Abstract
OBJECTIVE
Although early trials suggested that intensive glycemic targets reduce the number of complications with diabetes, contemporary trials indicate no cardiovascular benefit and potentially higher mortality risk. As patients with advanced chronic kidney disease (CKD) transitioning to treatment with dialysis were excluded from these studies, the optimal glycemic level in this population remains uncertain. We hypothesized that glycemic status, defined by hemoglobin A1c (HbA1c) and random glucose levels, in the pre–end-stage renal disease (ESRD) period is associated with higher 1-year post-ESRD mortality among patients with incident diabetes who have ESRD.
RESEARCH DESIGN AND METHODS
Among 17,819 U.S. veterans with diabetic CKD transitioning to dialysis from October 2007 to September 2011, we examined the association of mean HbA1c and random glucose levels averaged over the 1-year pre-ESRD transition period with mortality in the first year after dialysis initiation. All-cause mortality hazard ratios (HRs) were estimated using multivariable survival models. Secondary analyses examined cardiovascular mortality using competing risks methods.
RESULTS
HbA1c levels ≥8% (≥64 mmol/mol) were associated with higher mortality in the first year after dialysis initiation (reference value 6% to <7% [42–53 mmol/mol]): adjusted HRs [aHRs] 1.19 [95% CI 1.07–1.32] and 1.48 (1.31–1.67) for HbA1c 8% to <9% [64–75 mmol/mol] and ≥9% [≥75 mmol/mol], respectively). Random glucose levels ≥200 mg/dL were associated with higher mortality (reference value 100 to <125 mg/dL): aHR 1.34 [95% CI 1.20–1.49]). Cumulative incidence curves showed that incrementally higher mean HbA1c and random glucose levels were associated with increasingly higher cardiovascular mortality.
CONCLUSIONS
In patients with diabetes and CKD transitioning to dialysis, higher mean HbA1c and random glucose levels during the pre-ESRD prelude period were associated with higher 1-year post-ESRD mortality. Clinical trials are warranted to examine whether modulating glycemic status improves survival in this population.
Introduction
Diabetes is the leading cause of kidney disease in the U.S., accounting for ∼40% of patients with non–dialysis-dependent chronic kidney disease (NDD-CKD) and end-stage renal disease (ESRD) (1,2). United States Renal Data System (USRDS) data show that the number of patients with diabetes who have ESRD continues to rise and that their survival is markedly worse compared with their counterparts without diabetes (1). Hence, there is a compelling need to determine whether factors prior to the development of ESRD contribute to the exceedingly poor survival of patients with diabetes who have NDD-CKD and are transitioning to dialysis.
In the general population, adequate glycemic control is a cornerstone of averting and ameliorating the microvascular and macrovascular complications of diabetes. Early randomized controlled trials and long-term corollary follow-up studies have shown that intensive glycemic control in patients with type 1 diabetes (3–5) and type 2 diabetes (6–8) with minimal end-organ damage reduces microvascular and macrovascular complications. However, contemporary trials (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation [ADVANCE] and Veterans Affairs Diabetes Trial [VADT]) show that intensive glycemic control does not provide cardiovascular benefit (9,10), and the Action to Control Cardiovascular Risk (ACCORD) trial showed higher mortality among patients with type 2 diabetes with underlying cardiovascular risk (9–11), who are more akin to the NDD-CKD and ESRD populations.
The optimal glycemic level in patients with diabetes who have NDD-CKD and ESRD remains unknown, as most trials of glycemic control have excluded patients with advanced kidney disease. Multiple large observational studies (12–17) suggest that both lower and higher glycemic levels, defined by hemoglobin A1c (HbA1c) level, are associated with a higher risk of death in cohorts of patients with diabetes undergoing dialysis. Although these data advance our understanding of the relationship between post-ESRD HbA1c levels with survival in dialysis patients, they fail to inform how glycemic status immediately preceding dialysis transition (pre-ESRD) impacts mortality risk in this population. There has been sparse study of glycemic status and mortality in patients with NDD-CKD, which has shown mixed findings (18,19). Moreover, because dialysis patients experience the highest mortality rates in the first few months of dialysis initiation, there has been considerable interest in identifying factors in advanced stages of NDD-CKD (immediate pre-ESRD period) that influence dialysis mortality during this precarious post-ESRD period (20,21). However, it has yet to be determined whether glycemic status prior to the development of irreversible kidney failure influences post-ESRD mortality among patients with very late-stage CKD who transition to treatment with dialysis.
A major barrier to this end has been the paucity of pre-ESRD transition data across large post-ESRD databases. Linkage of pre-ESRD data from the national Veterans Affairs (VA) database with post-ESRD registries (USRDS) provides a unique opportunity to fill this knowledge gap. To better inform the field, we examined the association of pre-ESRD glycemic status, defined by mean HbA1c and random blood glucose levels averaged over the immediate predialysis transition period, with 1-year post-ESRD mortality among U.S. veterans with diabetes and NDD-CKD transitioning to dialysis.
