Abstract
OBJECTIVE
During the Diabetes Control and Complications Trial (DCCT), intensive diabetes therapy achieving a mean HbA1c of ∼7% was associated with a threefold increase in the rate of severe hypoglycemia (defined as requiring assistance) compared with conventional diabetes therapy with a mean HbA1c of 9% (61.2 vs. 18.7 per 100 patient-years). After ∼30 years of follow-up, we investigated the rates of severe hypoglycemia in the DCCT/Epidemiology of Diabetes Inverventions and Complications (EDIC) cohort.
RESEARCH DESIGN AND METHODS
Rates of severe hypoglycemia were reported quarterly during DCCT and annually during EDIC (i.e., patient recall of episodes in the preceding 3 months). Risk factors influencing the rate of severe hypoglycemia over time were investigated.
RESULTS
One-half of the DCCT/EDIC cohort reported episodes of severe hypoglycemia. During EDIC, rates of severe hypoglycemia fell in the former DCCT intensive treatment group but rose in the former conventional treatment group, resulting in similar rates (40.8 vs. 36.6 episodes per 100 patient-years, respectively) with a relative risk of 1.12 (95% CI 0.91–1.37). A preceding episode of severe hypoglycemia was the most powerful predictor of subsequent episodes. Entry into the DCCT study as an adolescent was associated with an increased risk of severe hypoglycemia, whereas insulin pump use was associated with a lower risk. Severe hypoglycemia rates increased with lower HbA1c similarly among participants in both treatment groups.
CONCLUSIONS
Rates of severe hypoglycemia have equilibrated over time between the two DCCT/EDIC treatment groups in association with advancing duration of diabetes and similar HbA1c levels. Severe hypoglycemia persists and remains a challenge for patients with type 1 diabetes across their life span.
Introduction
Intensive treatment of type 1 diabetes (T1D) prevents and slows the progression of the long-term complications of diabetes, reducing vision loss, kidney failure, nerve dysfunction, cardiovascular disease, and mortality (1–7). The major adverse effect associated with intensive therapy has been an increased risk of severe hypoglycemia (SH), which is defined as episodes requiring assistance. During the 6.5-year average study follow-up in the Diabetes Control and Complications Trial (DCCT), participants randomized to intensive therapy and achieving a mean hemoglobin A1c (HbA1c) of 7.2% (55.6 mmol/mol) reported a threefold increased risk of SH (61.2 vs. 18.7 per 100 patient-years) compared with participants randomized to conventional therapy and achieving a mean HbA1c of 9.1% (76 mmol/mol) (8). A threefold elevated risk was also observed for the subset of SH that resulted in seizure or coma (8). More than two decades later, hypoglycemia remains the leading adverse effect of intensive diabetes therapy for patients with T1D (9–11). Data from the T1D Exchange Clinic Registry indicated that the risk of SH increases sharply in patients with >20 years duration of diabetes (12).
At the end of the DCCT, conventional treatment group participants were offered instruction in intensive therapy, and all participants were referred to their community health care providers for diabetes care. Ninety-seven percent of the original DCCT cohort enrolled in the observational Epidemiology of Diabetes Interventions and Complications (EDIC) study with annual follow-up evaluations (13). Over the course of EDIC, intensive diabetes management has evolved (14–17), and participants have adopted many advances in diabetes technology, including the introduction of rapid- and long-acting insulin analogs and improved insulin pumps and blood glucose meters. During EDIC, HbA1c levels have been ∼8.0% in both former treatment groups. We now have investigated the rates of SH and associated risk factors in DCCT/EDIC participants over the past 30 years and, importantly, the long-term impact of intensive diabetes management on rates of SH.
