Abstract
To investigate familial influences on the full range of variability in attention and activity across adolescence, we collected maternal ratings of 339 twin pairs at ages 12, 14 and 16, and estimated the transmitted and new familial influences on attention and activity as measured by the Strengths and Weaknesses of ADHD Symptoms and Normal Behavior Scale (SWAN). Familial influences were substantial for both traits across adolescence: genetic influences accounted for 54–73% (attention) and 31–73% (activity) of the total variance, and shared environment accounted for 0–22% of the attention variance and 13–57% of the activity variance. The longitudinal stability of individual differences in attention and activity was largely accounted for by familial influences transmitted from previous ages. Innovations over adolescence were also partially attributable to familial influences. Studying the full range of variability in attention and activity may facilitate our understanding of ADHD’s etiology and intervention.
Attention Deficit Hyperactivity Disorder (ADHD) is one of the most common child neurodevelopmental disorders with symptoms in two essential are as: attention and activity, affecting about 3.4% of children and adolescents worldwide (Polanczyk, 2015). ADHD symptoms manifest as an impaired ability to sustain attention and inhibit impulsive/hyperactive behavior, respectively (Barkley, 2003). These symptoms arise in childhood, with DSM-IV requiring symptoms present by age 7 (American Psychiatric Association, 1994) and DSM-5 extending the age of onset up to age 12 (American Psychiatric Association, 2013). Importantly, follow-up studies of children with ADHD into adolescence show that, although the symptoms of ADHD may shift to better adapted ranges with the onset of puberty, 70–85% of diagnosed children have continued issues with attention and activity levels during adolescence (Barkley et al., 1990; Biederman et al., 1996; Centers for Disease Control and Prevention, 2005; Pingault et al., 2015). Thus, it is very important to study the genetic etiology of ADHD development in adolescence.
Attention and activity are two continua expressed quantitatively from the well-adapted end to the extremely abnormal end in the general population (Levy et al., 1997). A dimensional description of attention and activity is in line with the Research Domain Criteria (RDoC) initiative that aims at developing, for research purposes, new ways of classifying mental disorders based on behavioral dimensions and neurobiological measures (Cuthbert, 2014). In fact, attention is included as one of the core constructs of the Cognitive Systems domain, while activity (and its regulation) maps well onto another construct of the same domain, cognitive (effortful) control. A dimensional description of psychopathology also presents significant advantages for genetic studies, such as greater power to identify specific genetic variant s; therefore, it is crucial that research studies consider a full range of variation in phenotypic manifestation of attention and activity for investigating the nature of ADHD (van der Sluis et al., 2013). Avoiding an artificial restriction of the range of variance in the underlying liability existing in the general population may help shed light on the processes underlying developmental shifts in ADHD from dysregulation to highly-adaptive behaviors. Unfortunately, the “adaptive ends” of these full range continuums of attention and activity in the general population have been largely neglected.
Most previous twin studies on ADHD, which have generally focused on the symptomatic portions of these dimensions by using behavior rating scales, have reported substantial heritability estimates for inattention (31% to 82%) and hyperactivity/impulsivity (36% to 88%) (Chang et al., 2013; Freitag et al., 2010; Greven, Rijsdijk & Plomin, 2011b; McLoughlin et al., 2007; Pingault et al., 2015; Swanson et al., 2001; Thapar et al., 2000). Even though it has been suggested that their manifestations are affected by interplay of multiple genetic and environmental factors that provide either risk or protection during development (Thapar et al., 2007), polygenic liability studies should not be limited to the symptomatic direction only, since the effects of single genes on behavior may manifest themselves not only in the problematic range, but also in the adaptive, normative range (Flint, 1998).
Cross-sectional twin research using continuous measures provided by SWAN reported heritability estimates of 0.82 and 0.89 for attention and 0.31 and 0.90 for activity (Hay et al., 2007; Polderman et al., 2007). Interestingly, Hay et al (Hay et al., 2007) found significant shared environmental contribution that explained 53–66% of the variability for activity in both study groups (6–9 and 12–20 years old), and 28% of the variability for attention in younger age group. However, shared environmental influences have rarely been observed in previous ADHD genetic studies using measures that focused on the symptomatic end (Brikell, Kuja-Halkola & Larsson, 2015; Burt, 2009; Burt et al., 2012; Posthuma & Polderman, 2013).