Research Design and Methods
Source Cohort
We conducted a cohort study with longitudinal data from the Transition of Care in CKD (TC-CKD) study (22–25), a retrospective cohort study specifically examining U.S. veterans with advanced CKD who newly transitioned to treatment with dialysis. Our source population consisted of 52,172 patients from the national VA database who transitioned to treatment with dialysis over the period from 1 October 2007 to 30 September 2011 (1). Our primary cohort was designated as the “Overall HbA1c Cohort,” which was intended to capture all patients with diabetes, using a history of HbA1c measurement as a sensitive proxy. The Overall HbA1c Cohort included patients who did not have missing censoring event dates, were ≥18 years of age, and underwent one or more HbA1c measurements up to 1 year preceding dialysis initiation (“prelude period”) (22). Because HbA1c levels may have been measured in patients without diabetes (prediabetic patients), we also designated a “Restricted HbA1c Cohort” using more specific criteria, including ICD-9 codes for diabetes, prior to dialysis initiation and/or cause of ESRD due to diabetes, that may potentially misclassify (i.e., under-capture) patients with diabetes (Supplementary Fig. 1). Given the controversy regarding the use of HbA1c level as a glycemic metric in patients with CKD (2,26–30), we also designated a complementary “Mean Random Glucose Cohort” that was composed of patients who did not have missing censor data, were ≥18 years of age or older, underwent one or more random blood glucose measurements during the 1-year prelude period, and had either an ICD-9 code or cause of ESRD due to diabetes. The study was approved by the Institutional Review Boards of the University of California Irvine, VA Long Beach Healthcare System, and Memphis VA Medical Center.
Exposure Ascertainment
To determine the impact of pre-ESRD glycemic status upon post-ESRD mortality, we examined serial measures of glycemic status averaged over the prelude period. Our primary exposure was mean HbA1c level averaged over the 1-year prelude period, which was categorized as <5%, 5% to <6%, 6% to <7%, 7% to <8%, 8% to <9%, and ≥9% (<31, 31 to <42, 42 to <53, 53 to <64, 64 to <75, and ≥75 mmol/mol, respectively). Our secondary exposure was mean random glucose level averaged over the 1-year prelude period, which was categorized as <100, 100 to <125, 125 to <150, 150 to <175, 175 to <200, and ≥200 mg/dL.
Outcome Ascertainment
Our primary and secondary outcomes of interest were 1-year post-ESRD all-cause and cardiovascular mortality, respectively. Follow-up began the day after dialysis initiation and ended 1 year afterward. Patients were censored for kidney transplantation, loss to follow-up, or the last date of available follow-up data (27 December 2012 and 6 October 2011 for all-cause and cardiovascular mortality, respectively) (23–25), whichever occurred first. All-cause mortality data, censoring events, and associated dates were obtained from VA, Center for Medicare and Medicaid Services (CMS), and USRDS data sources (1,31–33). Cardiovascular mortality data were obtained from USRDS sources only.
Sociodemographic, Comorbidity, Medication, and Laboratory Data
Data from the USRDS Patient and Medical Evidence files were used to determine patients’ baseline sociodemographic information (age, sex, race, ethnicity) at the time of dialysis initiation. Cause of ESRD was obtained from CMS data, and information on initial dialysis modality was obtained from USRDS sources. Information about comorbidities at the time of dialysis initiation was extracted from the VA Inpatient and Outpatient Medical SAS data sets (34) and CMS data sets using ICD-9 diagnostic and procedure codes and Current Procedural Terminology codes as well as from VA/CMS data (1,31–33). Charlson comorbidity index (CCI) scores were estimated using the Deyo modification for administrative data sets without including kidney disease (35). BMI data were obtained from the VA Vital Status file. Medication data were obtained from both CMS Part D and VA pharmacy dispensation records (36). HbA1c level, random glucose level, and other laboratory data were obtained from the Decision Support System-National Data Extracts Laboratory Results files (37) and were defined as the average of each covariate during the 1-year prelude period preceding dialysis initiation. VA Corporate Data Warehouse-LabChem data files were used to extract data about predialysis serum creatinine levels (38). Using serum creatinine and demographic data, estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (39).
Statistical Analysis
We estimated the association between mean HbA1c level and mortality using Cox models with four adjustment levels with the following covariates:
Minimally adjusted model: patient’s calendar quarter of dialysis initiation to account for secular changes in care over time;
Case-mix model: minimally adjusted model covariates, plus age, sex, race, ethnicity, cause of ESRD, CCI, diabetes, congestive heart failure (CHF), and cerebrovascular disease (CVD);
Expanded case-mix model: case-mix model covariates, plus residential region, initial dialysis modality, and BMI; and
Expanded case-mix plus laboratory model: expanded case-mix model covariates, plus serum albumin level, hemoglobin level, serum bicarbonate level, eGFR level averaged over the 1-year prelude period (proxy of residual kidney function), and last eGFR level measured prior to dialysis initiation (proxy of dialysis practice patterns).
