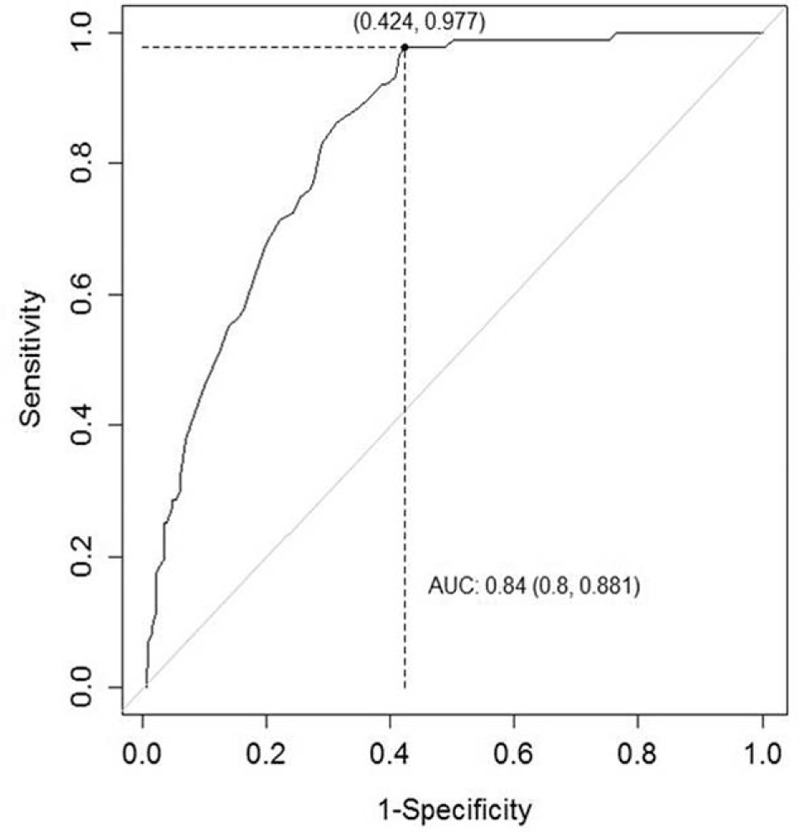
Receiver operating characteristic curve of the serum PG I/II ratio for the prediction of gastric neoplasms. The optimal cutoff value was 4.5, derived from the Youden index. The area under the curve was 0.840 (CI = 0.800–0.881). CI = confidence interval.
4. Discussion
Serum PG levels differed according to the gastric mucosal histology, providing a so-called serological biopsy.[7] In mild to moderate gastritis, both the PG I and II levels are increased by H pylori induced stimulation of the gastric glands.[13] With a prominent increase in the PG II level, the PG I/II ratio decreases. When gastric atrophy progresses to a severe stage, chief cells in the corpus are replaced by pyloric gland cells, leading to a decreased level of PG I. Consequently, a further decrease in the PG I/II ratio is observed.
Miki et al[14] reported that the serum PG I level and PG I/II ratio were significantly lower in patients with gastric cancer than in cancer-free subjects. The serum PG level was considered low when the PG I level was ≤70 ng/mL and the PG I/II ratio was ≤3. When these cutoff values were used for the detection of gastric cancer, the sensitivity and specificity were 84.6% and 73.5%, respectively.[15] In a cohort study, mass screening for gastric cancer was performed using both serum PG measurements and X-ray methods; 23.6% of those screened by serum PG measurements, and 11.7% screened by X-ray, required further endoscopic screening.[16] In total, 10 gastric cancers were detected. The measurement of serum PG showed a higher rate of gastric cancer detection (0.18%) than the X-ray method (0.05%).