Previous developmental studies focusing on the symptomatic end of ADHD have reported substantial stability of individual differences in inattention and hyperactivity/impulsivity across development, which was accounted for primarily by genetic factors, where as developmental changes were mostly attributed to environmental influences (Costello, Copeland & Angold, 2011; Kan et al., 2013; Kuntsi et al., 2005; Ramtekkar et al., 2010; Reiersen, 2005; Todd et al., 2008). Longitudinal twin studies suggest continuity of genetic influences on ADHD symptoms, i.e. some of the genes that influence ADHD symptom dimensions at an early age continue to operate in later age (Chang et al., 2013; Greven et al., 2011a; Kuntsi et al., 2005; Nadder et al., 2002; Price et al., 2005; Saudino & Cherny, 2001) including the adolescent period (Larsson, Lichtenstein & Larsson, 2006; Larsson, Larsson & Lichtenstein, 2004).
However, these studies were mainly confined to the symptomatic end. To our knowledge, no previous longitudinal studies have investigated the familial influences on the full range of variability of attention and activity, using a developmental, genetically sensitive design. The present study is the first to investigate the contributions of genetic and environmental influences to the stability and changes of attention and activity during adolescence based on their full range variability. We hypothesize d that continuous, full-range measures of attention and activity are strongly influenced by stable and enduring genetic and environmental factors that are transmitted from prior ages (versus those that are transient and period-specific) and account for developmental stability of individual differences along these two dimensions. We further hypothesized that developmental change is brought by new genetic and environmental influences that enter at each age.
METHOD
Subjects
The present data were collected as part of a larger study of Genetics, Neurocognition, and Adolescent Substance Abuse (GNASA), a population-based, longitudinal cohort-sequential study of adolescent twins involving bi-annual laboratory visits. Twin pairs were recruited through the Missouri Family Registry, (a database of twin pairs from a population-based twin registry in the state of Missouri, USA), which has a demographic composition that is broadly representative of the local population. The present analyses utilize data from a subset of twin pairs for whom maternal reports of twin behavior were available. Maternal reports of twin attention and activity were available for both members of 217 twin pairs at age 12 (122 MZ and 95 DZ pairs), for 294 twin pairs at age 14 (140 MZ and 154 DZ pairs), and for 184 pairs at age 16 (88 MZ and 96 DZ pairs). Data from a total of 339 twin pairs were included, with data available at all three ages for 75 pairs, at two ages for 206 pairs (N=104 at ages 12 and 14; N=92 at ages 14 and 16, and N=10 at ages 12 and 16), and at a single age for 58 pairs (N=28 at age 12, N=23 at age 14, and N=7 at age 16 exclusively). The retention rate for 14 year old phase was above 80%, but a significant drop in the number of 16 year old participants was caused by a gap in funding. Since SWAN was added to the assessment battery when baseline assessments had already been in progress, the number of participants at age 14 (first follow-up) is larger than at age 12 (baseline) (Sample characteristics are listed in Table 1). Zygosity for these twin pairs was determined using genotyping on 160 DNA markers. Parents signed an informed consent form as approved by the Institutional Review Board of Washington University School of Medicine. Mothers completed the SWAN while their children performed psychological tasks.
Table 1.
Sample characteristics and cross-age correlations for Attention and Activity scores (above and below the diagonal, respectively)
| N | % male | % MZ | ATTa M (SD) |
ACTb M (SD) |
Cross-age within individual correlations
|
|||
|---|---|---|---|---|---|---|---|---|
| Age 12 | Age 14 | Age 16 | ||||||
| Age 12 | 434 | 52.3% | 56.2% | 32.7 (8.5) | 31.5 (8.5) | -- | 0.71 (0.66–0.76)* | 0.66 (0.56–0.74)* |
| Age 14 | 589 | 51.4% | 47.5% | 31.0 (9.7) | 29.8 (9.9) | 0.65 (0.59–0.71)* | -- | 0.74 (0.69–0.78)* |
| Age 16 | 370 | 52.7% | 47.8% | 28.7 (10.3) | 27.4 (10.3) | 0.63 (0.53–0.71)* | 0.67 (0.60–0.72)* | -- |
Note: The 12-year-olds consisted of 122 MZ and 95 DZ pairs; the 14-year-olds consisted of 140 MZ and 154 DZ pairs; and the 16-year-olds group consisted of 89 MZ and 96 DZ pairs;
significant at the 0.05 level. In brackets: SDs for ATT and ACT scores and 95% confidence intervals for cross-age correlations.