We a priori defined the case-mix model as our preferred model, which included core sociodemographic measures and comorbidity confounders of the association between glycemic status and mortality. For the HbA1c analyses, there were no missing data for age or sex; remaining covariates had <1% missing values, except for cause of ESRD (7.7%), residential region (2.4%), modality (9.7%), BMI (18.4%), serum albumin levels (30.7%), hemoglobin levels (31.0%), serum bicarbonate levels (27.9%), and eGFRs averaged over the 1-year prelude period (37.3%). Hence, further adjustments for potential confounders in expanded case-mix and expanded case-mix plus laboratory models were conducted as sensitivity analyses. For cardiovascular mortality, we conducted sensitivity analyses using Fine and Gray competing risks regression (40) and cumulative incidence curves to account for noncardiovascular deaths as competing events. We also conducted subgroup analyses of HbA1c and all-cause mortality across clinically relevant subgroups. To address missing covariate data, we implemented multiple imputation using five imputed data sets. Proportional hazards assumptions were confirmed by graphical analysis. Analogous analyses were conducted for the association of mean random glucose levels with mortality. Analyses and figures were generated using SAS version 9.4 (SAS Institute Inc., Cary, NC), Stata version 13.1 (Stata Corporation, College Station, TX), and SigmaPlot Version 12.5 (Systat Software, San Jose, CA).
Results
Baseline Characteristics
Among 17,819 patients who met eligibility criteria for the Overall HbA1c Cohort (Supplementary Fig. 1), the mean ± SD, median (interquartile range [IQR]), and minimum–maximum of prelude HbA1c values were 6.9% ± 1.4%, 6.6% (5.9%, 7.5%), and 2.7–18.4%, respectively. The median (IQR) and minimum–maximum number of HbA1c (both Overall and Restricted Cohorts) measurements per patient were 2 (1, 3) and 1–15, respectively. The median (IQR) and minimum–maximum of glucose measurements per patient were 6 (2, 16) and 1–637, respectively.
Compared with patients in the lowest HbA1c category (<5% [<31 mmol/mol]), those in the highest category (≥9% [≥75 mmol/mol]) were more likely to be Hispanic; have diabetes as the cause of ESRD; have CHF and CVD; have higher mean BMI and 1-year averaged and last eGFR prior to dialysis initiation, hemoglobin, and bicarbonate levels; and have lower albumin levels (Table 1).
Table 1.
Baseline characteristics among 17,819 patients with HbA1c levels in the 1-year prelude period
| Overall | HbA1c (%) [mmol/mol] |
P value | ||||||
|---|---|---|---|---|---|---|---|---|
| <5 [<31] | 5 to <6 [31 to <42] | 6 to <7 [42 to <53] | 7 to <8 [53 to <64] | 8 to <9 [64 to <75] | ≥9 [≥75] | |||
| N (%) | 17,819 | 491 (2.8) | 4,530 (25.4) | 6,065 (34.0) | 3,561 (20.0) | 1,808 (10.1) | 1,364 (7.7) | NA |
| Age, years (mean [SD]) | 68 (11) | 65 (12) | 69 (11) | 70 (11) | 68 (10) | 66 (10) | 62 (10) | <0.001 |
| Female sex (%) | 2 | 2 | 2 | 1 | 1 | 2 | 3 | 0.007 |
| Race (%) | ||||||||
| White | 70 | 60 | 69 | 71 | 73 | 69 | 63 | 0.4 |
| Black | 28 | 39 | 29 | 27 | 25 | 28 | 33 | 0.8 |
| Other | 2 | 1 | 2 | 2 | 2 | 3 | 3 | 0.002 |
| Hispanic ethnicity (%) | 7 | 6 | 6 | 7 | 8 | 9 | 10 | <0.001 |
| Cause of ESRD (%) | ||||||||
| Diabetes | 62 | 30 | 41 | 61 | 77 | 80 | 82 | <0.001 |
| Hypertension | 23 | 38 | 37 | 24 | 13 | 12 | 10 | <0.001 |
| Glomerulonephritis | 4 | 11 | 8 | 4 | 2 | 1 | 1 | <0.001 |
| Other | 11 | 22 | 15 | 11 | 8 | 8 | 7 | <0.001 |
| Residential region (%) | ||||||||
| Northeast | 14 | 11 | 14 | 16 | 13 | 13 | 11 | 0.05 |
| Midwest | 23 | 25 | 22 | 24 | 24 | 23 | 22 | >0.9 |
| South | 45 | 46 | 46 | 43 | 46 | 45 | 47 | 0.5 |
| West | 18 | 19 | 18 | 17 | 17 | 19 | 20 | 0.3 |
| Initial dialysis modality (%) | ||||||||
