Abstract
Objective
To assess the utility of CT perfusion for selection of patients for endovascular therapy up to 18 hours after symptom onset.
Methods
We conducted a multicenter cohort study of consecutive acute stroke patients scheduled to undergo endovascular therapy within 90 min after a baseline CTP. Patients were classified as ‘target mismatch’ if they had a small ischemic core and a large penumbra on their baseline CT perfusion. Reperfusion was defined as >50% reduction in critical hypoperfusion between the baseline CT perfusion and the 36-hour follow-up MRI.
Results
Of the 201 patients enrolled, 190 patients with an adequate baseline CT perfusion study who underwent angiography were included; mean age 66 years, median NIHSS 16, median time from symptom onset to endovascular therapy 5.2 hours. Rate of reperfusion was 89%. In patients with target mismatch (n=131), reperfusion was associated with higher odds of favorable clinical response, defined as an improvement of ≥8 points on the NIH Stroke Scale (83% vs 44%, p=0.002; adjusted OR=6.6; 95% CI 2.1–20.9). This association did not differ between patients treated within 6 hrs (OR = 6.4; 95% CI 1.5–27.8) and those treated beyond 6 hrs after symptom onset (OR = 13.7; 95% CI 1.4–140).
Interpretation
The robust association between endovascular reperfusion and good outcome among patients with the CT perfusion target mismatch profile treated up to 18 hours after symptom onset supports a randomized trial of endovascular therapy in this patient population.
INTRODUCTION
Recent randomized controlled trials have demonstrated benefit from endovascular therapy for patients with acute ischemic stroke who were treated predominantly in the less than six-hour time-window.[1–5] Based on these studies, current guidelines recommend endovascular therapy for patients with occlusion of the ICA or MCA who can be treated within six hours after symptom onset.[6] Whether endovascular therapy is also beneficial for patients outside of the six-hour time-window is unknown.
While most studies have demonstrated a gradual decrease in the effectiveness of endovascular therapy with longer onset-to-treatment times[7–9], our group has shown that good outcome rates in reperfused patients who meet MRI diffusion-perfusion mismatch criteria remain relatively constant over time.[10] These findings suggest that patient selection criteria will play a key role in determining the outcome of endovascular thrombectomy trials in the extended time-window.
A main drawback of MRI-based patient selection is the small percentage of hospitals that have MR readily available for the assessment of acute stroke patients. Therefore, the time it takes to obtain an MRI scan to triage acute stroke patients is unacceptably long in most hospitals. Another drawback of MRI is the relatively large percentage of patients who have contra-indications to MRI because of metal implants (eg pacemakers) or claustrophobia. To overcome the limitations of MRI-based patient selection, CT perfusion imaging can be used instead. CT is much more widely available and has fewer contra-indications than MRI. It is, however, not known if CT perfusion -based patient selection is comparable to MRI. Before embarking on a randomized trial with image-based patient selection, we therefore conducted the CT perfusion to predict response to Recanalization in Ischemic Stroke Project (CRISP) study to examine the utility of CT perfusion in identifying patients who are likely to benefit from endovascular therapy.
METHODS
Study design
The CT perfusion to Predict Response to Recanalization in Ischemic Stroke Project (CRISP) was a multi-center prospective cohort study of acute stroke patients who underwent a CT perfusion scan before endovascular therapy. The institutional review board at each site approved the study, and informed consent for participation was obtained from each patient or, if the patient was mentally incompetent to consent, their proxy.
Study patients and protocol
Patients were enrolled at six US hospitals between 2012 and 2015. Patients were eligible for participation in the study if they: 1) were at least 18 years old; 2) had an ischemic stroke with an NIHSS score of 5 or more; 3) were scheduled to undergo endovascular therapy for the stroke; and 4) had undergone a CT perfusion and CT angiogram within 90 minutes prior to scheduled endovascular therapy. Patients who had a pre-existing illness resulting in a modified Rankin Scale Score of 2 or higher prior to the qualifying stroke were excluded.