In addition to the serum PG level, the serum level of anti-H pylori IgG antibody was considered a predictive marker for the development of gastric cancer.[17] Gastric cancer screening has been performed using a combination of serum PG levels and the H pylori antibody status. In 1 study, the risk of gastric cancer was divided into the following 4 groups: normal PG level and negative H pylori antibody status, normal PG level and positive H pylori antibody status, low PG level and positive H pylori antibody status, and low PG level and negative H pylori antibody status. The group with a low PG level and negative H pylori antibody status showed the highest hazard ratio for gastric cancer (8.2, 95% CI = 3.2–21.5).[18]
In Korea, the participation rate in gastric cancer screening programs has increased, and the proportion of screeners preferring endoscopy has increased gradually from 2002 to 2011.[19] Biennial endoscopic screening can lead to earlier detection of gastric cancer.[20] A shorter endoscopic surveillance interval is recommended for high-risk populations with atrophic gastritis or intestinal metaplasia.[21] However, a precise method to stratify gastric cancer risk has not been determined.[22] For the diagnosis of atrophic gastritis and intestinal metaplasia, a histological analysis of gastric mucosa is considered the gold standard. The operative links for gastritis and gastric intestinal metaplasia assessment staging systems were proposed recently.[23,24] However, the use of multiple biopsy specimens can be time-consuming. With regard to atrophic gastritis, low interobserver agreement among pathologists remains.[25] Recently, image-enhanced endoscopy systems showed high accuracy for the diagnosis of premalignant gastric conditions.[26–28] However, these systems are not available in all endoscopy units, and their diagnostic accuracy may be operator-dependent. In contrast, the measurement of serum PG levels is a simple and noninvasive test for detecting gastric diseases.[29] Thus, the serum PG level can be considered a surrogate marker for the mass screening of gastric cancer.
Few studies have used serum PG measurements and/or the H pylori antibody status to predict gastric neoplasms in Korea. Kang et al[30] reported that the sensitivity and specificity of a low PG I/II ratio (≤3) for detecting gastric cancer were 59.2% and 61%, respectively. A low PG I level (≤70 ng/mL) had an adequate sensitivity (72.4%) but a low specificity (20.2%). In a study by Choi et al,[31] the risk of gastric neoplasms was evaluated by combination of the serum PG level and anti-H pylori IgG antibody status. Patients with a low PG level and negative H pylori antibody status had the highest OR (25.8, 95% CI = 2.26–294.77) for gastric neoplasms. However, the number of subjects in this group was quite small (0.7%, n = 24/3328), including only 1 subject with low-grade dysplasia. For gastric neoplasm screening, Park et al[32] proposed using a cutoff serum PG I/II ratio ≤3.1 with negative H pylori antibody status, and ≤4.1 with positive H pylori antibody status. However, 27.5% (n = 50/182) of the patients with gastric neoplasms were not categorized in the high-risk group.
In our study, the serum PG I level and H pylori antibody status were not used for the assessment of gastric neoplasm risk. Haj-Sheykholeslami et al[33] reported that the serum PG I level was not a suitable marker for atrophic gastritis screening among first-degree relatives of patients with gastric cancer. In a study by Kim et al,[34] a serum PG I level ≤70 ng/mL in combination with a low PG I/II ratio had a low sensitivity for predicting histologically confirmed atrophic gastritis (22.7% in the antrum and 42.1% in the corpus). Previously, those with a low PG level and negative H pylori antibody status were considered to be at the highest risk of gastric cancer. However, the proportion of such subjects in previous studies was small (2.4–4.1%).[35–37] Recently, no significant difference in the cumulative incidence of gastric cancer was found among subjects with low PG levels, regardless of the H pylori antibody status.[38–40] Subjects with low PG levels are often categorized into the same group.[41] Therefore, we divided the risk of gastric neoplasms into 4 groups according to the serum PG I/II ratio alone.