| Hemodialysis | 87 | 87 | 86 | 86 | 85 | 86 | 87 | 0.7 |
| Missing | 9 | 10 | 10 | 10 | 10 | 9 | 8 | 0.1 |
| Peritoneal dialysis | 2 | 2 | 4 | 4 | 4 | 5 | 4 | 0.1 |
| Home hemodialysis | <1 | <1 | <1 | <1 | <1 | <1 | <1 | 0.8 |
| Comorbidities | ||||||||
| CCI (median [IQR]) | 5 (4, 7) | 4 (2, 6) | 5 (3, 6) | 5 (4, 7) | 6 (4, 7) | 6 (4, 7) | 5 (4, 7) | <0.001 |
| Diabetes (%) | 87 | 60 | 67 | 90 | 99 | 99 | 99 | <0.001 |
| CHF (%) | 62 | 30 | 56 | 64 | 66 | 64 | 62 | <0.001 |
| CVD (%) | 23 | 18 | 23 | 24 | 24 | 23 | 23 | 0.5 |
| Body anthropometry | ||||||||
| BMI (mean [SD]) | 31 (7) | 28 (6) | 29 (7) | 31 (7) | 32 (7) | 32 (7) | 32 (8) | <0.001 |
| Laboratory results* (mean [SD]) | ||||||||
| eGFR (averaged over the 1-year prelude period) (mL/min/1.73 m2) | 19 (14) | 18 (17) | 17 (14) | 19 (14) | 19 (13) | 20 (13) | 22 (16) | <0.001 |
| eGFR at dialysis initiation (mL/min/1.73 m2) | 17 (15) | 15 (14) | 16 (14) | 17 (14) | 18 (14) | 18 (14) | 20 (16) | <0.001 |
| Serum albumin (g/dL) | 3.5 (0.6) | 3.4 (0.6) | 3.5 (0.6) | 3.5 (0.6) | 3.5 (0.6) | 3.4 (0.6) | 3.2 (0.6) | <0.001 |
| Hemoglobin (g/dL) | 11.2 (1.5) | 10.5 (1.5) | 11.0 (1.5) | 11.1 (1.5) | 11.3 (1.5) | 11.4 (1.5) | 11.5 (1.6) | <0.001 |
| Bicarbonate (mg/dL) | 24 (4) | 22 (4) | 23 (4) | 24 (4) | 24 (4) | 24 (4) | 25 (4) | <0.001 |
NA, not applicable.
*All laboratory results were averaged over the 1-year prelude period except for eGFR at dialysis initiation.
HbA1c and Mortality
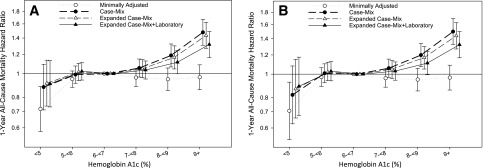
Patients contributed a total of 15,119 patient-years of follow-up during which 4,374 all-cause deaths occurred. Median (IQR) at-risk time was 365 days (305, 366 days). In case-mix analyses, higher HbA1c levels of 8% to <9% (64 to <75 mmol/mol) and ≥9% (≥75 mmol/mol) were associated with a 19% and 48% higher all-cause mortality risk, respectively (reference value HbA1c 6% to <7% [42 to <53 mmol/mol]) (Fig. 1A and Supplementary Table 1). These associations persisted in case-mix and expanded case-mix plus laboratory analyses. Similar findings were observed in the Restricted HbA1c Cohort (Fig. 1B and Supplementary Table 1).
Figure 1.
Association between HbA1c levels over the 1-year prelude period and 1-year all-cause mortality rate in the Overall Cohort (A) and Restricted Cohort (B). HbA1c levels of <5%, 5% to <6%, 6% to <7%, 7% to <8%, 8% to <9%, and ≥9%, which are equivalent to <31, 31 to <42, 42 to <53, 53 to <64, 64 to <75, and ≥75 mmol/mol, respectively. The minimally adjusted model includes the patient’s calendar quarter of dialysis initiation. The case-mix–adjusted model includes minimally adjusted covariates plus age, sex, race, ethnicity, cause of ESRD, CCI, diabetes, CHF, and CVD. The expanded case-mix model includes case-mix covariates plus residential region, initial dialysis modality, and BMI. The expanded case-mix plus laboratory model includes expanded case-mix covariates plus serum albumin, hemoglobin, serum bicarbonate, eGFR averaged over the 1-year prelude period, and the last eGFR level measured prior to dialysis initiation.
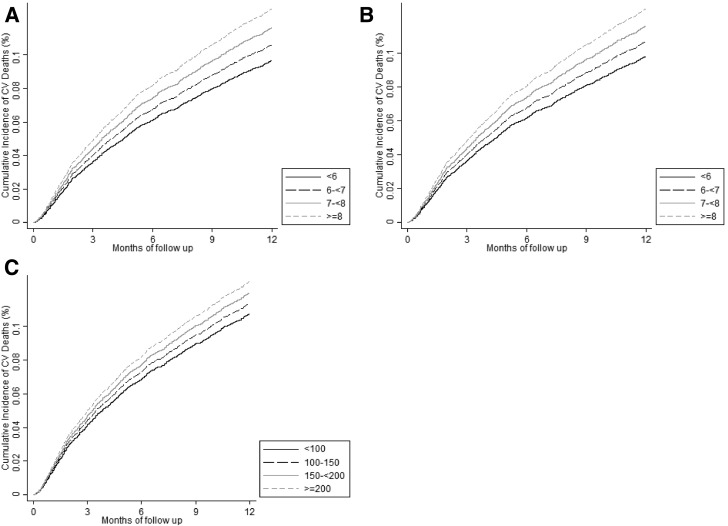
In secondary analyses using Fine and Gray competing risk regression to estimate cardiovascular mortality, case-mix, expanded case-mix, and expanded case-mix plus laboratory analyses showed that higher HbA1c levels ≥9% (≥75 mmol/mol) were associated with higher cardiovascular death risk in both the Overall and Restricted HbA1c Cohorts (Supplementary Fig. 2 and Supplementary Table 2). In case-mix–adjusted cumulative incidence curves of HbA1c (<6%, 6% to <7%, 7% to <8%, and ≥8% [<42, 42 to <53, 53 to <64, and ≥ 64 mmol/mol, respectively]) and cardiovascular mortality, higher HbA1c categories were incrementally associated with higher cardiovascular mortality in both the Overall and Restricted HbA1c Cohorts (Fig. 2A and B).