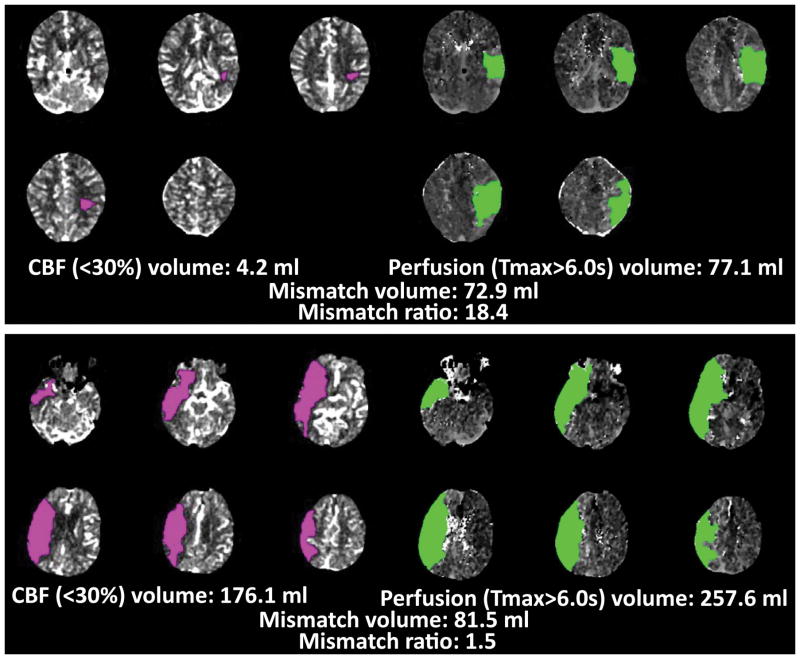
CT perfusion imaging was performed using the institutions’ routine protocols. CT perfusion studies were post-processed using RAPID (iSchemaView, Menlo Park) to generate maps of the ischemic core (rCBF<30%) and critically hypoperfused tissue (Tmax>6s). RAPID automatically segmented and calculated volumes of the ischemic core and of the critically hypoperfused tissue. These maps were available for viewing by local investigators, who determined patients’ target mismatch status based on the RAPID CT perfusion maps. Target mismatch was defined as a CBF core <70 mL, a Tmax>6s – core difference >15mL, a Tmax>6s/core ratio >1.8, and a Tmax>10s lesion <100 mL. (Figure 1)
Figure 1. CT perfusion target mismatch examples.
The top panel shows the CT perfusion study of a patient who has the target mismatch pattern. The ischemic core (pink lesion) is 4.2 mL. The volume of critically hypoperfused tissue (green lesion) is 77.1 mL. The absolute mismatch volume is 72.9 mL and the mismatch ratio is 18.4. All these values meet target mismatch criteria. The bottom panel shows the CT perfusion study of a patient who does not meet target mismatch criteria. The core lesion exceeds the 70 mL threshold and the mismatch ratio does not meet the >1.8 criterion.
While physicians could use the results of the non-contrast CT, the CT angiogram and their routine CT perfusion maps to guide treatment decisions, they were instructed not to use the RAPID maps to make treatment decisions. Consequently, patients underwent endovascular therapy regardless of their target mismatch status. The device and method used for endovascular therapy was based on operator preference and included treatment with stent retrievers, manual aspiration, intra-arterial thrombolytics, and/or angioplasty with or without stenting.
Patients underwent an early follow-up MRI, obtained within 36 hours after the baseline CT perfusion scan. Patients, who could not undergo a follow-up MRI because of a contra-indication, underwent a follow-up CT scan instead.
Patients returned for clinical follow-up on days 30 and 90. During these visits, trained investigators rated patients on the NIHSS, the modified Rankin Scale, the Glasgow Outcome Scale and the Barthel Index. If patients were unable to return to clinic, study coordinators made every attempt to visit the patients at their residence. If this was not possible, assessments were made by phone.
