Abstract
Background
Previous studies of cardiovascular disease (CVD) among HIV-infected individuals have been limited by the inability to validate and differentiate atherosclerotic type 1 myocardial infarctions (T1MIs) from other events. We sought to define the incidence of T1MIs and risk attributable to traditional and HIV-specific factors among participants in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), and compare adjusted incidence rates to the general population Atherosclerosis Risk in Communities (ARIC) cohort.
Methods
We ascertained and adjudicated incident MIs among individuals enrolled in seven NA-ACCORD cohorts between 1995–2014. We calculated incidence rates (IR), adjusted incidence rate ratios (aIRRs), and 95% confidence intervals ([,]) of risk factors for T1MI using Poisson regression. We compared aIRRs of T1MIs in NA-ACCORD with those from ARIC.
Results
Among 29,169 HIV-infected individuals, the IR for T1MIs was 2.57[2.30–2.86] per 1000 person-years, and the aIRR was significantly higher compared with participants in ARIC (1.30[1.09–1.56]). In multivariable analysis restricted to HIV-infected individuals and including traditional CVD risk factors, the rate of T1MI increased with decreasing CD4 count (≥500 cells/μL: ref; 350–499 cells/μL: aIRR=1.32[0.98–1.77]; 200–349 cells/μL: aIRR=1.37[1.01–1.86]; 100–199 cells/μL: aIRR=1.60[1.09–2.34]; <100 cells/μL: aIRR=2.19[1.44–3.33]). Risk associated with detectable HIV RNA (<400 copies/mL: ref; ≥400 copies/mL: aIRR=1.36 [1.06–1.75]) was significantly increased only when CD4 was excluded.
Conclusions
The higher incidence of T1MI in HIV-infected individuals and increased risk associated with lower CD4 count and detectable HIV RNA suggest that early suppressive antiretroviral treatment and aggressive management of traditional CVD risk factors are necessary to maximally reduce MI risk.
Keywords: HIV, cardiovascular disease, myocardial infarction, MI, CVD
Introduction
The introduction of effective combination antiretroviral therapy (ART) transformed HIV infection from a rapidly progressive fatal illness into a chronic manageable disease. However, HIV-infected individuals remain at increased risk for comorbid conditions that are associated with inflammation and aging in the general population, including cardiovascular disease (CVD)1,2. Although traditional CVD risk factors such as smoking are prevalent among HIV-infected individuals3, cumulative exposure to chronic inflammation and immune activation that persists in persons with treated HIV infection4–6 may also contribute to the development of atherosclerotic CVD (ASCVD)7–9.
Increases in subclinical atherosclerosis10–16, endothelial dysfunction17,18 and levels of inflammatory biomarkers19,20 that are associated with myocardial infarction (MI) in the general population occur in HIV-infected individuals. HIV infection has also been associated with risk for clinical CVD outcomes21–25. However, previous studies have relied on unvalidated MI events21,23–25 and not classified MIs by pathophysiologic mechanism as defined by the Universal Definition of MI (UDMI), a standard endorsed by international cardiology societies26, in order to focus on atherosclerotic type 1 MIs (T1MIs) and exclude type 2 MIs (T2MIs). Distinguishing between types is important because T2MIs result from an imbalance of myocardial oxygen supply and demand caused by a diverse set of clinical conditions, including sepsis and cocaine-induced vasospasm27, whereas T1MIs are due to spontaneous atherosclerotic plaque rupture, erosion, or dissection with associated intraluminal thrombus26. We have shown28 that T2MIs may account for a greater proportion of MIs among HIV-infected individuals as compared with what is seen in the general population due in part to the high prevalence of illicit drug use29 and concurrent infections among HIV-infected individuals.
Unvalidated or poorly defined outcomes in studies of the association of HIV infection with CVD22,23,30–32 may have contributed to inconsistent findings, and studies of MI incidence in HIV-infected individuals conducted in single healthcare systems22–24,33 may not have broad generalizability. To account for these limitations, we implemented a state-of-the-art centralized MI ascertainment, adjudication and classification protocol in the largest and most diverse cohort of HIV-infected individuals in North America. Classification of MI type enabled us to define the incidence and predictors of validated T1MI, while excluding those events that were secondary to conditions other than atherosclerosis. A primary aim of this study was to determine the risk of MI associated with HIV disease severity measured by current CD4 count and effective antiretroviral treatment measured by undetectable HIV RNA. In addition, we sought to compare adjusted MI incidence rates in HIV-infected individuals to those in the general population.
Methods
HIV-Infected Study Population: NA-ACCORD
The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) is the largest consortium of HIV cohorts in North America as previously described34. Briefly, NA-ACCORD consists of single and multi-site clinical and interval cohorts that prospectively collect data on >150,000 HIV-infected adults (≥18 years old) from more than 200 sites in the US and Canada. Each cohort has standardized methods of data collection and submits data on enrolled participant characteristics, diagnoses, laboratory measures, prescribed medications and vital status to the Data Management Core (University of Washington, Seattle WA) where they undergo quality control and are harmonized across cohorts. Data are then transmitted to the Epidemiology/Biostatistics Core (Johns Hopkins University, Baltimore, MD), which conducted the analyses presented here. For the present study, seven US clinical cohorts within NA-ACCORD with complete access to both inpatient and outpatient electronic medical record (EMR) data contributed information about 29,169 individuals enrolled on or after January 1, 1995 and followed up to March 31, 2014. NA-ACCORD has been approved by the local institutional review boards (IRB) of all participating cohorts.
General Population CVD Study Cohort: ARIC
We examined data collected on individuals aged ≥40 from a large, multi-center prospective, observational cohort study designed to assess CVD risk, the Atherosclerosis Risk in Communities (ARIC)35. ARIC was chosen because it is relatively contemporaneous with NA-ACCORD, has well-defined clinical outcomes, and captures an ethnically and racially diverse patient population comparable to NA-ACCORD. ARIC contributed 14,308 individuals aged 45–64 at baseline who enrolled between 1987–1989 and were followed through 2010. De-identified data were obtained through the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). While ARIC does not determine the HIV status of participants, the prevalence of HIV infection should be similar to that of the general population. NA-ACCORD IRB approval was provided prior to receipt of the BioLINCC data.