Figure 2.
Cumulative incidence curves of the association between HbA1c over the 1-year prelude period and 1-year cardiovascular (CV) mortality in the Overall Cohort (A) and Restricted Cohort (B). Association between mean random glucose over the 1-year prelude period and 1-year cardiovascular mortality (C). HbA1c levels of <6%, 6% to <7%, 7% to <8%, and ≥8%, which are equivalent to <42, 42 to <53, 53 to <64, and ≥64 mmol/mol, respectively. Random glucose levels of <100, 100 to <150, 150 to <200, and ≥200 mg/dL. Analyses adjusted for case-mix covariates including the patient’s calendar quarter of dialysis initiation, age, sex, race, ethnicity, cause of ESRD, CCI, diabetes, CHF, and CVD.
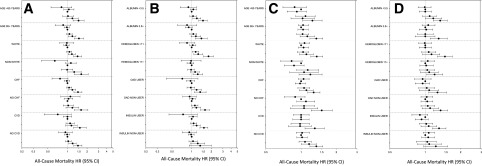
In case-mix–adjusted subgroup analyses, HbA1c levels ≥8% (≥64 mmol/mol) or ≥9% (≥75 mmol/mol) were associated with higher all-cause death risk across subgroups of age, race, CHF, CVD, serum albumin level, and oral antidiabetes drug use (Fig. 3A and B and Supplementary Table 3). Notably, among insulin users, HbA1c levels ≥8% (≥64 mmol/mol) were associated with higher all-cause mortality, whereas no association between HbA1c level and mortality was observed in nonusers.
Figure 3.
Subgroup analyses of the association between HbA1c (A and B) and mean random glucose (C and D) over the 1-year prelude period and 1-year all-cause mortality rate adjusted for case-mix covariates. HbA1c levels of <5%, 5% to <6%, 6% to <7% (reference), 7% to <8%, 8% to <9%, and ≥9% (<31, 31 to <42, 42 to <53 [reference], 53 to <64, 64 to <75, and ≥75 mmol/mol, respectively) shown as filled circles in descending order. Glucose levels <100, 100 to <125 (reference), 125 to <150, 150 to <175, 175 to <200, and ≥200 mg/dL shown as filled circles in descending order. The case-mix model includes patient’s calendar quarter of dialysis initiation, age, sex, race, ethnicity, and cause of ESRD, CCI, diabetes, CHF, and CVD. OAD, oral antidiabetes drug.
Mean Random Glucose and Mortality
In case-mix analyses, mean random glucose levels ≥200 mg/dL were associated with a 34% higher mortality; these associations persisted in expanded case-mix and expanded case-mix plus laboratory analyses (Supplementary Fig. 3A and Supplementary Table 4). Case-mix–adjusted Fine and Gray competing risks regression models showed that higher mean random glucose levels ≥200 mg/dL were associated with higher cardiovascular death risk in case-mix, expanded case-mix, and expanded case-mix plus laboratory analyses (Supplementary Fig. 3B and Supplementary Table 4). Case-mix–adjusted cumulative incidence curves showed that higher mean random glucose levels (categorized as <100, 100-<150, 150-<200, and ≥200 mg/dL) were incrementally associated with higher cardiovascular mortality (Fig. 2C).
In case-mix–adjusted subgroup analyses, higher mean random glucose levels exceeding a threshold of ≥175 or ≥200 mg/dL were associated with higher all-cause mortality across most subgroups (Fig. 3C and D and Supplementary Table 5). Notably, mean random glucose levels ≥200 mg/dL were associated with higher all-cause death risk in insulin users, whereas no association was observed in nonusers.
Conclusions
In a large national cohort of U.S. veterans with diabetic kidney disease newly transitioning to dialysis with longitudinal measures of glycemic status, higher HbA1c levels of ≥8 (≥64 mmol/mol) or ≥9% (≥75 mmol/mol) during the pre-ESRD prelude period were associated with higher all-cause and cardiovascular post-ESRD mortality independent of case-mix covariates. We also found that higher mean random glucose levels of ≥200 mg/dL were associated with higher all-cause and cardiovascular death risk. These observations were robust across multiple secondary and sensitivity analyses, which used sensitive versus specific criteria to define the source population with diabetes and accounted for noncardiovascular mortality as competing risks.
