Abstract
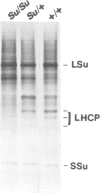
Su is a nuclear encoded, semi-dominant aurea mutation in Nicotiana tabacum L. The homozygous plants (Su/Su) are pale yellow and non-photosynthetic while the heterozygous (Su/+) are photosynthetically competent and have a yellow-green phenotype which is distinct from that of green wild-type plants (+/+). We have examined the RNA and protein levels for a number of nuclear and plastid encoded chloroplast proteins under high and low light plant growth conditions. Under high light conditions, the light-harvesting chlorophyll a/b binding proteins (LHCP) were undetectable in the homozygous Su/Su plants, and the large subunit (LSu) and the small subunit (SSu) of ribulose bisphosphate carboxylase (Rubisco) and cytochrome b559 were severely deficient. However, only the nuclear encoded cab and plastid encoded psbE mRNA (encoding LHCP and cytochrome b559 respectively) were reduced significantly. In heterozygous Su/+ plants, the level of LHCP was reduced to 25% of that in wild-type plants while cab and psbE mRNA, LSu, SSu and cytochrome b559 remained at normal levels, suggesting that LCHP is more immediately affected by the Su mutant gene product than the rest of the photosynthetic proteins and mRNA examined. Under low light conditions, the levels of cab and psbE mRNA, LSu, SSu and cytochrome b559 in homozygous Su/Su plants were equivalent to those in wild-type plants except LHCP which remained undetectable. Similarly, the LHCP level in low light grown Su/+ plants still remained at 25% of wild-type level. These results indicate that the decrease in LHCP is independent of light conditions and has not resulted from photooxidation, whereas the depletion of other proteins and mRNA examined under high light growth conditions is a consequence of photooxidative damage to Su/Su plastids. Furthermore, transgenic Su/Su and Su/+ plants with a cauliflower mosaic virus 35S (CaMV 35S)-cab construct constitutively maintained high levels of cab mRNA but displayed the same pattern of diminished LHCP accumulation as their non-transformed counterparts when grown under both high and low light conditions. These results indicate that the Su mutation primarily causes depletion of LHCP. The depletion of LHCP leads to photooxidative damage which results in decreased cab mRNA levels and other pleiotropic lesions in Su/Su plants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batschauer A., Mösinger E., Kreuz K., Dörr I., Apel K. The implication of a plastid-derived factor in the transcriptional control of nuclear genes encoding the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1986 Feb 3;154(3):625–634. doi: 10.1111/j.1432-1033.1986.tb09444.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Polypeptide turnover in darkness. Eur J Biochem. 1981 Aug;118(1):61–70. doi: 10.1111/j.1432-1033.1981.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Steinback K. E., Arntzen C. J. Analysis of the Light-harvesting Pigment-Protein Complex of Wild Type and a Chlorophyll-b-less Mutant of Barley. Plant Physiol. 1979 Feb;63(2):237–243. doi: 10.1104/pp.63.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia C. P., Duesing J. H., Arntzen C. J. Developmental Loss of Photosystem II Activity and Structure in a Chloroplast-Encoded Tobacco Mutant, Lutescens-1. Plant Physiol. 1986 Sep;82(1):19–27. doi: 10.1104/pp.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Bogorad L. Plastid Development in Pisum sativum Leaves during Greening : II. Post-Translational Uptake by Plastids as an Indicator System to Monitor Changes in Translatable mRNA for Nuclear-Encoded Plastid Polypeptides. Plant Physiol. 1987 Nov;85(3):816–822. doi: 10.1104/pp.85.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Scolnik P. A. Transcription of Two Photosynthesis-Associated Nuclear Gene Families Correlates with the Presence of Chloroplasts in Leaves of the Variegated Tomato ghost Mutant. Plant Physiol. 1988 Jan;86(1):7–9. doi: 10.1104/pp.86.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leto K. J., Bell E., McIntosh L. Nuclear mutation leads to an accelerated turnover of chloroplast-encoded 48 kd and 34.5 kd polypeptides in thylakoids lacking photosystem II. EMBO J. 1985 Jul;4(7):1645–1653. doi: 10.1002/j.1460-2075.1985.tb03832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P., Taylor W. C. Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur J Biochem. 1984 Oct 1;144(1):79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., Diner B. A., Williams JGK., Arntzen C. J. Deletion Mutagenesis of the Cytochrome b559 Protein Inactivates the Reaction Center of Photosystem II. Plant Cell. 1989 Jun;1(6):591–597. doi: 10.1105/tpc.1.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J., VAN Montagu M., Herrera-Estrella L. Photosynthesis-associated gene families: differences in response to tissue-specific and environmental factors. Science. 1986 Jul 4;233(4759):34–38. doi: 10.1126/science.233.4759.34. [DOI] [PubMed] [Google Scholar]
- Spreitzer R. J., Goldschmidt-Clermont M., Rahire M., Rochaix J. D. Nonsense mutations in the Chlamydomonas chloroplast gene that codes for the large subunit of ribulosebisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5460–5464. doi: 10.1073/pnas.82.16.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhaus J., Schell J., Willmitzer L. Correlation of the expression of the nuclear photosynthetic gene ST-LS1 with the presence of chloroplasts. EMBO J. 1989 Sep;8(9):2445–2451. doi: 10.1002/j.1460-2075.1989.tb08379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry C., Olive J., Drapier D., Recouvreur M., Wollman F. A. Posttranslational events leading to the assembly of photosystem II protein complex: a study using photosynthesis mutants from Chlamydomonas reinhardtii. J Cell Biol. 1989 Sep;109(3):991–1006. doi: 10.1083/jcb.109.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]