Clinical and radiological endpoints
The primary clinical endpoint was “favorable neurological response”, defined as an 8-point or more improvement on the NIHSS between baseline and day 30 or an NIHSS score of ≤1 at day 30. This endpoint was chosen because it is sensitive to the effects of early reperfusion and to match the primary endpoint of the DEFUSE 1 and 2 studies. The secondary clinical endpoint was functional independence, defined as mRS ≤2 on day 90. Symptomatic intracranial hemorrhage (sICH), defined as any intracranial hemorrhage associated with a ≥4-point worsening on the NIHSS, was used as a safety endpoint.
Core imaging laboratory
Stanford’s core imaging laboratory assessed 1) the ASPECT score on the baseline non-contrast CT scan; 2) the ischemic core and critical hypoperfused tissue volumes on the baseline CT perfusion maps, if needed after removal of imaging artifacts; 3) the location of the primary arterial occlusive lesion and the TICI reperfusion score on the digital subtraction angiography images; and 4) the volume of persistent critical hypoperfused tissue (Tmax>6s) and presence of hemorrhagic transformation on the follow-up MRI. Reperfusion was defined as >50% reduction in Tmax>6s lesion volume between baseline CT perfusion and the follow-up MRI, or TICI 2b/3 at completion of endovascular therapy if a follow-up MRI was not performed or technically inadequate.
Statistical analysis
Percentages of favorable neurological response were compared between groups using Fisher exact tests. The effect of reperfusion on binary outcomes was assessed using logistic regression with and without adjustment for imbalances in baseline variables. Baseline variables were entered into the multivariable model if they were significant at a p<0.1 level in univariate analysis, and were retained in the multivariate model if they were significant at a p<0.05. The effect of reperfusion on the overall distribution of outcomes on the modified Rankin Scale (shift analysis) was assessed with the assumption-free ordinal analysis that uses the Wilcoxan-Mann-Whitney generalized odds ratio.[11, 12] Analyses were conducted using SPSS and SAS software.
RESULTS
Of the 201 patients enrolled, 190 patients with anterior circulation strokes and a good quality baseline CT perfusion who underwent digital subtraction angiography were included in the primary analyses. Eleven patients were excluded; 2 withdrew from the study, 2 did not undergo catheter angiography, 6 because of inadequate baseline CT perfusion, and 1 had an occlusion in the posterior circulation. (figure 2) The mean age of the patient population was 66±15 yrs, median NIHSS 16 (IQR 12–20), and median time from symptom onset to endovascular therapy 5.2 hrs (IQR 3.8–7.9). Femoral puncture occurred >6hrs after symptom onset in 40% (n=75). The majority of these patients fell into the 6–9 hour window (61%), with fewer patients in the 9–12 hour (17%), and the >12 hour time window (21%). In 84% of the late (>6hr) treated patients, the exact time-of-onset was not known (i.e. wake-up strokes) and the time-of-onset was therefore based on the time last known well.
Figure 2. Flow diagram of study participants.