Although current clinical practice guidelines advise an HbA1c level of 7% (53 mmol/mol) for patients with CKD, with recommendations for a higher range among patients who are at risk for low glucose (patients with comorbidities, limited life expectancy, stage 4–5 CKD, and insulin/sulfonylurea use) (31,32,41,42), the upper HbA1c threshold remains undefined. Thus, there is considerable uncertainty with regard to the optimal precise glycemic level in patients with diabetes and CKD (2). Whereas multiple population-based studies show that both lower and higher HbA1c levels are associated with worse survival in patients with diabetes undergoing hemodialysis and peritoneal dialysis (12–17), there are comparatively fewer studies in those with NDD-CKD, which show conflicting findings (18,19). In a secondary analysis of ACCORD participants stratified according to CKD status by Papademetriou et al. (18), intensive versus conventional glycemic control (HbA1c target of <6% [<42 mmol/mol] vs. 7–9% [53–75 mmol/mol], respectively) was associated with higher all-cause and cardiovascular mortality in the CKD subgroup. In contrast, in a study of 23,296 patients with stage 3–4 CKD by Shurraw et al. (19), higher HbA1c categories >9% (>75 mmol/mol) were associated with CKD progression, cardiovascular events, hospitalization, and mortality, whereas spline analyses showed that both lower and higher HbA1c levels (<6.5% [48 mmol/mol] and >8% [>64 mmol/mol], respectively) were associated with a higher death risk.
As an extension of these prior works, our core objective was to focus on advanced CKD and newly transitioned ESRD patients with diabetes, and how their glycemic status in the immediate pre-ESRD period influences post-ESRD mortality within 1 year of dialysis initiation. Multiple large population-based studies of glycemic status in dialysis patients (12–17) have focused upon glycemic levels and outcomes restricted to the post-ESRD period only. For example, a U.K. study (43) of 3,157 patients with diabetes who initiated dialysis showed that higher HbA1c levels >8.5% (>69 mmol/mol) were associated with higher mortality among those who were <60 years of age; however, these observations do not inform the impact of glycemic status prior to the development of ESRD upon dialysis outcomes. Although the aforementioned study by Shurraw et al. (19) focused on glycemic status in predialysis CKD patients, only 401 patients progressed to ESRD and 3,665 patients died prior to the development of ESRD, providing limited insight into the potential sustained influence of glycemic control after development of end-organ kidney failure.
To our knowledge, ours is the first study to examine the association of glycemic status 1 year prior to transitioning to dialysis with post-ESRD mortality by focusing upon a large population of patients with diabetes and NDD-CKD transitioning to ESRD, thus providing insight into the impact of pre-ESRD glycemic status upon hard outcomes in dialysis patients. In case-mix analyses, higher mean HbA1c levels (≥8% [≥64 mmol/mol] or 9% [75 mmol/mol]) and random glucose levels (>200 mg/dL) in the pre-ESRD period were associated with higher all-cause and cardiovascular post-ESRD death risk, and these associations persisted across most subgroups. Our findings of an association between higher pre-ESRD glycemic status and worse post-ESRD survival are analogous to observations from the long-term follow-up of Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) and UK Prospective Diabetes Study (UKPDS) participants. In the DCCT/EDIC studies, despite a narrowing of HbA1c differences between intensive versus conventional control groups over time, those in the intensive arm had lower risks of fatal and nonfatal cardiovascular events (4) and all-cause mortality after a mean follow-up period of 27 years (5). Similarly, 10-year follow-up data from UKPDS survivors showed that the intensive group had a lower risk of cardiovascular events and all-cause death despite an attenuation in HbA1c differences by 1 year (8). Our data suggest that the avoidance of high glycemic levels (HbA1c level >8% [>64 mmol/mol] and random glucose level ≥200 mg/dL) in patients with diabetic kidney disease may have lasting benefit in reducing cardiovascular morbidity and mortality even after developing irreversible end-organ damage and transitioning to dialysis therapy, described as a “metabolic memory” or “legacy effect.” (8) Indeed, several potential pathways of glycemic status leading to a legacy effect on vascular outcomes relating to oxidative stress, advanced glycation processes, and epigenetic mechanisms have been described (40,44).
Another noteworthy observation was the differential association between glycemic status and survival according to antidiabetes medication use. Although HbA1c levels ≥8% (≥64 mmol/mol) and mean random glucose levels ≥200 mg/dL were associated with higher mortality among insulin users, no association was observed among nonusers. Although insulin use may be an indicator of having more severe diabetes, a recent study by Lin et al. (45) of 15,161 Taiwanese patients with diabetes undergoing dialysis showed that the use of certain oral antidiabetes drugs (e.g., sulfonylurea, meglitinides, and thiazolidinediones) was associated with a higher risk of myocardial infarction compared with insulin use. Hence, future studies are needed to determine whether the lowering of glycemic status with specific antidiabetes regimens improves survival among patients with diabetic kidney disease transitioning to ESRD.