Research Design and Methods
Study Design
The DCCT/EDIC has been described in detail in previous reports (1,13). In brief, between 1983 and 1989, 1,441 participants with T1D age 13–39 years were enrolled in the DCCT, a multicenter, randomized controlled clinical trial that compared the effects of intensive versus conventional blood glucose management on the development and progression of early microvascular complications. Approximately one-half of the cohort was randomized to intensive therapy (n = 711), which included three or more daily injections of insulin or treatment with insulin pumps, with dose selection guided by frequent self-monitoring of blood glucose. The glycemia goals of intensive therapy were 1) preprandial blood glucose concentrations between 70 and 120 mg/dL (3.9 and 6.7 mmol/L), 2) postprandial concentrations of <180 mg/dL (10 mmol/L), 3) a weekly 3:00 a.m. measurement >65 mg/dL, and 4) monthly HbA1c within the normal range, specifically <6.05% (1). Intensive therapy participants attended monthly study visits and were contacted frequently by telephone to review and adjust their insulin regimens. Participants initially chose either multiple daily injection (MDI) or pump therapy and could subsequently change to the other method if their glycemic goals were not achieved or as a result of personal preference. The remaining participants (n = 730) were randomized to conventional therapy. By using one or two daily injections of insulin, the goals of conventional therapy were 1) absence of symptoms attributable to glycosuria or hyperglycemia; 2) absence of ketonuria; 3) clinical well-being with maintenance of normal growth, development, and ideal body weight; 4) freedom from symptoms related to hyperglycemia and hypoglycemia; and 5) HbA1c <13.1%. Conventional therapy participants were examined every 3 months. The DCCT included two cohorts. Participants in the primary prevention cohort had diabetes for 1–5 years, albumin excretion rate <40 mg/24 h, and no retinopathy. Participants in the secondary intervention cohort had diabetes for 1–15 years, albumin excretion rate ≤200 mg/24 h, and very mild to moderate nonproliferative retinopathy. Ninety-eight percent (1,394 of 1,428) of the surviving cohort joined the EDIC observational follow-up study to examine the long-term effects of the original DCCT therapies on micro- and macrovascular complications.
Definition of SH
SH was defined as an episode with symptoms or signs consistent with hypoglycemia in which the patient required the assistance of another person (e.g., as a result of confusion, coma, or seizure) and which was associated with a blood glucose level <50 mg/dL or prompt recovery after administration of oral carbohydrate, glucagon, or intravenous glucose. During the DCCT, both intensive and conventional treatment group participants were asked to report all episodes of suspected SH immediately by phone or at visits, and all participants were interviewed about the episodes through a standard series of questions. In addition, all participants were asked at the quarterly follow-up visits about the occurrence of any SH. To avoid the potential for recall bias, we restricted the ascertainment of events during EDIC to a 3-month window before the annual visit to get a more accurate annualized rate of events.
Statistical Methods
The crude event rates are presented as number of events per 100 patient-years on the basis of the ratio of observed number of events to total patient-years of exposure. The log-relative risk and its variance were estimated from the ratio of the crude event rates and used to assess the differences between groups (intensive vs. conventional). The rates are presented for the DCCT and EDIC periods separately. Events in EDIC were ascertained in the 3-month interval before the annual visit; therefore, the total patient-years of exposure during EDIC is based on 0.25 years per participant per visit. Thirty-four participants who were originally enrolled in the DCCT did not have an EDIC visit. Therefore, the EDIC period is based on 1,407 participants (699 of the original 711 in the intensive treatment group and 708 of the original 730 in the conventional treatment group).
Smoothed estimates of the risk (rate per 100 patient-years) over time were obtained by fitting a smooth function to the monthly crude rates during DCCT and EDIC (18). The Kaplan-Meier method was used to estimate the cumulative incidence of an event on the basis of the time to the first event in EDIC, regardless of a history of SH during DCCT. Discrete time intervals during EDIC were used. The difference between the cumulative incidence curves was tested by using the Mantel-Haenszel log-rank test (19), and the relative risk was estimated from the Cox proportional hazards model (19).
To assess the influence of the baseline and time-dependent covariates, a proportional hazards model was used to assess the risk of any SH (first episode) during EDIC, and a Poisson regression model was used to assess the risk of all SH (all episodes) during EDIC. Because of the highly significant relative risks, the χ2 value, which is proportional to R2, is presented as a measure of the relative importance of each covariate. Poisson regression models of time to first event were also used to describe the covariate effects on the absolute risk of any SH (20).
Analyses that used time-dependent covariates updated at each annual visit were used to assess the association between the covariate values at a given visit and the risk of any SH (first episode) and all SH (all episodes) before the next visit. These variables included the current insulin regimen (continuous subcutaneous insulin infusion vs. MDI vs. one to two injections per day) and current HbA1c value. All analyses used the natural log of the HbA1c value. The regression models express the log(risk) as a linear function of the log(HbA1c); therefore, the risk gradient is expressed as the percent change in risk for a fixed 10% reduction in the HbA1c calculated as (0.9β – 1) × 100, where β is the coefficient (slope) for log(HbA1c) (8).