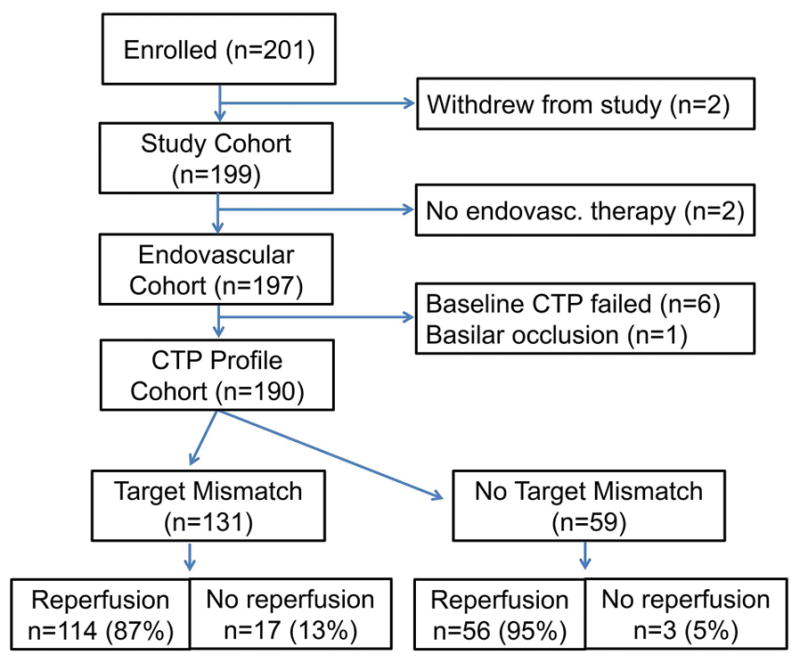
Of the 201 patients enrolled, 190 were included in the analyses of patients with baseline CT perfusion who underwent endovascular treatment. Of these, 131 had the target mismatch profile on CT perfusion, whereas 59 did not.
One hundred and thirty-one patients had the target mismatch profile. The baseline characteristics for patients with and without the target mismatch profile are listed in table 1. The percentage of patients with target mismatch was 62% in the <6 hours window, 83% in the 6–9 hour window, 85% in the 9–12 hour window, and 69% in the >12 hour window. Overall, the percentage of patients with target mismatch was lower in the <6-hour window than in the >6 hour-window (62% v 80%; p=0.01). Rate of reperfusion, determined primarily on the 36-hr perfusion scan was 90% (87% TICI 2b/3). The rate of reperfusion did not differ between patients with femoral puncture <6hrs versus >6hrs (90 v 89%; p=1.0) nor between patients with and without target mismatch (87 v 95%; p=0.13).
Table 1.
Baseline characteristics in patients included in the MRI profile cohort.
| Characteristic | Target Mismatch (n=131) | No Target Mismatch (n=59) |
|---|---|---|
| Mean (SD) age – yr | 66 (15) | 66 (15) |
| Female sex – no. (%)† | 75 (57) | 15 (25) |
| Hypertension – no. (%) | 86 (66) | 42 (71) |
| Diabetes mellitus – no. (%)* | 22 (17) | 20 (34) |
| Hyperlipidemia – no. (%) | 48 (37) | 28 (48) |
| Atrial fibrillation – no. (%) | 43 (33) | 17 (29) |
| Prior stroke/TIA – no. (%) | 12 (9) | 9 (15) |
| Median NIHSS score (IQR)† | 15 (11–19) | 20 (16–23) |
| Intravenous tPA pretreatment – no. (%) | 58 (44) | 30 (51) |
| Median (IQR) time from symptom onset to start of CTP – hrs* | 4·6 (3·2–7·0) | 3·5 (2·2–5~3) |
| Median (IQR) time from symptom onset to femoral puncture – hrs* | 5·6 (4·2–8·6) | 4·6 (3·3–6·0) |
| Median (IQR) time from arrival at study site to endovascular therapy – hrs | 1·3 (0·9–1·6) | 1.0 (0·8–1·6) |
| Median (IQR) volume of infarct core – ml† | 4 (0–13) | 23 (3–44) |
| Median (IQR) volume of perfusion lesion – ml† | 104 (69–144) | 194 (163–235) |
| Vessel occlusion on angiogram – no. (%) | ||
| Internal carotid artery, proximal | 13 (10) | 9 (15) |
| Internal carotid artery, distal | 22 (17) | 10 (17) |
| Middle cerebral artery, proximal | 73 (56) | 34 (58) |
| Middle cerebral artery, distal | 20 (15) | 6 (10) |
| Other | 2 (2) | 0 (0) |
| None | 0 (0) | 0 (0) |
p-value < 0.001;
p-value = 0.01
Among patients with the target mismatch (n=131), reperfusion was associated with increased odds of favorable clinical response (83% vs 44%, p=0.002; OR=6.1; 95% CI 2.0–18.4). This association remained significant when adjusted for age and NIHSS (OR=6.6; 95% CI 2.1–20.9). The results were similar with independence (mRS 0–2) as the outcome: patients with the target mismatch (n=131) had increased odds of independence with reperfusion in unadjusted analysis (66% vs 29%, p=0.007; OR=4.6; 95% CI 1.5–14.0) and after adjustment for NIHSS and age (OR=11.5; 95% CI 3.1–42.3). The shift analysis also showed improved functional outcome with reperfusion (Generalized OR = 2.7; 95% CI 2.4–3.0).