This study demonstrated that a decrease in the serum PG I/II ratio was significantly associated with a high risk of gastric neoplasms. The risk of gastric neoplasm increased in a stepwise manner from groups A to D. A serum PG I/II ratio ≤4.5 showed an excellent negative predictive value (98.9%), but a low positive predictive value (39.2%), for predicting gastric neoplasms in our study. However, measurement of serum PG levels is not a diagnostic method for gastric neoplasm itself, but rather a screening tool for those at a high risk of gastric neoplasms. In subjects with low PG I/II ratios, endoscopic examination is needed to confirm the presence of gastric neoplasms. Of the gastric neoplasm-free subjects with a PG I/II ratio ≤4.5, 87.1% (n = 115/132) had a current H pylori infection. In another study, the diagnostic accuracy of the PG I/II ratio for H pylori induced gastritis, using the same cutoff value, was >80%.[42] This suggests that a serum PG I/II ratio ≤4.5 may be adequate for identifying candidates for primary prevention of gastric cancer. According to the Asia-Pacific guidelines for the management of H pylori infection,[43] eradication therapy for gastric cancer prevention is strongly recommended in countries with a high incidence of gastric cancer. In 2013, the Japanese government approved the coverage by national health insurance of eradication therapy for all H pylori infected populations.[44] Although the “test and treat” strategy for H pylori infection has not been introduced in Korea, H pylori eradication for gastric cancer prevention may be permitted in the near future.
In addition, we examined the relationship between serum PG I and II levels and the serum PG I/II ratio. Notably, the serum PG I level was lowest in group D, compared with the other 3 groups, suggesting that a prominent decrease occurred when gastric atrophy was severe. With regard to the serum PG II level, a significant increase was consistently seen in groups B, C, and D compared with group A. There was no linear correlation between serum PG I, II levels and gastric atrophy. In contrast, the serum PG I/II ratio was inversely associated with the severity of gastric atrophy and intestinal metaplasia, in a stepwise manner. These results were consistent with those of other studies. Kiyohira et al[45] reported that a decrease in the serum PG I level was affected by marked atrophy and intestinal metaplasia. In H pylori induced active and chronic inflammation, the serum PG II level was increased significantly. When serum PG measurements and the H pylori antibody status were both assessed for gastric cancer screening, the changes in serum PG I and II levels according to risk group were similar to our results.[17,18,46]
Herein, gastric dysplasia was included when the risk assessment and cutoff value for gastric neoplasm were calculated. Previous studies reported that gastric dysplasia might involve foci of malignant adenocarcinoma. In a study by Kato et al,[47] 44% of biopsy-proven gastric dysplasia cases were upgraded to adenocarcinoma by the postresection pathology. Moreover, a synchronous cancer in another part of the stomach was found in up to 30% of patients with gastric dysplasia.[48] For the management of gastric dysplasia, endoscopic resection has been accepted as a good therapeutic option.[49] Therefore, in surveillance programs for gastric cancer, gastric dysplasia must be treated in high-risk groups.
This study had several limitations. First, gastric atrophy was not assessed histologically. However, histological examination of gastric atrophy is well-correlated with endoscopic findings according to the Kimura–Takemoto classification.[50] Second, this study was conducted at a single center. The number of enrolled subjects may be insufficient for determining an accurate serum PG level to predict gastric neoplasms. Third, the serum H pylori antibody status was not evaluated. Fourth, the risks of gastric neoplasms were not presented as hazard ratios in this case–control study. A large-scale cohort study is needed to determine the incidence rate of gastric neoplasms.
In conclusion, a decrease in the serum PG I/II ratio was strongly associated with an increased risk of gastric neoplasms, in a stepwise manner. The serum PG I/II ratio can be used to identify those at a high risk of gastric neoplasms in mass screenings. A serum PG I/II ratio ≤4.5 was found to be a reliable marker for predicting gastric neoplasms among the Korean population.
Footnotes
Abbreviations: CI = confidence interval, H pylori = Helicobacter pylori, OR = odds ratio, PG = pepsinogen, ROC = receiver operating characteristic, SD = standard deviation.
This work was supported by the Soonchunhyang University Research Fund.
There are no conflicts of interest.