The event rate and cumulative incidence were not estimated for the combined DCCT/EDIC period because of the differences in ascertainment of SH. Additional descriptions of absolute and relative risk models in this context are presented in the appendix of the prior DCCT hypoglycemia article (8). All results that are nominally significant at P < 0.05 with no adjustment for the effects of multiple tests or comparisons are presented.
Results
Participant Characteristics
The characteristics of the participants at DCCT closeout by original DCCT treatment group assignment are described in Supplementary Table 1. After an average 6.5 years of follow-up, men and women in the intensive treatment group had a significantly higher BMI than those in the conventional treatment group (P < 0.01). Participants in the intensive group had lower current and mean DCCT HbA1c levels (∼2% mean difference; P < 0.001) as well as higher insulin doses than those in the conventional group (mean difference 0.04 units/kg per day; P < 0.001). During the DCCT, pump use across all quarterly visits averaged 35.7% in the intensive group versus 0.7% in the conventional group and rose to 41% and 1.6%, respectively, by DCCT closeout (P < 0.001).
Frequency of SH
During the DCCT, the rates of SH, including episodes with seizure or coma, were approximately threefold greater in the intensive treatment group than in the conventional treatment group (Table 1). During EDIC, the frequency of SH increased in the former conventional group and decreased in the former intensive group so that the difference in SH event rates between the two groups was no longer significant (36.6 vs. 40.8 episodes per 100 patient-years, respectively; relative risk 1.12 [95% CI 0.91–1.35]) (Table 1).
Table 1.
Number of SH events and rate per 100 patient-years of follow-up in DCCT and EDIC by treatment group
| Conventional treatment |
Intensive treatment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Participants with an event | Number of Events | Rate | n | Participants with an event | Number of Events | Rate | RR (95% CI) (Intensive vs. conventional) | |
| Any hypoglycemia (first episode) | |||||||||
| SH | |||||||||
| DCCT | 730 | 255 (34.9) | 255 | 7.1† | 711 | 459 (64.6) | 459 | 19.0 | 2.67 (1.33–5.38)† |
| EDIC* | 708 | 351 (49.6) | 351 | 14.0 | 699 | 358 (51.2) | 358 | 14.7 | 1.05 (0.78–1.42) |
| Coma or seizure | |||||||||
| DCCT | 730 | 137 (18.8) | 137 | 3.3 | 711 | 271 (38.1) | 271 | 7.8 | 2.39 (0.86–6.68) |
| EDIC* | 708 | 192 (27.1) | 192 | 6.3 | 699 | 199 (28.5) | 199 | 6.5 | 1.04 (0.70–1.54) |
| All hypoglycemia (all episodes) | |||||||||
| SH | |||||||||
| DCCT | 730 | 255 (34.9) | 892 | 18.7† | 711 | 459 (64.6) | 2,896 | 61.2† | 3.28 (2.65–4.05)‡ |
| EDIC* | 708 | 351 (49.6) | 1,330 | 36.6 | 699 | 358 (51.2) | 1,483 | 40.8 | 1.12 (0.91–1.37) |
| Coma or seizure | |||||||||
| DCCT | 730 | 137 (18.8) | 257 | 5.4† | 711 | 271 (38.1) | 770 | 16.3 | 3.02 (2.36–3.86)‡ |
| EDIC* | 708 | 192 (27.1) | 433 | 11.9 | 699 | 199 (28.5) | 473 | 13.0 | 1.09 (0.83–1.44) |
Data are n (%) unless otherwise indicated. SH is defined as a hypoglycemic episode requiring assistance (see Research Design and Methods). Episodes with coma or seizure make up a subset of severe episodes. Rates are defined as episodes per 100 patient-years of follow-up. RR (intensive vs. conventional) was calculated as the ratio of event rates. The DCCT and EDIC periods were analyzed separately: First episode signifies the first episode during DCCT or the first episode during EDIC regardless of any prior DCCT hypoglycemic events, and the same is true for all episodes occurring distinctly within the two time periods. RR, relative risk.