Rates of functional independence were similar in target mismatch patients with reperfusion who were treated <6hrs versus >6hrs (70% vs 62%; p = 0.4 unadjusted and p=0.2 adjusted) suggesting that time does not modify the effect of reperfusion among patients with target mismatch. Also, in multivariate analysis, the effect of reperfusion on functional independence among patients with the target mismatch was not modified by time-to-treatment (p=0.4 for the interaction between reperfusion and time-to-treatment when time is dichotomized at 6 hours; p=0.1 when time is modeled as a continuous variable). The adjusted odds ratio for independence with reperfusion was 11.2 (95% CI 2.4–53.4) in patients treated within 6 hours (n=71) compared to 18.2 (95% CI 1.7–201) in patients treated beyond 6 hours (n=60). (figure 3) When favorable clinical response was the endpoint instead of functional independence, results were similar with an adjusted OR of 6.4 (95% CI 1.5–27.8) in patients treated within 6 hours compared to 13.7 (95% CI 1.4–140) in patients treated beyond 6 hrs (p=0.6 for difference between odds ratios).
Figure 3. Functional outcome in patients with target mismatch stratified by reperfusion and time-to-treatment.
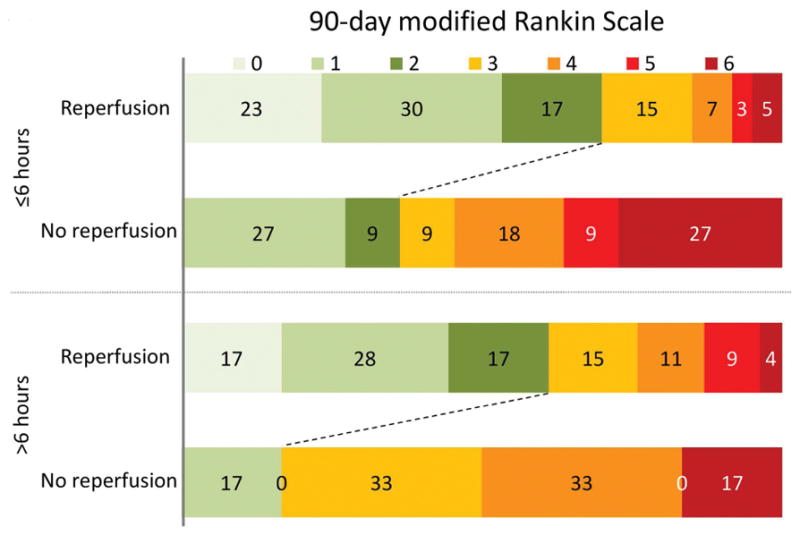
Among patients with the target mismatch (n=131), reperfusion was associated with an increased rate of functional independence on day 90. The adjusted odds ratio for independence with reperfusion was 11.2 (95% CI 2.4–53.4) in patients treated within 6 hours (n=71) compared to 18.2 (95% CI 1.7–201) in patients treated beyond 6 hours (n=60).