The strengths of our study include its examination of a large national cohort of NDD-CKD patients transitioning to ESRD; the comprehensive availability of detailed patient-level information, including longitudinal laboratory and prescription data; and reduced confounding by differential health care access and nonuniform medical care. However, several limitations should be acknowledged. First, because HbA1c level may be influenced by factors other than glucose concentration in patients with advanced CKD (2,28,29), there may have been misclassification of glycemic status and confounding of the HbA1c-mortality association by nonglycemic factors. For example, studies (29) comparing HbA1c levels to glucose levels in patients undergoing dialysis have shown that HbA1c level may lead to an underestimation of hyperglycemia. However, clinical practice guidelines recommend HbA1c as the preferred metric of long-term glycemic control in the CKD population in conjunction with glucose monitoring (41). Even after accounting for key confounders in multivariable analyses and examining mean random glucose as an alternative glycemic metric, we observed persistent associations between higher glycemic level and death risk. Second, our definition of glycemic status was based on one or more HbA1c and/or random glucose levels averaged over the prelude period, but we cannot exclude the possibility of sampling bias due to differential frequency of testing (i.e., more frequent testing may reflect greater burden of illness). Third, we lacked glycemic data collected outside of the VA clinical setting (i.e., self-monitored blood glucose levels, laboratory tests conducted outside of the VA). However, the likelihood of missing data would have applied equally across patients of varying glycemic status (i.e., nondifferential misclassification), rendering estimates conservative. Fourth, given the observational nature of our study, our findings cannot confirm a causal relationship between glycemic status and mortality.
In conclusion, in a large national cohort of U.S. veterans with diabetic kidney disease newly transitioning to dialysis, higher HbA1c levels >8% and mean random glucose levels ≥200 mg/dL were independently associated with higher mortality in the first year after dialysis initiation. Future studies are needed to more granularly define the lower threshold of the optimal glycemic range in this population.
Supplementary Material
Article Information
Funding. This study was supported by a University of California Irvine Department of Medicine Chairman’s Award (C.M.R.), National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant U01-DK-102163 (K.K.-Z.), and resources from the U.S. Department of Veterans Affairs. The authors are supported by NIH/NIDDK research grants K23-DK-102903 (to C.M.R.), R01-DK-096920 (to C.P.K. and K.K.-Z.), U01-DK-102163 (to C.P.K. and K.K.-Z.), R01-DK-092232 (to D.V.N.), and K24-DK-091419 (to K.K.-Z.) and NIH/National Center for Advancing Translational Sciences grant UL1-TR-001414 (to D.V.N.). E.S. is supported by a career development award from the Office of Research & Development of the U.S. Department of Veterans Affairs (IK2-CX001266-01). The data reported here have been supplied by the USRDS. Support for VA/CMS data is provided by the U.S. Department of Veterans Affairs Veterans Health Administration, Office of Research & Development, and VA Information Resource Center (project nos. SDR 02-237 and 98-004).
The sponsors were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
C.P.K., E.S., G.A.B., and K.K.-Z. are employees of the U.S. Department of Veterans Affairs.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.M.R. designed the study, drafted the initial manuscript, and had the final responsibility for the decision to submit the manuscript. C.P.K. assisted in the study design, obtained study data, and critically reviewed the manuscript. V.A.R. and E.S. performed the analyses and critically reviewed the manuscript. S.M.B., M.S., K.S., M.Z.M., G.A.B., and D.V.N. critically reviewed the manuscript. K.K.-Z. designed the study, obtained study data, critically reviewed the manuscript, and had the final responsibility for the decision to submit the manuscript. All authors approved the final manuscript as submitted. C.M.R. and K.K.-Z. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a “Top Oral Abstracts by Trainees” oral abstract at Kidney Week 2015 (American Society of Nephrology), San Diego, CA, 3–8 November 2015 and as an abstract at the 18th International Congress on Renal Nutrition and Metabolism 2016, Okinawa, Japan, 19–23 April 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0110/-/DC1.
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as the official policy or interpretation of the U.S. Department of Veterans Affairs or the U.S. Government.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.United States Renal Data System USRDS 2015 Annual Data Report. Vol. 2. End-Stage Renal Disease in the United States. Bethesda, MD, United States Renal Data System, 2015 [Google Scholar]
- 2.Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial 2014;27:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orchard TJ, Nathan DM, Zinman B, et al.; Writing Group for the DCCT/EDIC Research Group . Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United Kingdom Prospective Diabetes Study (UKPDS) United Kingdom Prospective Diabetes Study (UKPDS). 13: relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995;310:83–88 [PMC free article] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 8.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 9.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 10.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 11.Ismail-Beigi F, Craven T, Banerji MA, et al.; ACCORD trial group . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duong U, Mehrotra R, Molnar MZ, et al. . Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol 2011;6:1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill CJ, Maxwell AP, Cardwell CR, et al. . Glycated hemoglobin and risk of death in diabetic patients treated with hemodialysis: a meta-analysis. Am J Kidney Dis 2014;63:84–94 [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. . A1C and survival in maintenance hemodialysis patients. Diabetes Care 2007;30:1049–1055 [DOI] [PubMed] [Google Scholar]