*Number of events in 3-month interval before annual visit. Exposure is 0.25 years per patient per visit. Thirty-four DCCT participants did not have an EDIC visit.
†Difference between rates (DCCT vs. EDIC) is significant, P < 0.05.
‡Difference between RR values (DCCT vs. EDIC) is significant, P < 0.001.
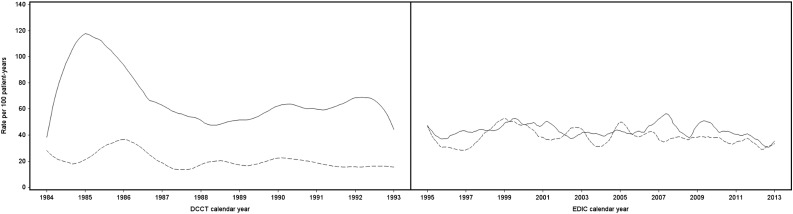
By the end of DCCT, with an average of 6.5 years of follow-up, 65% of the intensive group versus 35% of the conventional group experienced at least one episode of SH. In contrast, ∼50% of participants within each group reported an episode of SH during the 20 years of EDIC. The rates of SH were stable during this time and were similar for the former treatment groups (Fig. 1). Of these participants reporting episodes of SH, during the DCCT, 54% of the intensive group and 30% of the conventional group experienced four or more episodes, whereas in EDIC, 37% of the intensive group and 33% of the conventional group experienced four or more events (Supplementary Fig. 1). Moreover, a subset of participants (14% [99 of 714]) experienced nearly one-half of all SH episodes (1,765 of 3,788) in DCCT, and a subset of 7% (52 of 709) in EDIC experienced almost one-third of all SH episodes (888 of 2,813) (Supplementary Fig. 1). The cumulative incidence over time of the first SH event (Supplementary Fig. 2A) and SH causing seizure or coma (Supplementary Fig. 2B) during EDIC in the two treatment groups were virtually identical.
Figure 1.
Spline-smoothed estimates of SH per 100 patient-years by calendar time during DCCT (left) and EDIC (right). Solid lines represent rates for the intensive treatment group; dashed lines represent rates for the conventional treatment group.
Analyses Within Subgroups From DCCT Closeout
Supplementary Tables 2A and B describe the risk of all episodes of SH during EDIC and the relative risk of intensive versus conventional treatment within subgroups defined by DCCT closeout characteristics. During EDIC, the relative risk for SH after intensive versus conventional treatment during DCCT was ∼1.0 for all subgroup comparisons. The significant sex and HbA1c differences with regard to SH between the intensive and conventional groups during DCCT did not persist throughout EDIC. Similar to observations during the DCCT, participants with prior episodes of SH during EDIC had higher rates of subsequent SH. Other factors from DCCT closeout, including cohort (primary prevention vs. secondary intervention), age, education, intensity of exercise, BMI, duration of diabetes, insulin dose, and presence of cardiac autonomic neuropathy, did not have a demonstrable impact on the rate of SH during EDIC (Supplementary Table 2A and B).
Influence of Covariates on the Risk of SH
As shown in Table 2, history of SH causing seizure or coma during DCCT and lower current HbA1c during EDIC as a time-dependent covariate increased the risk of SH during EDIC in both treatment groups. Even after adjusting for other factors, a history of SH during the DCCT was the dominant predictor of the risk of any SH (i.e., a first EDIC episode) during EDIC in both groups. Having entered the DCCT study as an adolescent increased the risk of SH during EDIC. Conversely, use of continuous subcutaneous insulin infusion versus standard treatment and MDIs reduced the risk of SH during EDIC (Supplementary Tables 4 and 5).
Table 2.