Primary Outcome: Type 1 MI
The protocol for ascertainment, validation and classification of MIs within NA-ACCORD has been previously published36. Potential MI events were centrally ascertained within the NA-ACCORD data repository using a standard protocol based on the presence of an MI diagnosis or elevated cardiac biomarkers. We have shown among HIV-infected adults that screening based on cardiac biomarkers in addition to diagnoses increases the sensitivity of identifying confirmed T1MIs compared with relying on diagnosis codes alone36. Comprehensive medical records pertaining to each potential event including clinician progress notes, electrocardiograms, laboratory measures, echocardiography results, and coronary catheterization and operative reports were abstracted from EMRs at the contributing site, de-identified and uploaded to the NA-ACCORD data repository. Information regarding antiretroviral (ARV) drugs used was redacted to avoid potential reviewer bias during adjudication. Sites attempted to obtain complete clinical data from potential events that occurred outside of their hospital system. Each potential event was adjudicated by at least two physician reviewers who have extensive experience adjudicating MIs in other CVD cohorts37,38. A third review was conducted if the adjudications of the first two reviewers differed. Potential events were classified as atherosclerotic T1MI or as T2MI according to the UDMI26. Reviewers also identified individuals who screened positive by diagnosis or cardiac biomarkers and underwent a cardiac intervention consistent with treatment of severe underlying coronary artery disease (coronary artery bypass graft or percutaneous coronary intervention with stent placement) but did not meet MI criteria. We excluded participants with prevalent MIs and those who had a T2MI in order to focus the analysis on atherosclerotic T1MIs rather than MIs that occur via other mechanisms. The primary outcome was an incident T1MI or invasive cardiac intervention.
ARIC has an established protocol for MI validation that incorporates clinical data35. However cardiac biomarkers are not used to screen for MI in ARIC, whereas they are part of the screening algorithm in NA-ACCORD. While this could lead to more potential MIs identified in NA-ACCORD compared with ARIC, we anticipated that a substantial portion of events identified by biomarkers alone would be T2MIs, which likely occur infrequently in ARIC by design since biomarkers are not used for ascertainment. Thus, by excluding T2MIs in NA-ACCORD from these analyses, we sought to maximize similarities between the two cohorts. Lastly, individuals with prevalent CVD events were excluded from ARIC in order to focus on incident MIs.
Covariates
For analyses of HIV-infected individuals in NA-ACCORD, we assessed the association of demographic and clinical variables with T1MI defined as follows. Race/ethnicity was self-reported and categorized as black, white, Hispanic, and other/unknown. An individual was classified as having ever or never smoked cigarettes based on clinician-recorded diagnoses and patient-reported responses to validated questionnaire items. Hypertension requiring pharmacologic treatment was defined as a clinical diagnosis of hypertension and prescription of antihypertensive medication. Diabetes mellitus was defined as a diagnosis of diabetes and prescription of a diabetes-related medication, or prescription of a diabetes-specific medication, or a glycated hemoglobin ≥6.5%. Dyslipidemia was defined based on serum lipid values prior to lipid-lowering treatment if applicable; elevated total cholesterol was defined as ≥240 mg/dL and low high-density lipoprotein (HDL) cholesterol was defined as ≤40 mg/dL for men and ≤50 mg/dL for women. Statin-treated dyslipidemia was defined as prescription of an HMG-CoA reductase inhibitor. We calculated estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease (CKD) Epidemiology Collaboration equation39 and required two measurements separated by 90 days, and dichotomized eGFR to represent CKD severity (eGFR <30 mL/min or ≥30 mL/min). Hepatitis C virus (HCV) coinfection was defined as ever having a positive HCV RNA, antibody or documented HCV genotype. History of an AIDS-defining illness was based on clinical diagnoses defined according to the 1993 CDC case definition40. CD4 counts were categorized using clinically meaningful cut points (<100, 100–199, 200–349, 350–499, and ≥500 cells/μL). Virologic suppression was defined as HIV RNA <400 copies/mL. ART was defined as three ARV agents from at least two classes or a triple nucleoside/nucleotide reverse transcriptase inhibitor regimen containing abacavir or tenofovir disoproxil fumarate.
For the analysis comparing HIV-infected adults in NA-ACCORD to participants in ARIC, age (40–49, 50–59, ≥60 years), sex, race, hypertension, diabetes mellitus, elevated total cholesterol (≥240 mg/dL) and cigarette smoking were assessed at study entry. In ARIC, self-reported race was categorized as black vs. non-black. Hypertension was defined as diastolic blood pressure >95mmHg, systolic blood pressure >160mmHg or self-report of current antihypertensive medication use. Diabetes was defined as random glucose ≥200mg/dL, fasting glucose ≥140mg/dL, or self-report of diabetes diagnosis or current diabetes medication use. An individual was classified as having ever or never smoked cigarettes based on responses to questionnaire items.
Statistical Analysis
In NA-ACCORD, person-time accrued for individuals from study entry defined as the latter of enrollment in the cohort or the date a cohort began full capture of inpatient and outpatient laboratory and diagnosis data (MI observation start date) until study exit defined as incident T1MI, death, cohort MI observation end date, one year after last CD4 count or HIV RNA measurement which was considered to be the time an individual was lost to follow-up or administrative censoring in 2014. Individuals who had a T2MI were excluded from the primary analysis, but were included in a sensitivity analysis and censored at the time of T2MI. In ARIC, person-time was accrued from enrollment (which initiated in 1987) until date of MI, death, lost to follow-up or censoring in 2010.