Phenotype assessment
The Strengths and Weaknesses of ADHD Symptoms and Normal Behavior Scale (SWAN) (Swanson et al., 2001) was used to assess the full range of variability of attention (ATT) and activity (ACT). The SWAN contains 18 items to assess attention (9 items) and activity/impulsivity (9 items). Mothers were asked to indicate on a 7-point Likert scale how each twin (rated separately) compared to “other children the same age” over the preceding month. As in the original SWAN measure, all questions were written so that it is beneficial to be “far above” average (e.g., “organize tasks and activities”, “stay seated (when required by class rules/social conventions”)). The nine items on each scale were summed to create a total score (possible range 9–63 for each subscale). Previous genetic studies using SWAN used variable scales to present the results. To facilitate the comparison with clinical studies that used symptomatic measures, in the present analyses all items were reverse-coded, such that higher scores correspond to the dysfunctional end of the distribution (inattention and hyperactivity), while lower scores correspond to the adaptive end (high attentional skills and well-regulated behavior). The SWAN has been found to have strong internal consistency (0.80 to 0.95), acceptable test-retest reliability (0.72–0.90), construct validity and a normal distribution (Arnett et al., 2013; Lakes, Swanson & Riggs, 2012; Polderman et al., 2007; Reiersen & Todorov, 2013; Swanson, 2005). In the present data, Crobach’s alpha ranged from 0.93 to 0.96 for two subscales. Its two -factor structure was confirmed at all three age points (CFI ranged from 0.986 to 0.989, TLI ranged from 0.986 to 0.988) by conducting Confirmatory Factor Analysis using Mplus version 7 (Muthen & Muthen, 2012).
Statistical Analyses
The mean scores of the two subscales within individuals at consecutive time points were compared by paired t-tests using Stata version 9.2 (StataCorp, 2005), and the equivalence of the MZ and DZ mean scores at the same time point was tested using the regression procedure in Stata with the cluster option to control for the non-independence of twins. To assess stability of ATT and ACT throughout development, we calculated within-person phenotypic correlations over time. To explore the genetic architecture, cross-twin correlations for MZ and DZ groups were calculated at three time points and quantitative genetic modeling was conducted to assess the significance of familial influences.
Genetic Analysis
Our genetic analysis was based on standard assumptions of the twin study method (Plomin et al., 2013). These models assume that phenotypic variance arises from additive genetic influences (A), non-additive genetic influences (D), environmental influences shared by family members (C), and individually unique (non-shared) environmental influences (E). Genetic influences are indicated when MZ twin correlations are larger than DZ twin correlations. If all twin pair similarity were attributable to A, the MZ correlation would be about twice the DZ correlation, because MZ twins share all of their genes and DZ twins share half of their segregating genes (on average). Non-additive genetic influences are indicated when the MZ correlation is more than twice the DZ correlation (because MZs again share 100% of non-additive genetic effects, but DZ twins only share 25% of such effects). Shared environmental influences are indicated when the DZ correlation is more than 50% of the MZ correlation. If all twin pair similarity were attributable to C, the MZ and DZ correlations would be equal in magnitude because shared familial components are shared equally among MZ and DZ twin pairs. When only data from twin pairs reared together is available, it is not possible to test C and D simultaneously, and a decision regarding whether to test an ADE or an ACE model is made based upon the observed twin correlations (Rijsdijk & Sham, 2002). A detailed description of the model fitting approach and assessment of heritability can be found elsewhere (Neale & Cardon, 1992; Rijsdijk & Sham, 2002). Structural equation models were used to examine the pattern of familiality using the Mx package, which was specifically developed to model genetically informative data (Neale, 2004). As in a longitudinal design, data from one or more time points or from one twin may be missing from the data set, multivariate structural equation models were fitted to the raw data by a Maximum Likelihood (ML) method (Lange, Westlake & Spence, 1976). As a first step to multivariate analysis, we tested a Cholesky (lower triangular) model, in which influences at time 1 are also allowed to load directly onto all other assessments, new influences enter the model at each subsequent assessment, and these influences are also allowed to load onto all later assessments (Rijsdijk & Sham, 2002); the path loadings for the E components of the attention scale in Figure 1 depict a Cholesky parameterization. This model provides a first glance into the genetic architecture and serves as a base model to which more restricted models can be compared. A particularly useful model for longitudinal data is an autoregressive (or Simplex) model, as it specifies that a latent factor at time t is influenced directly the immediately preceding time (t – 1) in addition to any new influences (Boomsma & Molenaar, 1987; Eaves, Long & Heath, 1986; Neale MC, 1992); the A components in both the attention and activity scales of Figure 1 depict a Simplex pattern. The Simplex model takes full advantage of the time series nature of longitudinal data (Boomsma & Molenaar, 1987) and is a stronger test of developmental hypotheses. A hallmark of a Simplex data structure is a pattern where the correlations are highest among adjacent assessments and decrease systematically as the span between assessments increases (Rijsdijk & Sham, 2002). The Simplex structure fits well with our hypothesis that the stability of both attention and activity is maintained by prior influences that are transmitted to subsequent ages, and that change may be brought by new influences that enter at each age which suggests a Simplex (autoregressive) model. The significance of paths is tested by examining the decrement in fit when individual paths are eliminated from the model. Fit of the sub-models was determined by calculating the difference in –2 times the log -likelihood of the full model and the sub-model, which is interpreted as a Chi-square test for the given degrees of freedom. Fit statistics for the reduced developmental models were compared with those for the saturated models. If the decrement in fit for a reduced model was not significant, that path was deleted from the model and we tested the significance of additional parameters.