Influence of history of SH during the DCCT and current (most recent) HbA1c on the risk of any SH (first episode) and all SH (all episodes) during EDIC
| Conventional therapy |
Intensive therapy |
|||||
|---|---|---|---|---|---|---|
| RR (95% CI) | P value | χ2 | RR (95% CI) | P value | χ2 | |
| Any SH (first episode) | ||||||
| SH | ||||||
| History of hypoglycemia (coma or seizure) during DCCT (yes/no) | 2.77 (2.17–3.55) | <0.0001 | 65.42 | 2.52 (2.03–3.14) | <0.0001 | 68.31 |
| Current HbA1c (%), time-dependent, per 10% decrease | 1.28 (1.23–1.33) | <0.0001 | 38.66 | 1.26 (1.22–1.31) | <0.0001 | 36.28 |
| Coma or seizure | ||||||
| History of hypoglycemia (coma or seizure) during DCCT (yes/no) | 2.86 (2.09–3.92) | <0.0001 | 42.79 | 2.74 (2.05–3.66) | <0.0001 | 45.91 |
| Current HbA1c (%), time-dependent, per 10% decrease | 1.37 (1.30–1.44) | <0.0001 | 37.12 | 1.20 (1.14–1.26) | 0.0003 | 13.15 |
| All SH (all episodes) | ||||||
| SH | ||||||
| History of hypoglycemia (coma or seizure) during DCCT (yes/no) | 2.15 (1.64–2.82) | <0.0001 | 30.84 | 3.18 (2.37–4.28) | <0.0001 | 58.68 |
| Current HbA1c (%), time-dependent, per 10% decrease | 1.28 (1.23–1.33) | <0.0001 | 41.48 | 1.31 (1.26–1.36) | <0.0001 | 47.35 |
| Coma or seizure | ||||||
| History of hypoglycemia (coma or seizure) during DCCT (yes/no) | 2.19 (1.61–2.99) | <0.0001 | 24.53 | 4.81 (3.34–6.93) | <0.0001 | 71.06 |
| Current HbA1c (%), time-dependent, per 10% decrease | 1.29 (1.23–1.35) | <0.0001 | 30.87 | 1.38 (1.29–1.47) | <0.0001 | 24.95 |
RRs were calculated from coefficients shown in Supplementary Tables 3 and 4 and jointly adjusted for other factors. Time to event used discrete time intervals during EDIC (regardless of prior DCCT hypoglycemia). Information about EDIC events was collected for the 3-month window before the annual visit. The RR, presented per 10% decrease in HbA1c, is 0.9β. χ2 test, which is proportional to a measure of R2, is presented to differentiate the effect of one covariate from another. RR, relative risk.
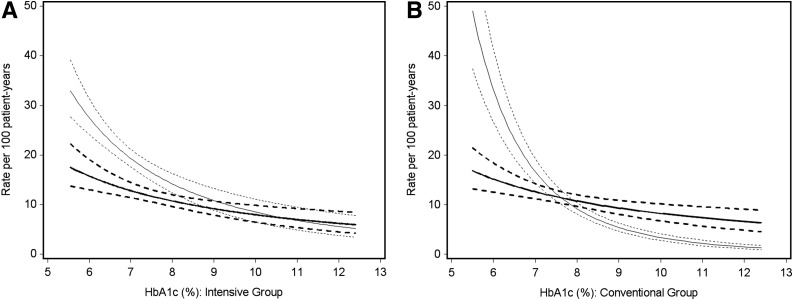
Figure 2 contrasts the absolute risk of SH (first episode) between DCCT and EDIC, as estimated from a Poisson regression model as a function of the HbA1c measured at the preceding visit during DCCT and EDIC, respectively. Within the intensive group, the risk of SH during DCCT increased by 27% for a 10% lower HbA1c during the DCCT (e.g., from 8.0 to 7.2%). For the same decrement in HbA1c during EDIC, the increased risk of SH in the intensive treatment group increased by only 15%. Within the conventional treatment group, the increase in risk for SH was 60% during the DCCT and only 13% during EDIC for a 10% lower HbA1c. In addition, the risk of SH was lower during EDIC than during DCCT in both groups for those who achieved HbA1c lower than the current target range of <7.0%. The unadjusted and HbA1c-adjusted relative risks of SH (first episode) during EDIC for intensive versus conventional treatment were 1.05 (95% CI 0.90–1.22) and 1.06 (95% CI 0.91–1.23), respectively.
Figure 2.
Risk of any SH (first episode) as a function of the HbA1c values for the intensive treatment group (monthly during DCCT and annually during EDIC) (A) and conventional treatment group (quarterly during DCCT and annually during EDIC) (B). The regression line and its 95% confidence band are provided by the simple exponential Poisson models presented in Supplementary Table 2. Bold lines represent the EDIC period; light lines represent the DCCT period.