Age-specific crude incidence rates per 1,000 person years (IRs) and 95% confidence intervals (95% CI or [,]) were estimated for NA-ACCORD and ARIC. In analyses restricted to NA-ACCORD participants, adjusted incidence rate ratios (aIRR) and 95% CIs for T1MIs were estimated for the following time-fixed covariates: sex, race/ethnicity, HIV transmission risk, year of enrollment, cigarette smoking, HCV coinfection, and cohort. Time-updated covariates in the multivariable models included: age, hypertension, statin-treated dyslipidemia, diabetes mellitus, CKD, total and HDL cholesterol, CD4 count, detectable HIV RNA, AIDS-defining illness, and ART. HCV was omitted from the final model to avoid collinearity with injection drug use as a risk factor for HIV transmission, as was ART in order to evaluate the impact of effective ART use measured by undetectable HIV RNA. We hypothesized that the effect of HIV RNA on MI risk is at least partially mediated through CD4 count, so in a sensitivity analysis we analyzed HIV RNA without adjusting for CD4 count. Nearly a third of the study population was on ART at study entry inhibiting our ability to examine measures of cumulative HIV RNA. Finally, we examined IRs by calendar year in order to determine if the rate of T1MIs varied over calendar time.
We estimated aIRR and 95% CIs for MIs comparing HIV-infected participants in NA-ACCORD to participants in ARIC using Poisson regression to account for key baseline risk factors including age, sex, race, hypertension, diabetes, elevated total cholesterol, and cigarette smoking. All analyses were performed using SAS version 9.3 (SAS Institute) and a p-value <0.05 guided statistical interpretations.
Results
NA-ACCORD Incidence Rates
Among the 29,169 HIV-infected individuals in NA-ACCORD, 335 had an incident T1MI during 131,534 person-years of follow-up. Excluded from the analysis were 257 individuals who had a T2MI, nearly half of which were caused by sepsis and drug-induced vasospasm (e.g. cocaine). Median follow-up was 3.2 [IQR 1.3, 5.9] years among individuals with a T1MI and 3.6 [IQR 1.5, 7.0] years among those without a T1MI. The crude IR [95% CI] for T1MIs was 2.57 [2.30–2.86] per 1,000 person-years and increased significantly with each decade of age. Incidence rates for T1MIs did not vary significantly across calendar periods (data not shown). At study entry, NA-ACCORD participants who went on to have a T1MI were more likely to have been older, male, white, to have enrolled in the cohort in the early ART era (1995–2000), and to have had a history of cigarette smoking, hypertension, diabetes mellitus, statin-treated dyslipidemia, elevated total cholesterol, low HDL cholesterol, eGFR <30 mL/min, prior ARV use and an AIDS-defining illness (Table 1). A sensitivity analysis that included individuals with T2MI and censored them at the time of T2MI did not substantively change our results.
Table 1.
Characteristics of HIV-infected individuals in NA-ACCORD at study entry who experienced a Type 1 myocardial infarction (T1MI) and those who did not
| Characteristic | T1MI n=335 |
No T1MI n=28,577 |
|||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | <40 | 60 | 18 | 12,560 | 44 |
| 40–49 | 136 | 41 | 10,397 | 36 | |
| 50–59 | 109 | 33 | 4,591 | 16 | |
| ≥60 | 30 | 9 | 1,029 | 4 | |
|
| |||||
| Sex | Male | 287 | 86 | 22,997 | 80 |
| Female | 48 | 14 | 5,580 | 20 | |
|
| |||||
| Race/ethnicity | White | 188 | 56 | 13,245 | 46 |
| Black | 119 | 36 | 10,564 | 37 | |
| Hispanic | 18 | 5 | 2,997 | 10 | |
| Other/unknown | 10 | 3 | 1,771 | 6 | |
|
| |||||
| HIV transmission risk | MSM | 182 | 54 | 15,249 | 53 |
| IDU | 52 | 16 | 3,439 | 12 | |
| Heterosexual | 75 | 22 | 7,382 | 26 | |
| Other/unknown | 26 | 8 | 2,507 | 9 | |
|
| |||||
| Enrollment into cohort | 1995–2000 | 188 | 56 | 9,708 | 34 |
| 2001–2005 | 96 | 29 | 8,340 | 29 | |
| 2006–2014 | 51 | 15 | 10,529 | 37 | |
|
| |||||
| Cigarette smoking* | Never | 52 | 16 | 6,730 | 24 |
| Ever | 283 | 84 | 21,847 | 76 | |
|
| |||||
| Hypertension† | No | 191 | 57 | 24,092 | 84 |
| Yes | 144 | 43 | 4,485 | 16 | |
|
| |||||
| Diabetes mellitus‡ | No | 285 | 85 | 27,277 | 95 |
| Yes | 50 | 15 | 1,300 | 5 | |
|
| |||||
| Elevated total cholesterol§ | No | 260 | 78 | 23,519 | 82 |
| Yes | 72 | 21 | 2,638 | 9 | |
| Unknown | 3 | 1 | 2,420 | 8 | |
|
| |||||
| Low HDL cholesterol|| | No | 196 | 59 | 15,020 | 53 |
| Yes | 126 | 38 | 8,311 | 29 | |
| Unknown | 13 | 4 | 5,246 | 18 | |
|
| |||||
| Statin-treated dyslipidemia¶ | No | 295 | 88 | 27,401 | 96 |
| Yes | 40 | 12 | 1,176 | 4 | |
|
| |||||
| Chronic kidney disease** | eGFR ≥30 | 315 | 94 | 28,390 | 99 |
| eGFR <30 | 20 | 6 | 187 | 1 | |
|
| |||||
| CD4 count (cells/mm3) | <100 | 71 | 21 | 4,369 | 15 |
| 100–199 | 49 | 15 | 3,175 | 11 | |
| 200–349 | 72 | 21 | 6,003 | 21 | |
| 350–499 | 51 | 15 | 5,809 | 20 | |
| ≥500 | 87 | 26 | 8,965 | 31 | |
| Unknown | 5 | 1 | 256 | 1 | |
|
| |||||
| HIV viral load (copies/mL) | <400 | 98 | 29 | 8,676 | 30 |
| 400–9,999 | 84 | 25 | 6,591 | 23 | |