- 15.Ramirez SP, McCullough KP, Thumma JR, et al. . Hemoglobin A(1c) levels and mortality in the diabetic hemodialysis population: findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Diabetes Care 2012;35:2527–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricks J, Molnar MZ, Kovesdy CP, et al. . Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes 2012;61:708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams ME, Lacson E Jr, Wang W, Lazarus JM, Hakim R. Glycemic control and extended hemodialysis survival in patients with diabetes mellitus: comparative results of traditional and time-dependent Cox model analyses. Clin J Am Soc Nephrol 2010;5:1595–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papademetriou V, Lovato L, Doumas M, et al.; ACCORD Study Group . Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int 2015;87:649–659 [DOI] [PubMed] [Google Scholar]
- 19.Shurraw S, Hemmelgarn B, Lin M, et al.; Alberta Kidney Disease Network . Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med 2011;171:1920–1927 [DOI] [PubMed] [Google Scholar]
- 20.Foley RN, Chen SC, Solid CA, Gilbertson DT, Collins AJ. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int 2014;86:392–398 [DOI] [PubMed] [Google Scholar]
- 21.Lukowsky LR, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol 2012;35:548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Kovesdy CP, Streja E, et al. . Transition of care from prelude to renal replacement therapy in chronic kidney disease: the blueprints of an emerging field. Nephrol Dial Transplant 2017;32(Suppl. 2):ii91–ii98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molnar MZ, Gosmanova EO, Sumida K, et al. . Predialysis cardiovascular disease medication adherence and mortality after transition to dialysis. Am J Kidney Dis 2016;68:609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumida K, Molnar MZ, Potukuchi PK, et al. . Association of slopes of estimated glomerular filtration rate with post-end-stage renal disease mortality in patients with advanced chronic kidney disease transitioning to dialysis. Mayo Clin Proc 2016;91:196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumida K, Molnar MZ, Potukuchi PK, et al. Association between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal disease. Nephrol Dial Transplant. 30 May 2016 [Epub ahead of print]. DOI: 10.1093/ndt/gfw220 [DOI] [PMC free article] [PubMed]
- 26.Freedman BI. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: time to dispense with the hemoglobin A1C in end-stage kidney disease. Diabetes Care 2012;35:1621–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: A1C remains the gold standard outcome predictor in diabetic dialysis patients. Counterpoint. Diabetes Care 2012;35:1625–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovesdy CP, Sharma K, Kalantar-Zadeh K. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis 2008;52:766–777 [DOI] [PubMed] [Google Scholar]
- 29.Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients with diabetes: glycosylated hemoglobin or glycated albumin? Clin J Am Soc Nephrol 2011;6:1520–1522 [DOI] [PubMed] [Google Scholar]
- 30.Speeckaert M, Van Biesen W, Delanghe J, et al.; European Renal Best Practice Guideline Development Group on Diabetes in Advanced CKD . Are there better alternatives than haemoglobin A1c to estimate glycaemic control in the chronic kidney disease population? Nephrol Dial Transplant 2014;29:2167–2177 [DOI] [PubMed] [Google Scholar]
- 31.Kovesdy CP, Lott EH, Lu JL, et al. . Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation 2012;125:677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu JL, Molnar MZ, Naseer A, Mikkelsen MK, Kalantar-Zadeh K, Kovesdy CP. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol 2015;3:704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravel V, Ahmadi SF, Streja E, et al. . Pain and kidney function decline and mortality: a cohort study of US veterans. Am J Kidney Dis 2016;68:240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Department of Veterans Affairs; Health Services Research and Development Service . VA Information Resource Center. VIReC Research User Guide: VHA Medical SAS Datasets FY2006-2007. Hines, IL, VA Information Resource Center, 2007 [Google Scholar]
- 35.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 36.U.S. Department of Veterans Affairs; Health Services Research and Development Service . VA Information Resource Center. VIReC Research User Guide: VHA Pharmacy Prescription Data. 2nd ed. Hines, IL, VA Information Resource Center, 2008 [Google Scholar]
- 37.U.S. Department of Veterans Affairs; Health Services Research and Development Service . VA Information Resource Center. VIReC Research User Guide: VHA Decision Support System Clinical National Data Extracts. 2nd ed. Hines, IL, VA Information Resource Center, 2009 [Google Scholar]
- 38.U.S. Department of Veterans Affairs; Health Services Research and Development Service . VA Information Resource Center. VIReC Resource Guide: VA Corporate Data Warehouse. Hines, IL, VA Information Resource Center, 2012 [Google Scholar]
- 39.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray P, Chune GW, Raghavan VA. Legacy effects from DCCT and UKPDS: what they mean and implications for future diabetes trials. Curr Atheroscler Rep 2010;12:432–439 [DOI] [PubMed] [Google Scholar]
- 41.National Kidney Foundation KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis 2012;60:850–886 [DOI] [PubMed] [Google Scholar]
- 42.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150 [Google Scholar]
- 43.Adler A, Casula A, Steenkamp R, et al. . Association between glycemia and mortality in diabetic individuals on renal replacement therapy in the U.K. Diabetes Care 2014;37:1304–1311 [DOI] [PubMed] [Google Scholar]
- 44.Bianchi C, Miccoli R, Del Prato S. Hyperglycemia and vascular metabolic memory: truth or fiction? Curr Diab Rep 2013;13:403–410 [DOI] [PubMed] [Google Scholar]
- 45.Lin TT, Wu CC, Yang YH, et al. . Anti-hyperglycemic agents and new-onset acute myocardial infarction in diabetic patients with end-stage renal disease undergoing dialysis. PLoS One 2016;11:e0160436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.