Major Accidents
Fifty-one major accidents occurred during the 6.5 years of DCCT and 143 during the 20 years of EDIC (Supplementary Table 6). Of these events, the number of participants with an event and the number of events of each type were similar between the two treatment groups. The most frequent type of major accident was that involving a motor vehicle (operator or nonoperator). Supplementary Table 6 also examines the role of SH in these major accidents. Hypoglycemia played a role as a possible, probable, or principal cause in 18 of 28 operator-caused motor vehicle accidents (MVAs) during DCCT (9 in both treatment groups) and in 23 of 54 operator-caused MVAs during EDIC (13 in the conventional group, 10 in the intensive group).
Conclusions
The overall rate of SH demonstrated during DCCT has decreased and the relatively higher rate of SH associated with intensive treatment during DCCT has dissipated during EDIC. The equalization of SH rates between the two original treatment groups is largely attributable to their similar HbA1c levels during EDIC. During EDIC, the risk of SH remains strongly related to HbA1c, with a 13–15% rise in SH risk for every 10% decrement in HbA1c. The findings also illustrate the dangers of hypoglycemia in causing major MVAs because SH was the possible, probable, or principal cause of one-half of the MVAs when the participant was the driver (41 of 82 accidents). The number of MVAs per year (with the participant being the operator) was slightly less during EDIC than DCCT (2.0 vs. 4.3 per year), which may be related to fewer adolescent and younger adult drivers during EDIC than DCCT. Unfortunately, no age-adjusted national statistics exist by which the rate per person per year can be compared.
The rates of SH did not increase in either former treatment group during the 20 years of EDIC, and achievement of target HbA1c <7.0% during EDIC was associated with much lower rates of SH than during DCCT. This finding is consistent with the results of randomized clinical trials of new insulin analogs and improved methods of glucose monitoring that have shown that lower HbA1c could be obtained without increasing the risk of SH compared with control subjects (14–17). Advancements in the tools for diabetes management and additional clinical trials have also demonstrated the importance of educational programs to support intensive diabetes therapy (21). Thus, with increasing years of experience, participants have likely benefited from tailored educational efforts provided by treating physicians and certified diabetes educators to minimize hypoglycemia.
The T1D Exchange Clinic Registry recently reported that 8% of 4,831 adults with T1D living in the U.S. had a seizure or coma event during the 3 months before their most recent annual visit (11). During EDIC, we observed that 27% of the cohort experienced a coma or seizure event over the 20 years of 3-month reporting intervals (∼1.4% per year), a much lower annual risk than in the T1D Exchange Clinic Registry. In part, the open enrollment of patients into the T1D Exchange may be reflected without the exclusion of participants with a history of SH as in the DCCT and other clinical trials. The current data support the clinical perception that a small subset of individuals is more susceptible to SH (7% of patients with 11 or more SH episodes during EDIC, which represents 32% of all SH episodes in EDIC) (Supplementary Fig. 1). This observation calls for careful consideration of interventions, such as individualized glycemic goals, targeted education, and continuous glucose monitoring to reduce SH among those most susceptible. Moreover, with a history of SH, which is the best predictor of recurrent episodes independent of treatment intensity, early recognition of high-risk individuals and intervention are clinical imperatives.
Rates of SH remained remarkably stable in EDIC participants (Fig. 1) despite a mean duration of diabetes >28 years in the DCCT primary prevention cohort and >35 years in the secondary intervention cohort at the most recent annual EDIC assessment. This finding is in stark contrast to the report from the T1D Exchange (12) of a marked 50% increase in the risk of SH in adults with a T1D duration of 20–40 years and by >100% in patients with diabetes duration >40 years compared with adults with <20 years of T1D. In addition, during EDIC the rates of SH did not increase, even though the prevalence of cardiac autonomic neuropathy increased over time in EDIC participants (6,22). These observations may be related to the benefits of translating improved methods of treatment into better care of patients with long-standing T1D.