| 10,000–99,999 | 87 | 26 | 7,691 | 27 | |
| ≥100,000 | 63 | 19 | 5,264 | 18 | |
| Unknown | 3 | 1 | 355 | 1 | |
|
| |||||
| History of AIDS-defining illness†† | No | 230 | 69 | 22,475 | 79 |
| Yes | 105 | 31 | 6,102 | 21 | |
|
| |||||
| Prior ARV use‡‡ | No | 135 | 40 | 14,465 | 51 |
| Yes | 200 | 60 | 14,112 | 49 | |
|
| |||||
| Hepatitis C infection§§ | No | 248 | 74 | 23,317 | 82 |
| Yes | 87 | 26 | 5,260 | 18 | |
NA-ACCORD: North American AIDS Cohort Collaboration on Research and Design
HDL: high-density lipoprotein
ARV: antiretroviral
An individual was classified as having ever or never smoked cigarettes based on clinician-recorded diagnoses and patient-reported responses to validated questionnaire items
Hypertension requiring pharmacologic treatment was defined as a clinical diagnosis of hypertension and prescription of antihypertensive medication
Diabetes mellitus was defined as a diagnosis of diabetes and prescription of a diabetes-related medication, or prescription of a diabetes-specific mediation, or a glycated hemoglobin (HbA1c) ≥6.5%
Elevated total cholesterol was defined as ≥240 mg/dL based on serum lipid values prior to lipid-lowering treatment if applicable
Low HDL cholesterol was defined as ≤40 mg/dL for men and ≤50 mg/dL for women based on serum lipid values prior to lipid-lowering treatment if applicable
Statin-treated dyslipidemia was defined as prescription of an HMG-CoA reductase inhibitor
eGFR was calculated using the CKD-Epi equation (38) and required two measurements separated by 90 days
History of an AIDS-defining illness was based on clinical diagnoses defined according to the 1993 CDC case definition
Prior use of any antiretroviral medication(s)
Hepatitis C infection was defined as ever having a positive HCV RNA, antibody, or a documented HCV genotype
Risk Factors for Atherosclerotic Type 1 MIs in NA-ACCORD
In multivariable analysis examining factors associated with risk of T1MI among HIV-infected individuals in NA-ACCORD, traditional CVD risk factors (aIRR [95% CI]) including time-updated age, hypertension, diabetes, statin-treated dyslipidemia, and eGFR <30 mL/min were independent predictors of incident T1MI (Table 2). In addition to CVD risk factors, we found an increased risk of T1MI with lower time-updated CD4 count across strata. Detectable HIV RNA did not reach statistical significance (1.20 [0.92, 1.56] in the main model, whereas in sensitivity analysis excluding CD4 count, time-updated detectable HIV RNA was significantly associated with increased risk of T1MI (1.36 [1.06–1.75]).
Table 2.
Multivariable analysis of time-updated traditional CVD and HIV-related factors* in association with risk of Type 1 myocardial infarction (T1MI) among HIV-infected individuals in NA-ACCORD
| Characteristic | T1MI Risk | ||
|---|---|---|---|
| aIRR | 95% CI | ||
| Age (years) | <40 | 1.00 | |
| 40–49 | 2.92 | [1.88, 4.52] | |
| 50–59 | 4.04 | [2.57, 6.37] | |
| 60–69 | 6.47 | [3.91, 10.70] | |
|
| |||
| Sex | Male | 1.00 | |
| Female | 0.75 | [0.52, 1.07] | |
|
| |||
| Race/ethnicity | White | 1.00 | |
| Black | 0.72 | [0.54, 0.95] | |
| Hispanic | 0.65 | [0.39, 1.07] | |
| Other/unknown | 0.60 | [0.32, 1.14] | |
|
| |||
| HIV transmission risk | MSM | 1.00 | |
| IDU | 1.11 | [0.78, 1.57] | |
| Heterosexual | 0.87 | [0.62, 1.22] | |
| Other/unknown | 1.09 | [0.71, 1.67] | |
|
| |||
| Enrollment into cohort | 1995–2000 | 1.00 | |
| 2001–2005 | 0.65 | [0.50, 0.85] | |
| 2006–2014 | 0.55 | [0.39, 0.79] | |
|
| |||
| Cigarette smoking | Never | 1.00 | |
| Ever | 1.47 | [1.08, 2.00] | |
|
| |||
| Hypertension | No | 1.00 | |
| Yes | 2.49 | [1.93, 3.20] | |
|
| |||
| Diabetes mellitus | No | 1.00 | |
| Yes | 1.40 | [1.05, 1.86] | |
|
| |||
| Elevated total cholesterol | No | 1.00 | |
| Yes | 1.23 | [0.96, 1.58] | |
|
| |||
| Low HDL cholesterol | No | 1.00 | |
| Yes | 1.16 | [0.89, 1.52] | |
|
| |||
| Statin-treated dyslipidemia | No | 1.00 | |
| Yes | 1.90 | [1.46, 2.48] | |
|
| |||
| Chronic kidney disease | eGFR ≥30 | 1.00 | |
| eGFR <30 | 6.03 | [4.11, 8.85] | |
|
| |||
| CD4 count (cells/mm3) | <100 | 2.19 | [1.44, 3.33] |
| 100–199 | 1.60 | [1.09, 2.34] | |
| 200–349 | 1.37 | [1.01, 1.86] | |
| 350–499 | 1.32 | [0.98, 1.77] | |
| ≥500 | 1.00 | ||
|
| |||
| HIV viral load (copies/mL) | <400 | 1.00 | |
| ≥400 | 1.20 | [0.92, 1.56] | |
CVD: cardiovascular disease
NA-ACCORD: North American AIDS Cohort Collaboration on Research and Design
HDL: high-density lipoprotein
aIRR: adjusted incidence rate ratio
Bold signals statistical significance (p<0.05)
NA-ACCORD variable definitions, see Table 1
Comparing MI incidence in NA-ACCORD to ARIC
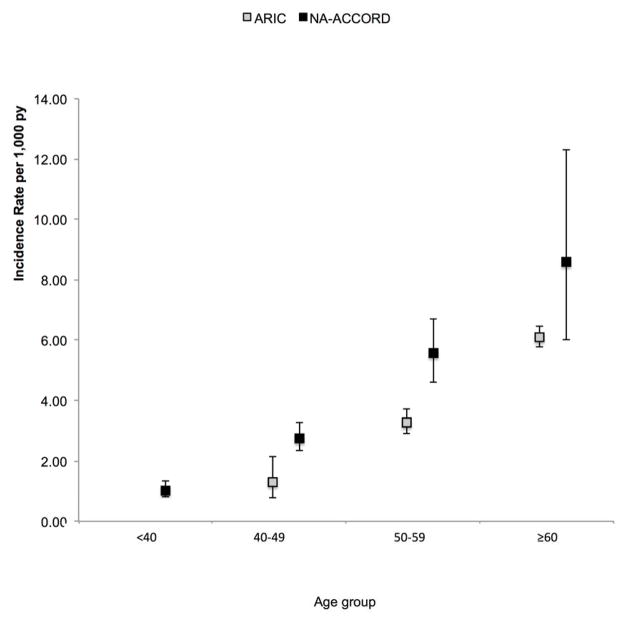