The effect of reperfusion could not be assessed in patients without the target mismatch because of the relatively small size (n=59) and the high rate of reperfusion (95%) of this subgroup. When compared to patients with target mismatch, the rate of functional independence in the setting of reperfusion was lower among patients without target mismatch in unadjusted analysis (45% vs 66%; p=0.01) but this difference did not remain significant after adjustment (p=0.2); the rate of favorable clinical response was lower in unadjusted (62% vs 83%; p=0.01) and adjusted (p=0.02) analysis. Patients who do not meet target mismatch criteria make up a heterogeneous population with large, small and matched lesion patterns (table 2). The most common pattern was a Tmax>10s lesion exceeding 100 mL and an ischemic core volume <70 mL (n=45). Forty-four of these patients reperfused and their rate of independence was not significantly different from target mismatch patients with reperfusion (52 v 66%; p=0.1).
Table 2.
Characteristics of patients without target mismatch.
| Type of lesion | Imaging Criteria | Total, n | Reperfusion, n | mRS 0–2 among reperfused (%) |
|---|---|---|---|---|
| No target mismatch | 59 | 56 | 45% | |
| Large (Tmax10) | rCBF <70mL Tmax10 >100mL |
45 | 44 | 52% |
| Large (ischemic core) | rCBF >70mL Tmax10 <100mL |
1 | 0 | - |
| Large (Tmax10 and ischemic core) | rCBF >70mL Tmax10 <100mL |
6 | 5 | 0% |
| Small | rCBF <15mL Tmax6 <15mL |
5 | 5 | 40% |
| Matched | rCBF 15–70mL Ratio Tmax6/rCBF <1.8 |
2 | 2 | 0% |
mRS indicates modified Rankin Scale; rCBF, relative cerebral blood flow; Tmax, time to the maximum of the residue function.
The overall rate of symptomatic ICH was 5.3%. Rates of symptomatic ICH were similar between patients who underwent endovascular therapy following intravenous tPA (n=88) and patients who did not receive intravenous tPA (n=102) (5.7% vs 4.9%, p=1.0) and between patients with symptom onset to groin puncture <6 and >6 hours (5.2 vs 5.3%, p=1.0). There was also no significant association between other baseline parameters (baseline NIHSS, age, core volume, Tmax6, time to treatment or reperfusion, target mismatch status, reperfusion) and sICH.
DISCUSSION
This study demonstrates a robust association between endovascular reperfusion and favorable clinical outcomes in patients who present with a target mismatch pattern on their baseline CT perfusion scan. It also shows that this association extends well beyond the currently established six-hour time-window for endovascular therapy for acute stroke. These results support randomized controlled trials of endovascular therapy in the extended time-window for patients with a target mismatch pattern on CT perfusion.
The findings of this study are consistent with prior studies that evaluated the response to reperfusion in patients selected with MR perfusion imaging. Specifically, in the DEFUSE 2 study, which had the same design as CRISP but used MRI instead of CT to classify patients, reperfusion was associated with a 26% (57% vs 31%) absolute increase of functional independence among patients with an MRI-based target mismatch profile. This is similar to the 37% (66% vs 29%) absolute increase in functional independence among patients with a CT-based target mismatch profile, observed in this study. These findings suggest that CT perfusion is as effective as MRI at selecting patients who are likely to benefit from endovascular therapy.
Our results are also consistent with the results of the EXTEND-IA study, a trial of early thrombectomy in patients selected with very similar CT perfusion imaging criteria.[3] Despite much earlier treatment in EXTEND-IA (median time 3.5 hrs) compared to CRISP (median time 5.6 hrs), the studies saw similar increases in functional independence with endovascular reperfusion (42% in EXTEND-IA and 37% in CRISP). The fact that treatment effects were similar despite a much shorter time-to-treatment in EXTEND-IA suggests that time may be a less critical factor among patients who meet mismatch criteria. This suggestion is supported by our finding that the positive association between reperfusion and rate of functional independence was similar among target mismatch patients who were treated early (<6 hours) and those who were treated late (>6 hours); a result that is consistent with our findings in DEFUSE 2, which demonstrated that the effect of reperfusion was similar among early and late treated patients with an MRI target mismatch pattern.[10] Our finding that rates of symptomatic intracranial hemorrhage (sICH) were almost identical among early and late treated patients (5.2 vs 5.3%) points to the safety of endovascular treatment of patients with target mismatch in the delayed time-window (>6 hours).