It was not surprising that a history of SH during DCCT and lower current HbA1c levels were the two major factors associated with an increased risk of SH during EDIC. Safety concerns were the reason why a history of frequent SH events was an exclusion criterion for enrollment in DCCT. We found that insulin treatment that used an insulin pump compared with injections was associated with a decreased risk of SH. This finding has been corroborated by numerous randomized clinical trials, clinical outcome studies, and patient registries (14–17). The rates of SH during EDIC were not affected by the level of education, usual level of physical activity, BMI, duration of diabetes, insulin dose, or the presence of cardiac autonomic neuropathy. During DCCT, intensive treatment of diabetes helped to sustain residual β-cell function and was associated with reduced SH rates (8). More recently, the long-term preservation of β-cell function, even after a duration of diabetes of two to three decades, has been reported (23,24). In our pilot study of 58 EDIC participants (i.e., all but one from the intensive treatment group), 17% had a clinically significant C-peptide response to mixed-meal tolerance testing (24). A full cohort investigation is ongoing in EDIC that will allow us to explore the long-term impact of preserved β-cell function on the frequency of SH and complications.
An increased risk of SH in T1D is associated with an altered physiologic response to low or falling blood glucose levels. In the individual without diabetes, the hierarchy of physiologic responses to hypoglycemia comprises 1) suppression of insulin secretion as the blood glucose level falls to ∼80–85 mg/dL and 2) a release of counterregulatory hormones (glucagon, epinephrine, cortisol, and growth hormone) when blood glucose falls to ∼65–70 mg/dL; the latter triggers 3) release of glucose from hepatic glycogen stores into the circulation and 4) neurogenic symptoms (at a blood glucose of ∼50–60 mg/dL), resulting in food-seeking behavior to prevent a further fall of blood glucose. In individuals with T1D (as well as those with insulin-deficient type 2 diabetes), the suppression of insulin as blood glucose levels fall cannot occur, and the release of glucagon in response to hypoglycemia is attenuated or absent. Because cortisol and growth hormone play small roles in the acute prevention of hypoglycemia, dependence on the release of epinephrine results as the final defense to trigger the hepatic release of glucose and the warning symptoms of impending hypoglycemia. If epinephrine release in response to hypoglycemia also fails or is attenuated or the glycemic threshold for its release is lowered, the patient is defenseless against neuroglycopenia-producing hypoglycemia (i.e., SH). This situation has been termed hypoglycemia-associated autonomic failure (HAAF) (25). HAAF is most notably triggered by recurrent antecedent hypoglycemia and increases the risk of subsequent SH. The results reported here show that the threefold increased rate of SH occurring during the mean 6.5 years of randomized intervention during DCCT did not translate to an increased risk of SH years or decades later during the 20-year follow-up in EDIC. For a similar HbA1c level, the rates of SH for a given HbA1c level below ∼7% are indeed lower during EDIC than during DCCT in both the intensive and the conventional therapy groups. In addition, the rates of SH in both groups during EDIC did not appear to increase over time. Although the presence or absence of HAAF was not directly determined during DCCT or EDIC, these observations suggest that the physiologic and behavioral responses to impending hypoglycemia remain stable (or at least do not worsen) over time and that the risk of developing HAAF does not increase with increasing duration of diabetes.
Of note, we found that participants who entered the DCCT as adolescents were more likely to experience SH during EDIC. During DCCT, adolescents experienced higher rates of SH than adults and worse glycemic control (8). Potential explanations of increased SH in adolescents during DCCT are both physiologic and behavioral challenges: 1) puberty wherein increased peripheral insulin resistance required larger doses of meal-time insulin (largely regular insulin at that time), which altered the pharmacokinetics and produced overshoot hyperinsulinemia that suppressed hepatic glucose production and resulted in hypoglycemia; 2) irregular bouts of exercise varying in time of day, intensity, and duration; 3) decreased counterregulatory responses during sleep; and 4) less diligent monitoring of blood glucose levels (26,27). Although newer insulin analogs have reduced overshoot hyperinsulinemia as a cause, one might speculate, as suggested above, that other physiologic and behavioral responses remained stable during EDIC, resulting in adolescents having an unfortunate increased risk of SH during EDIC.
The availability of a comprehensive set of longitudinal data related to hypoglycemia collected over 30 years in a dedicated group of patients with T1D is a major strength of this study. One limitation is the change in method of ascertaining SH during EDIC when we used a limited, retrospective review of the occurrence of SH over the 3 months before an EDIC annual visit. This approach lacks the rigor of the real-time reporting of SH that occurred during DCCT and raises the concern for underreporting of hypoglycemia during EDIC. If so, the true rates of SH would likely be higher in both treatment groups during EDIC than reported here.