ARIC participants contributed 1,448 MI events and 281,284 person-years of follow-up. HIV-infected individuals in NA-ACCORD were younger (NA-ACCORD age <40: 43%, 40–49: 36%, 50–59: 16%, ≥60: 4%; ARIC age 40–49: 32%, 50–59: 45%, ≥60: 23%), more likely to be male (NA-ACCORD 80%; ARIC 45%) and of black race (NA-ACCORD 37%; ARIC 26%) than participants in ARIC (Appendix Table 1), while ARIC participants had a greater prevalence of hypertension (NA-ACCORD 16%; ARIC 28%) and diabetes (NA-ACCORD 5%; ARIC 10%). Age-specific MI IRs were higher in NA-ACCORD than ARIC (Fig 1). In multivariable analysis, HIV-infected individuals in NA-ACCORD had significantly higher adjusted rates of MIs compared with participants in ARIC (Table 3). As expected, increased age, male sex, race, hypertension, diabetes, elevated total cholesterol, and cigarette smoking were all significantly associated with risk of MI independent of HIV infection status. HIV infection was significantly associated with increased risk of MI (aIRR 1.21 [95% CI 1.02, 1.45]). A sensitivity analysis excluding individuals <40 years of age from NA-ACCORD showed similar results.
Figure 1.
Incidence Rates of Myocardial Infarction by Age per 1,000 Person Years among HIV-infected individuals in NA-ACCORD and the general population in ARIC.
Table 3.
Adjusted risk of myocardial infarction among HIV-infected individuals (NA-ACCORD*) compared with the general population (ARIC†)
| Characteristic | NA-ACCORDvs. ARIC | ||
|---|---|---|---|
| aIRR | 95% CI | ||
| Cohort | General population (ARIC) | 1.00 | |
| HIV-infected population (NA-ACCORD) | 1.21 | [1.02, 1.45] | |
|
| |||
| Age (years) | 40–49 | 1.00 | |
| 50–59 | 1.72 | [1.39, 2.13] | |
| ≥60 | 2.97 | [2.38, 3.71] | |
|
| |||
| Sex | Male | 1.00 | |
| Female | 0.60 | [0.54, 0.66] | |
|
| |||
| Race | Non-black | 1.00 | |
| Black | 0.90 | [0.81, 1.00] | |
|
| |||
| Cigarette smoking | Never | 1.00 | |
| Ever | 1.53 | [1.38, 1.71] | |
|
| |||
| Hypertension | No | 1.00 | |
| Yes | 1.80 | [1.63, 1.99] | |
|
| |||
| Diabetes | No | 1.00 | |
| Yes | 2.51 | [2.22, 2.84] | |
|
| |||
| Elevated total cholesterol | No | 1.00 | |
| Yes | 1.51 | [1.36, 1.67] | |
NA-ACCORD: North American AIDS Cohort Collaboration on Research and Design
ARIC: Atherosclerosis Risk in Communities
aIRR: adjusted incidence rate ratio
Bold signals statistical significance (p<0.05)
NA-ACCORD variable definitions, see Table 1
ARIC variable definitions: race was self-reported and categorized as black vs. non-black; an individual was classified as having ever or never smoked cigarettes based on patient-reported responses to validated questionnaire items; hypertension was defined as diastolic blood pressure >95mmHg, systolic blood pressure <160mmHg, or self-report of current antihypertensive medication use; diabetes was defined as random glucose ≥200mg/dL, fasting glucose ≤140mg/dL, self-report of diabetes diagnosis or self-report of current diabetes medication use; elevated total cholesterol was defined as ≥240 mg/dL
Discussion
This study is the first to define the incidence of adjudicated T1MIs and associated clinical risk factors in HIV-infected individuals. Our analysis, from the largest cohort collaboration of HIV-infected persons in North America, found significantly higher adjusted rates of MIs than observed among the general population. The large number of well-characterized T1MIs observed in NA-ACCORD enabled us to examine multiple factors simultaneously, including known CVD risk factors, in order to define the independent association between HIV-specific factors and ASCVD.
After adjusting for traditional CVD risk factors, we found that having lower CD4 counts was significantly associated with increased risk of T1MI, and that this relationship was dose-dependent by CD4 strata. There was over 2-fold higher risk of MI among individuals with a CD4 <100 cells/uL compared to those with a CD4 ≥500 cells/uL, a magnitude similar to the risk associated with hypertension or cigarette smoking. Our findings suggest that individuals at successively lower CD4 count levels, indicative of increasing severity of poorly controlled HIV infection and immune dysfunction, are at greater risk of MI. Combined, the results of our main model and sensitivity analysis are consistent with our hypothesis that both undetectable HIV RNA, an accurate measure of effective ART use, and increased CD4 count are associated with decreased risk of MI. Furthermore, as expected, the risk associated with HIV RNA is partially mediated by CD4 count. Our results are consistent with the Strategies for Management of Antiretroviral Therapy (SMART) study that found significantly lower risk of major CVD events among persons randomized to continuous treatment with ART as opposed to delay or interruption of ART41. Similarly, our goal was to determine the impact of virally suppressive ART and so we did not examine individual ARV agents for which findings to date regarding CVD risk remain inconsistent21,42,43. Thus, while effective ART may reduce the risk of CVD, risk may vary by specific ARV agent. Our findings provide further evidence of the benefit of HIV treatment to prevent not only AIDS-defining illnesses44, but also important HIV-associated chronic conditions2,45,46 including ASCVD33,41 that can occur regardless of CD4 count, but are more common among individuals with lower CD4 counts47.