A pooled analysis of the endovascular treatment arms of 4 recent clinical trials showed a smaller gain in functional independence with reperfusion (12%) than the CRISP, DEFUSE 2 and EXTEND-IA studies.[13] Differences in study subjects and study methodology may account for this. First, most patients included in the pooled analysis were not selected with perfusion imaging and may therefore have had less tissue at risk of infarction than patients in the other studies. Second, in the pooled analysis reperfusion was defined as a TICI 2b or 3 score at the completion of the endovascular procedure, whereas reperfusion was assessed on early (<36 hours) follow-up perfusion scans in the other studies. While this is unlikely to account for large differences, the effect of reperfusion in the pooled analysis may have been attenuated some by patients with TICI 2b/3 reperfusion following mechanical thrombectomy who re-occluded the affected artery post-procedure.
Our study has limitations. First, because CRISP was a cohort study without a control group, no definitive conclusion can be drawn about the effect of endovascular treatment. The results are, however, suggestive that there may be benefit from endovascular therapy, even in the delayed time-window. Second, the very high reperfusion rate, a tribute to the efficacy of stent-retrievers, was unexpected and limited our ability to compare outcomes between patients with and without reperfusion. This was particularly true among patients without a target mismatch because virtually all patients without a target mismatch (68 of 71) reperfused. We can therefore not draw any direct conclusions about the effect of reperfusion among patients without target mismatch. The DEFUSE 2 study, which showed no association between reperfusion and good functional outcome in patients without target mismatch on MRI, suggests that there also is no (strong) association between reperfusion and good functional outcome in patients without target mismatch on CT. However, DEFUSE 2 was not powered to demonstrate an effect of reperfusion in patients without target mismatch. Moreover, results from the MR CLEAN study have shown no effect modification of endovascular treatment according to CT mismatch status.[14] Also, patients without target mismatch form a heterogeneous population of large, small and matched lesion patients (table 2), and it is likely that the response to reperfusion differs between these subsets. It is therefore possible that certain patients without a CT target mismatch do benefit from reperfusion, such as the subset of patients who had an ischemic core <70 mL but who were classified as non-target mismatch because of a large (>100 mL) lesion with a Tmax delay >10s. Third, because the exact time of stroke onset was unknown for most patients, a sub-analysis that excluded these patients was not feasible. Finally, while investigators were instructed not to use the perfusion maps generated by our automated CT perfusion analysis software for decision making regarding endovascular therapy, they could use their standard of care perfusion software for this purpose. Some patients were likely excluded from our study because they did not undergo endovascular therapy based on the results of the standard of care CT perfusion maps. Exclusion of patients on those grounds may have occurred preferentially in patients presenting late, given that the proportion of target mismatch was higher among patients who were treated beyond 6 hours and given that patients with target mismatch had longer symptom-onset to imaging times. While enrichment of our study population with target mismatch patients does not bias the main results of our study, the proportion of patients with target mismatch in the general stroke population is likely smaller than the 69% observed among all patients in this study and certainly lower than the 80% observed among patients treated after 6 hours.
In summary, the CRISP results provide compelling evidence that multimodal CT with CT perfusion can be used in an extended time-window to select patients who are likely to benefit from endovascular reperfusion. The favorable response to reperfusion among patients with a CT target mismatch pattern was similar to that observed in the DEFUSE 2 study among patients with an MR target mismatch pattern. Together these studies laid the foundation for the currently ongoing randomized controlled trial of endovascular therapy in patients selected with either multimodal CT or MR imaging (DEFUSE 3) (ClinicalTrials.gov, NCT02586415).