References
- [1].Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 2008;9:279–87. [DOI] [PubMed] [Google Scholar]
- [2].Oh CM, Won YJ, Jung KW, et al. Cancer statistics in Korea: incidence, mortality, survival, and Prevalence in 2013. Cancer Res Treat 2016;48:436–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim Y, Jun JK, Choi KS, et al. Overview of the National Cancer Screening Programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011;12:725–30. [PubMed] [Google Scholar]
- [4].Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–9. [DOI] [PubMed] [Google Scholar]
- [5].Kuipers EJ, Uyterlinde AM, Pena AS, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet 1995;345:1525–8. [DOI] [PubMed] [Google Scholar]
- [6].Samloff IM. Pepsinogens I and II: purification from gastric mucosa and radioimmunoassay in serum. Gastroenterology 1982;82:26–33. [PubMed] [Google Scholar]
- [7].Samloff IM, Varis K, Ihamaki T, et al. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology 1982;83:204–9. [PubMed] [Google Scholar]
- [8].Mukoubayashi C, Yanaoka K, Ohata H, et al. Serum pepsinogen and gastric cancer screening. Intern Med 2007;46:261–6. [DOI] [PubMed] [Google Scholar]
- [9].Miki K, Ichinose M, Shimizu A, et al. Serum pepsinogen as a screening test of extensive chronic gastritis. Gastroenterol Jpn 1987;22:133–41. [DOI] [PubMed] [Google Scholar]
- [10].Kodoi A, Yoshihara M, Sumii K, et al. Serum pepsinogen in screening for gastric cancer. J Gastroenterol 1995;30:452–60. [DOI] [PubMed] [Google Scholar]
- [11].Correa P. Human gastric carcinogenesis: a multistep and multifactorial process: First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735–40. [PubMed] [Google Scholar]
- [12].Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969;3:87–97. [Google Scholar]
- [13].Lorente S, Doiz O, Trinidad Serrano M, et al. Helicobacter pylori stimulates pepsinogen secretion from isolated human peptic cells. Gut 2002;50:13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miki K, Ichinose M, Kawamura N, et al. The significance of low serum pepsinogen levels to detect stomach cancer associated with extensive chronic gastritis in Japanese subjects. Jpn J Cancer Res 1989;80:111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kitahara F, Kobayashi K, Sato T, et al. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut 1999;44:693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Miki K, Morita M, Sasajima M, et al. Usefulness of gastric cancer screening using the serum pepsinogen test method. Am J Gastroenterol 2003;98:735–9. [DOI] [PubMed] [Google Scholar]
- [17].Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 2004;109:138–43. [DOI] [PubMed] [Google Scholar]
- [18].Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut 2005;54:764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choi KS, Suh M. Screening for gastric cancer: the usefulness of endoscopy. Clin Endosc 2014;47:490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Choi KS, Jun JK, Suh M, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer 2015;112:608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shin WG, Kim HU, Song HJ, et al. Surveillance strategy of atrophic gastritis and intestinal metaplasia in a country with a high prevalence of gastric cancer. Dig Dis Sci 2012;57:746–52. [DOI] [PubMed] [Google Scholar]
- [22].Choi IJ. Endoscopic gastric cancer screening and surveillance in high-risk groups. Clin Endosc 2014;47:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut 2007;56:631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010;71:1150–8. [DOI] [PubMed] [Google Scholar]
- [25].el-Zimaity HM, Graham DY, al-Assi MT, et al. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol 1996;27:35–41. [DOI] [PubMed] [Google Scholar]
- [26].Anagnostopoulos GK, Yao K, Kaye P, et al. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy 2007;39:202–7. [DOI] [PubMed] [Google Scholar]
- [27].Inoue T, Uedo N, Ishihara R, et al. Autofluorescence imaging videoendoscopy in the diagnosis of chronic atrophic fundal gastritis. J Gastroenterol 2010;45:45–51. [DOI] [PubMed] [Google Scholar]
- [28].Pimentel-Nunes P, Libânio D, Lage J, et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016;48:723–30. [DOI] [PubMed] [Google Scholar]