Traditional CVD risk factors including metabolic derangements, such as diabetes and dyslipidemia, were also independent predictors of incident T1MI. Analysis of many modifiable CVD risk factors in HIV-infected individuals is complex given that both HIV infection itself48,49 and some older ARV drugs25,50 have been linked to metabolic changes that are associated with atherosclerosis in the general population. Our results are consistent with an independent benefit of ART-mediated viral suppression on MI risk after accounting for the effect of traditional CVD risk factors, regardless of their etiology. While one study performed in a large HMO showed decreasing MI risk in HIV-infected individuals in recent years33, we did not observe a similar trend, likely owing to more inclusive event ascertainment in NA-ACCORD, adjudication of outcomes, and greater demographic and socioeconomic diversity within our study population. Furthermore, a newer study found increasing CVD mortality among HIV-infected persons in recent years.51 For clinicians caring for HIV-infected persons, these findings provide additional evidence to support the importance of aggressive management of both modifiable HIV-specific and traditional CVD risk factors, including early suppressive ART and a renewed clinical focus on smoking cessation, as well as screening for and treatment of hypertension, dyslipidemia and diabetes mellitus to reduce the overall burden of ASCVD in HIV-infected individuals.
Our analysis has several strengths. We conducted this study in the largest, most diverse cohort of HIV-infected individuals in North America. In contrast to previous studies conducted in single healthcare systems, the diversity of our cohort with regard to geographic, demographic and clinical characteristics including the full spectrum of HIV disease severity and comorbidities make our findings more broadly applicable to HIV-infected persons in settings where treatment with ART is readily available. To our knowledge, ours is the first study of MI rates in HIV-infected individuals to incorporate cardiac biomarker data as a means of screening for potential MIs (as opposed to use in event validation) that might have been missed had we relied on diagnoses alone. This allowed us to more completely capture the burden of ASCVD and define robust age-specific rates that demonstrate the absolute rate of T1MI in the aging HIV-infected population. Although contemporary troponin assays may also increase the sensitivity of detecting T2MIs52, we excluded these events in order to focus our analysis on ASCVD risk, which made our outcome more comparable to the general population cohort where the vast majority of MIs were likely T1MIs. An expert panel of physicians centrally reviewed detailed medical records for each potential MI event to adjudicate and classify confirmed MIs by type according to the UDMI. In contrast, most prior studies involving HIV-infected persons defined MI outcomes using diagnosis codes without undertaking MI event validation21,23–25 leading to potential misclassification and under or over estimation of true event rates53–55. The few studies conducted among HIV-infected persons that did validate outcomes may have also underestimated MI incidence by relying on diagnosis codes alone to ascertain events22,32 or review of case report forms completed by local site personnel32 rather than centralized expert adjudication of comprehensive primary clinical data, which is unique to our study.
The importance of distinguishing T1MIs and T2MIs is increasingly recognized in the general population52, and essential in HIV-infected populations given the large proportion of T2MIs identified in our cohort that would have been misclassified as presumptive atherosclerotic outcomes in previous studies. Because prior studies in HIV-infected persons did not differentiate T1MIs from T2MIs, our estimates provide the most accurate assessment to date of the impact of HIV infection on atherosclerotic MIs in HIV-infected individuals. The magnitude of excess risk seen when comparing NA-ACCORD to ARIC is somewhat smaller than that seen in prior studies that did not differentiate MIs by type, suggesting that some of the excess MI risk in HIV-infected individuals found in previous studies was the result of higher rates of T2MI in this population. Because the mechanisms and prevention of these types of events differ, clinicians will need distinct approaches to minimizing risk of T1MI and T2MI in HIV-infected persons.
Our study has important limitations. We examined known clinical and behavioral CVD risk factors, but diet and exercise were unmeasured and data regarding cigarette smoking may have been incomplete. Our analysis did not include silent MIs or sudden fatal MIs that may have occurred outside of the healthcare setting and could not have been captured by our protocol. By ascertaining potential events using both outpatient and inpatient MI diagnoses and collecting records from outside hospitals for independent review, we attempted to capture events that may have been managed outside of our sites’ hospital systems. While the possibility remains that we may not have captured all events in NA-ACCORD, were this to be the case, we would have underestimated MI incidence and found an even greater difference in incidence rates between HIV-infected individuals and those seen in the general population. Our comparison to ARIC was potentially limited by differences in variable definitions and event ascertainment, specifically that ARIC did not use cardiac biomarkers in screening or differentiate between T1MIs and T2MIs. However, excluding T2MIs from the NA-ACCORD analysis likely increased the comparability of outcomes since cardiac biomarkers would be expected to disproportionately identify T2MIs. Furthermore, had we included T2MIs in our analysis, the higher risk of MIs seen in HIV-infected individuals would have been even more pronounced. We adjusted for key traditional CVD risk factors, but other factors including potential socioeconomic differences between cohorts, may have impacted our results. However, our findings are consistent with estimates from comparisons between HIV-infected and uninfected individuals within a single health care system22–24.
In summary, by focusing our analysis on T1MIs and comparing incidence rates among HIV-infected individuals within a large and diverse cohort with rates from a well-characterized general population-based CVD cohort, we have shown with broad generalizability that HIV infection is independently associated with MI risk and provided robust estimates of the risk associated with HIV-specific factors compared with traditional CVD factors. Our results suggest that clinicians need to both modify traditional CVD risk factors, and suppress HIV viral replication and boost CD4 count by initiating early and continuous ART to maximally reduce the risk of ASCVD in HIV-infected individuals.