Acknowledgments
We appreciate the contributions of the members of the Data and Safety Monitoring board (Drs W. Smith, University of California at San Francisco (chair); J. English, California Pacific Medical Center; G. Howard, University of Alabama Birmingham; and Pina Sanelli, North Shore LIJ Health System). The study was funded by two grants from the National Institute for Neurological Disorders and Stroke (NINDS, Principal Investigators Drs M. Lansberg and G. Albers).
Footnotes
Author Contributions: MGL, SK, MM, RB, MS, GZ, MM and GA conceived and designed the study. MGL, SC, SK, MM, NM, CF, JPT, SK, RGN, TJ, TGD, NA, DRY, DH, SD, MPM, and GWA acquired and analyzed the data, and ML drafted the manuscript. The CRISP study group is further composed of D. Thai (Stanford Medical Center, Stanford, CA); K. Armbruster and S. DeCesare (University of Pittsburgh Medical Center, Pittsburgh, PA); B. Brion (St. Luke’s Hospital, Kansas City, MO); K. Schindler, and L. Craft (Emory University, Atlanta, GA); K. Barton and T. Massengale (Chattanooga Center for Neurologic Research, Chattanooga, TN); and K. Ramdas (University of Miami, Miami, FL)
Potential Conflicts of Interest
S. Christensen receives consultant fees from iSchemaView which produces the software used in this trial for post-processing of CT perfusion studies. T. Devlin received consulting fees and grant funding from Covidien/Medtronic which manufactures stent retrievers used to treat patients in this study. D.R. Yavagal serves on the trial steering committee of Covidien/Medtronic and received consulting fees from Covidien/Medtronic. T. Jovin received a research Grant from Stryker Neurovascular, consulting fees from Covidien/Medtronic, and payment for travel expenses from Stryker Neurovascular; all three companies manufacture stent retrievers used to treat patients in this study. M.P. Marks received consulting fees from Covidien/Medtronic. R.G. Nogueira received a research Grant from Stryker Neurovascular, serves on the Trial Exec. Committee for Penumbra, serves on the Trial Steering Committee for Covidien/Medtronic, and serves as the angiographic Core Lab for the STAR trial funded by Covidien/Medtronic; all three companies manufacture stent retrievers used to treat patients in this study. G.W. Albers received consulting fees from Covidien/Medtronic. G.W. Albers, R. Bammer and M. Straka received consulting fees and are shareholders of iSchemaView, which produces the software used in this trial for post-processing of CT perfusion studies and they hold a patent related to that software. None of the other authors (NA, CF, SD, DH, SK, SK, NM, MM, JT and GZ) have conflicts of interest.
References
- 1.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 7.Saver JL, Goyal M, van der Lugt A, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316:1279–1288. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 8.Khatri P, Abruzzo T, Yeatts SD, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khatri P, Yeatts SD, Mazighi M, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014;13:567–574. doi: 10.1016/S1474-4422(14)70066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lansberg MG, Cereda CW, Mlynash M, et al. Response to endovascular reperfusion is not time-dependent in patients with salvageable tissue. Neurology. 2015;85:708–714. doi: 10.1212/WNL.0000000000001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agresti A. Generalized Odds Ratios for Ordinal Data. Biometrics. 1980;36:59–67. [Google Scholar]
- 12.Churilov L, Arnup S, Johns H, et al. An improved method for simple, assumption-free ordinal analysis of the modified Rankin Scale using generalized odds ratios. Int J Stroke. 2014;9:999–1005. doi: 10.1111/ijs.12364. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BC, Hill MD, Rubiera M, et al. Safety and Efficacy of Solitaire Stent Thrombectomy: Individual Patient Data Meta-Analysis of Randomized Trials. Stroke. 2016;47:798–806. doi: 10.1161/STROKEAHA.115.012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borst J, Berkhemer OA, Roos YB, et al. Value of Computed Tomographic Perfusion-Based Patient Selection for Intra-Arterial Acute Ischemic Stroke Treatment. Stroke. 2015;46:3375–3382. doi: 10.1161/STROKEAHA.115.010564. [DOI] [PubMed] [Google Scholar]