In summary, although event rates in the DCCT/EDIC cohort seem to have fallen and stabilized over time, SH remains an ever-present threat for patients with T1D who use current technology, occurring at a rate of ∼36–41 episodes per 100 patient-years, even among those with longer diabetes duration. Having experienced one or more such prior events is the strongest predictor of a future SH episode. Efforts to prevent SH must continue. Further advances in the physiologic replacement of insulin, real-time continuous glucose monitoring, and continued behavioral support are needed to reduce the risk of SH in patients with T1D.
Supplementary Material
Article Information
Acknowledgments. David M. Nathan is acknowledged as the editor for DCCT/EDIC publications.
Funding. The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993, 2012–2017) and contracts (1982–2012) with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grant numbers U01-DK-094176 and U01-DK-094157) and through support by the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and the Clinical Translational Science Center Program (2006–present), Bethesda, MD.
Duality of Interest. Industry contributors played no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater NJ). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.A.G.-K. and J.M.L. obtained funding for the study and wrote the manuscript. B.H.B. and J.M.L. directed and conducted the statistical analyses. R.A.G.-K., B.H.B., N.H.W., F.J.S., J.M.L., and W.V.T. interpreted the data, wrote sections of the manuscript, and reviewed and edited the manuscript. R.S.S. contributed to interpreting the results and reviewing the manuscript. B.H.B. and J.M.L. contributed to the analysis plan specifications for the manuscript. B.H.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00360815 and NCT00360893, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-2723/-/DC1.
A complete list of the participants in the DCCT/EDIC Research Group can be found at http://www.nejm.org/doi/suppl/10.1056/NEJMoa1409463/suppl_file/nejmoa1409463_appendix.pdf.
References
- 1.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiello LP; DCCT/EDIC Research Group . Diabetic retinopathy and other ocular findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Boer IH; DCCT/EDIC Research Group . Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lachin JM, Orchard TJ, Nathan DM; DCCT/EDIC Research Group . Update on cardiovascular outcomes at 30 years of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group . Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orchard TJ, Nathan DM, Zinman B, et al.; Writing Group for the DCCT/EDIC Research Group . Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 9.Tamborlane WV. Severe hypoglycemia in youth with T1DM: going, going … but not yet gone. Pediatr Diabetes 2011;12:1–3 [DOI] [PubMed] [Google Scholar]
- 10.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014;63:2188–2195 [DOI] [PubMed] [Google Scholar]
- 11.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 12.Weinstock RS, Xing D, Maahs DM, et al.; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 13.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003;26:1079–1087 [DOI] [PubMed] [Google Scholar]
- 15.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765–774 [DOI] [PubMed] [Google Scholar]
- 16.Bergenstal RM, Tamborlane WV, Ahmann A, et al.; STAR 3 Study Group . Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 17.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCulloch PNJ. Generalized Linear Models. 2nd ed New York, Chapman and Idall, 1989 [Google Scholar]
- 19.Lee E. Statistical Methods for Survival Data Analysis. Belmont, CA, Lifetime Learning Publications, 1980 [Google Scholar]
- 20.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. 2nd ed. New York, John Wiley and Sons, 2011 [Google Scholar]
- 21.Little SA, Leelarathna L, Walkinshaw E, et al. . Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014;37:2114–2122 [DOI] [PubMed] [Google Scholar]
- 22.Pop-Busui R, Low PA, Waberski BH, et al.; DCCT/EDIC Research Group . Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009;119:2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keenan HA, Sun JK, Levine J, et al. . Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGee P, Steffes M, Nowicki M, et al.; DCCT/EDIC Research Group . Insulin secretion measured by stimulated C-peptide in long-established type 1 diabetes in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) cohort: a pilot study. Diabet Med 2014;31:1264–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagogo-Jack S. Philip E. Cryer, MD: seminal contributions to the understanding of hypoglycemia and glucose counterregulation and the discovery of HAAF (Cryer Syndrome). Diabetes Care 2015;38:2193–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones TW, Porter P, Sherwin RS, et al. . Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med 1998;338:1657–1662 [DOI] [PubMed] [Google Scholar]
- 27.Tamborlane, WV. Triple jeopardy: nocturnal hypoglycemia after exercise in the young with diabetes. J Clin Endocrinol Metab 92:815–816 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.