Supplementary Material
Acknowledgments
The authors acknowledge NA-ACCORD Collaborating Cohorts and Representatives including AIDS Link to the IntraVenous Experience: Gregory D. Kirk; Adult AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson, Ronald J. Bosch, and Ann C. Collier; Fenway Health HIV Cohort: Stephen Boswell, Chris Grasso, and Kenneth H. Mayer; HAART Observational Medical Evaluation and Research: Robert S. Hogg, P. Richard Harrigan, Julio SG Montaner, Angela Cescon, and Hasina Samji; HIV Outpatient Study: John T. Brooks and Kate Buchacz; HIV Research Network: Kelly A. Gebo and Richard D. Moore; Johns Hopkins HIV Clinical Cohort: Richard D. Moore; John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez; Kaiser Permanente Mid-Atlantic States: Michael A. Horberg; Kaiser Permanente Northern California: Michael J. Silverberg; Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne; Multicenter Hemophilia Cohort Study-II: James J. Goedert; Multicenter AIDS Cohort Study: Lisa P. Jacobson and Gypsyamber D’Souza; Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein; Ontario HIV Treatment Network Cohort Study: Sean B. Rourke, Ann N. Burchell, and Anita R. Rachlis; Retrovirus Research Center, Bayamon Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor; Southern Alberta Clinic Cohort: M. John Gill; Studies of the Consequences of the Protease Inhibitor Era: Steven G. Deeks and Jeffrey N. Martin; The Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Pragna Patel and John T. Brooks; University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig; University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik; University of Washington HIV Cohort: Mari M. Kitahata, Heidi M. Crane, and Daniel R. Drozd; Veterans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin; Vanderbilt-Meharry Centers for AIDS Research Cohort: Timothy R. Sterling, David Haas, Sally Bebawy, and Megan Turner; Women’s Interagency HIV Study: Stephen J. Gange and Kathryn Anastos; NA-ACCORD Study Administration; Executive Committee: Richard D. Moore, Michael S. Saag, Stephen J. Gange, Mari M. Kitahata, Keri N. Althoff, Rosemary G. McKaig, Amy C. Justice, and Aimee M. Freeman; Administrative Core: Richard D. Moore, Aimee M. Freeman, and Carol Lent; Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Daniel R. Drozd, Liz Morton, Justin McReynolds, and William B. Lober; Epidemiology and Biostatistics Core: Stephen J. Gange, Keri N. Althoff, Alison G. Abraham, Bryan Lau, Jinbing Zhang, Yuezhou Jing, Elizabeth Golub, Shari Modur, Cherise Wong, Adell Mendes, and Brenna Hogan.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflicts of Interest and Sources of Funding:
This work was supported by the National Institutes of Health (Grants U01-AI069918, U01-AA013566, U24-AA020794, U01-AA020790, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-AI69432, U01-AI69434, U01-HD32632, U10-EY08057, U10-EY08052, U10- EY08067, UL1-RR024131, UL1-TR000083, U54- MD007587, F31-DA035713, G12- MD007583, KL2-TR000421, K01-AI070001, K01-AI071754, K01-AI093197, K23 EY013707, K24-00432, MO1-RR-00052, N02-CP55504, P30-AI027763, P30-AI094189, P30-AI27757, P30-AI27767, P30-AI50410, P30-AI54999, P30-AI036219, P30-MH62246, R01-CA165937, R01-AA16893, R01-DA11602, R01-DA04334, R01-DA12568, R24-AI067039, R56-AI102622, R56-HL 126538, R01-HL 126538, Z01-CP010214, R01-DA026770 and Z01-CP010176 from the National Institutes of Health, USA); contract CDC200-2006-18797 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; grants TGF-96118, HCP-97105, CBR-86906, CBR-94036 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. The following authors report consultancies: GAB (Definicare), LPJ (Bristol-Myers Squibb), JJE (Gilead Sciences, Merck, ViiV Healthcare, Janssen Pharmaceuticals, Bristol-Myers Squibb), DBK (Gilead Sciences, Merck, Viiv Healthcare, Bristol-Myers Squibb, Abbvie), MJS (Gilead Sciences, Merck, Bristol-Myers Squibb, Tivo); honoraria for advice or public speaking: FJP (Gilead Sciences, Janssen Pharmaceuticals, Merck, Bristol-Myers Squibb), RAE (Gilead Sciences, Merck, ViiV Healthcare), JJE (Gilead Sciences, Merck, ViiV Healthcare, Janssen Pharmaceuticals, Bristol-Myers Squibb); expert testimony: RAE (Gilead Sciences); grants: KNA (NIH), GAB (Bristol-Myers Squibb, Amgen), MJS (Pfizer, Merck), SRH (NIH, American Heart Association), MJB (NIH, General Electric), JACD (NIH), CW (NIH), RAE (Gilead Sciences, Merck, Janssen Pharmaceuticals, ViiV Healthcare), MJS (Merck, Bristol-Myers Squibb, Abbvie, ViiV Healthcare, Janssen Pharmaceuticals), RDM (NIH); royalties: RAE (Gilead Sciences, Merck, ViiV Healthcare); advisory board: DRD (Gilead Sciences), KNA (Gilead Sciences), RAE (Gilead Sciences, Merck, ViiV Healthcare), DBK (Abbvie, Bristol-Myers Squibb, ViiV Healthcare), MJS (Gilead Sciences, Merck, Bristol-Myers Squibb); medical education: RAE (Gilead Sciences, Merck, Janssen Pharmaceuticals, ViiV Healthcare), RDM (Medscape). For the remaining authors none were declared.