- [29].di Mario F, Cavallaro LG. Non-invasive tests in gastric diseases. Dig Liver Dis 2008;40:523–30. [DOI] [PubMed] [Google Scholar]
- [30].Kang JM, Kim N, Yoo JY, et al. The role of serum pepsinogen and gastrin test for the detection of gastric cancer in Korea. Helicobacter 2008;13:146–56. [DOI] [PubMed] [Google Scholar]
- [31].Choi HS, Lee SY, Kim JH, et al. Combining the serum pepsinogen level and Helicobacter pylori antibody test for predicting the histology of gastric neoplasm. J Dig Dis 2014;15:293–8. [DOI] [PubMed] [Google Scholar]
- [32].Park CH, Kim EH, Jung da H, et al. The new modified ABCD method for gastric neoplasm screening. Gastric Cancer 2016;19:128–35. [DOI] [PubMed] [Google Scholar]
- [33].Haj-Sheykholeslami A, Rakhshani N, Amirzargar A, et al. Serum pepsinogen I, pepsinogen II, and gastric 17 in relatives of gastric cancer patients: comparative study with type and severity of gastritis. Clin Gastroenterol Hepatol 2008;6:174–9. [DOI] [PubMed] [Google Scholar]
- [34].Kim EH, Kang H, Park CH, et al. The optimal serum pepsinogen cut-off value for predicting histologically confirmed atrophic gastritis. Dig Liver Dis 2015;47:663–8. [DOI] [PubMed] [Google Scholar]
- [35].Mizuno S, Miki I, Ishida T, et al. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci 2010;55:3132–7. [DOI] [PubMed] [Google Scholar]
- [36].Shimoyama T, Aoki M, Sasaki Y, et al. ABC screening for gastric cancer is not applicable in a Japanese population with high prevalence of atrophic gastritis. Gastric Cancer 2012;15:331–4. [DOI] [PubMed] [Google Scholar]
- [37].Yamaguchi Y, Nagata Y, Hiratsuka R, et al. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels: the ABC method. Digestion 2016;93:13–8. [DOI] [PubMed] [Google Scholar]
- [38].Terasawa T, Nishida H, Kato K, et al. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: a systemic review and meta-analysis. PLoS One 2014;9:e109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Charvat H, Sasazuki S, Inoue M, et al. Prediction of the 10-year probability of gastric cancer occurrence in the Japanese population: the JPHC study cohort II. Int J Cancer 2016;138:320–31. [DOI] [PubMed] [Google Scholar]
- [40].Ikeda F, Shikata K, Hata J, et al. Combination of Helicobacter pylori antibody and serum pepsinogen as a good predictive tool of gastric cancer incidence: 20-year prospective data from the Hisayama study. J Epidemiol 2016;26:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Inoue K. Suzuki H, Warren R, Marshall B. Stratification of gastric cancer risk by H pylori infection. Helicobacter pylori. Tokyo: Springer; 2016. 169–79. [Google Scholar]
- [42].Kitamura Y, Yoshihara M, Ito M, et al. Diagnosis of Helicobacter pylori-induced gastritis by serum pepsinogen levels. J Gastroenterol Hepatol 2015;30:1473–7. [DOI] [PubMed] [Google Scholar]
- [43].Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol 2009;24:1587–600. [DOI] [PubMed] [Google Scholar]
- [44].Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J Gastroenterol 2014;49:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kiyohira K, Yoshihara M, Ito M, et al. Serum pepsinogen concentration as a marker of Helicobacter pylori infection and the histologic grade of gastritis; evaluation of gastric mucosa by serum pepsinogen levels. J Gastroenterol 2003;38:332–8. [DOI] [PubMed] [Google Scholar]
- [46].Yoshida T, Kato J, Inoue I, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer 2014;134:1445–57. [DOI] [PubMed] [Google Scholar]
- [47].Kato M, Nishida T, Tsutsui S, et al. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: a multicenter study by Osaka University ESD Study Group. J Gastroenterol 2011;46:325–31. [DOI] [PubMed] [Google Scholar]
- [48].Carmack SW, Genta RM, Graham DY, et al. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009;6:331–41. [DOI] [PubMed] [Google Scholar]
- [49].Goddard AF, Badreldin R, Pritchard DM, et al. British Society of Gastroenterology. The management of gastric polyps. Gut 2010;59:1270–6. [DOI] [PubMed] [Google Scholar]
- [50].Liu Y, Uemura N, Xiao SD, et al. Agreement between endoscopic and histological gastric atrophy scores. J Gastroenterol 2005;40:123–7. [DOI] [PubMed] [Google Scholar]