References
- 1.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. Bmj. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 2.Currier JS, Lundgren JD, Carr A, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008 Jul 8;118(2):e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saves M, Chene G, Ducimetiere P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003 Jul 15;37(2):292–298. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 4.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009 Oct 15;200(8):1212–1215. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 5.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010 Jun 15;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003 May 15;187(10):1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 7.Maggi P, Maserati R, Antonelli G. Atherosclerosis in HIV patients: a new face for an old disease? AIDS Rev. 2006 Jan 1;8(4):204–209. [PubMed] [Google Scholar]
- 8.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012 Jun;205(Suppl 3):S375–382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013 Oct 17;39(4):633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsue PY, Scherzer R, Hunt PW, et al. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. Journal of the American Heart Association. 2012 Apr;1(2) doi: 10.1161/JAHA.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009 Sep 10;23(14):1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. Aids. 2008 Aug 20;22(13):1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009 Jun 1;23(9):1059–1067. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metkus TS, Brown T, Budoff M, et al. HIV Infection is Associated with Increased Coronary Non-Calcified Plaque Among Participants with Coronary Artery Calcium Score of Zero: Multicenter AIDS Cohort Study (MACS) Journal of the American College of Cardiology. 2014;63(12_S) [Google Scholar]
- 15.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014 Apr 1;160(7):458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010 Jan 16;24(2):243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho JE, Deeks SG, Hecht FM, et al. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. AIDS. 2010 Jul 31;24(12):1897–1905. doi: 10.1097/QAD.0b013e32833bee44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. Journal of the American College of Cardiology. 2008 Aug 12;52(7):569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. Journal of acquired immune deficiency syndromes. 2009 Jul 1;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. Journal of acquired immune deficiency syndromes. 2011 Jul 1;57(3):245–253. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 22.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013 Apr 22;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. Journal of acquired immune deficiency syndromes. 2014 Feb 1;65(2):160–166. doi: 10.1097/QAI.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 24.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007 Jul;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Currier JS, Taylor A, Boyd F, et al. Coronary Heart Disease in HIV-Infected Individuals. Journal of acquired immune deficiency syndromes. 2003 Aug 1;33(4):506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 26.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Journal of the American College of Cardiology. 2012 Oct 16;60(16):1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Saaby L, Poulsen TS, Hosbond S, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013 Sep;126(9):789–797. doi: 10.1016/j.amjmed.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Crane H, Paramsothy P, Drozd D, et al. Types of myocardial infarction among HIV-infected individuals in the United States. JAMA Cardiol. 2016 doi: 10.1001/jamacardio.2016.5139. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cofrancesco J, Jr, Scherzer R, Tien PC, et al. Illicit drug use and HIV treatment outcomes in a US cohort. AIDS. 2008 Jan 30;22(3):357–365. doi: 10.1097/QAD.0b013e3282f3cc21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang S, Mary-Krause M, Simon A, et al. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis. 2012 Aug;55(4):600–607. doi: 10.1093/cid/cis489. [DOI] [PubMed] [Google Scholar]
- 31.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010 Dec 15;55(5):615–619. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007 Apr 26;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 33.Klein DB, Leyden WA, Xu L, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015 Apr 15;60(8):1278–1280. doi: 10.1093/cid/civ014. [DOI] [PubMed] [Google Scholar]
- 34.Gange SJ, Kitahata MM, Saag MS, et al. Cohort Profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) International journal of epidemiology. 2007 Jan 8; doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989 Apr;129(4):687–702. [PubMed] [Google Scholar]
- 36.Crane HM, Heckbert SR, Drozd DR, et al. Lessons learned from the design and implementation of myocardial infarction adjudication tailored for HIV clinical cohorts. Am J Epidemiol. 2014 Apr 15;179(8):996–1005. doi: 10.1093/aje/kwu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Multi-Ethnic Study of Atherosclerosis (MESA) Coordinating Center. MESA Field Center Manual of Operations. 2001. [Google Scholar]
- 38.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology. 2002 Nov 1;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports/Centers for Disease Control. 1992 Dec 18;41(RR-17):1–19. [PubMed] [Google Scholar]
- 41.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 42.Klein D, Hurley L, Quesenberry CJ, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002 Aug 15;30(5):471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 43.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010 Feb 1;201(3):318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 44.Palella F, Jr, Delaney K, Moorman A, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 45.Moore RD, Gebo KA, Lucas GM, Keruly JC. Rate of comorbidities not related to HIV infection or AIDS among HIV-infected patients, by CD4 cell count and HAART use status. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 Oct 15;47(8):1102–1104. doi: 10.1086/592115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi AI, Shlipak MG, Hunt PW, Martin JN, Deeks SG. HIV-infected persons continue to lose kidney function despite successful antiretroviral therapy. Aids. 2009 Oct 23;23(16):2143–2149. doi: 10.1097/QAD.0b013e3283313c91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T-cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010 Aug 15;51(4):435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 48.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992 May;74(5):1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 49.Baker JV, Henry WK, Patel P, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Oct;53(8):826–835. doi: 10.1093/cid/cir497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003 Nov 20;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 51.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. The American journal of cardiology. 2016 Jan 15;117(2):214–220. doi: 10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shroff GR. Acute Myocardial Infarction: What’s in a Name? Ann Intern Med. 2015 Mar 17;162(6):448–449. doi: 10.7326/M14-2259. [DOI] [PubMed] [Google Scholar]
- 53.Rosamond WD, Chambless LE, Sorlie PD, et al. Trends in the sensitivity, positive predictive value, false-positive rate, and comparability ratio of hospital discharge diagnosis codes for acute myocardial infarction in four US communities, 1987–2000. American journal of epidemiology. 2004 Dec 15;160(12):1137–1146. doi: 10.1093/aje/kwh341. [DOI] [PubMed] [Google Scholar]
- 54.Pladevall M, Goff DC, Nichaman MZ, et al. An assessment of the validity of ICD Code 410 to identify hospital admissions for myocardial infarction: The Corpus Christi Heart Project. International journal of epidemiology. 1996 Oct;25(5):948–952. doi: 10.1093/ije/25.5.948. [DOI] [PubMed] [Google Scholar]
- 55.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American heart journal. 2004 Jul;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.