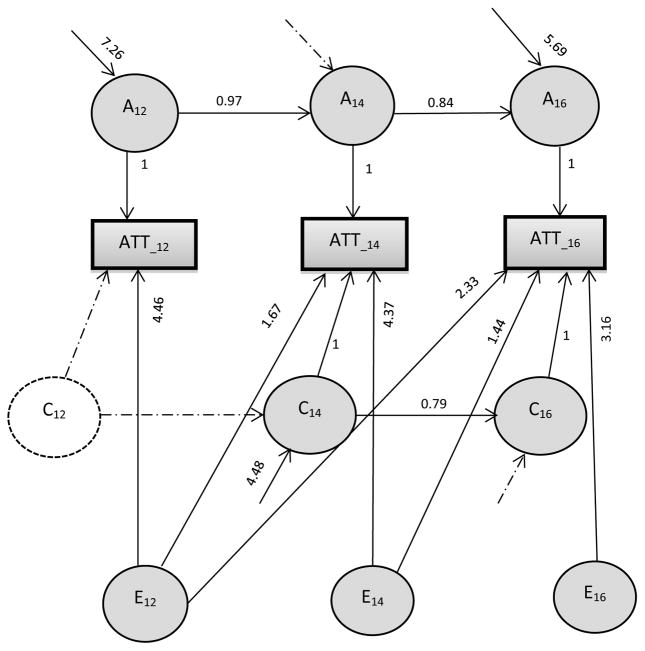
Figure 1. Best fitting structural equation model for genetic and environmental determinants of attention score, controlling for gender.
Note: Rectangles represent the observed variance for each age, and circles represent the latent factors. For additive genetic influences (A12, A14 and A16) and shared environmental influences (C12, C14 and C16), simplex model is shown. A Cholesky (triangular decomposition) model is shown for the non-shared environmental effects (E12, E14 and E16).
Given that the power to detect gender differences in variance components was low with the current sample size (Polderman et al., 2006), the data from male and female twins were combined in the present analyses, and gender was controlled for in all genetic models.
RESULTS
Descriptive Statistics
The mean values for attention (ATT) and activity (ACT) (Table 1) decreased significantly with age (tested using paired t-tests in Stata 9.2 (StataCorp, 2005), with clustering on family to control for the inclusion of data from both twins). Among the subset of 358 individuals (179 pairs) with data at both ages 12 and 14, the means declined from 32.6 to 30.9 for inattention, and from 31.5 to 29.3 for hyperactivity (t(357)=4.63 and 5.40 respectively, p<0.001) Among the subset of 336 individuals (168 pairs) with data at both ages 14 and 16, the means declined from 30.5 to 28.7 for inattention, and from 29.3 to 27.3 for hyperactivity (t(357)=4.59 and 4.29 respectively, p<0.001). There was no significant difference between MZ and DZ twins in their mean scores on either ATT and ACT at any age (p-value range: 0.08 – 0.97; tested using simple regression analyses in Stata, version 9.2, with clustering on family to control for the non-independence of twins; means not shown but available upon request). The skewness and Kurtosis scores for both scale scores suggested minimal departure from normality (ATT: Skewness −0.09 to −0.39, Kurtosis −0.86 to 0.33; ACT: skewness −0.36 to −0.63, Kurtosis −1.1 to 0.25).
Correlations
Test-retest phenotypic correlations across the time points (Table 1) were large (ATT: r=0.66–0.74; ACT: r=0.63–0.67; tested in SAS, version 9.2 (SAS Institute Inc, 2008), indicating high longitudinal stability of these traits over a 4-year period. Furthermore, phenotypic cross-age correlations were slightly lower for the longer interval (ages 12–16) than correlations for shorter intervals (ages 12–14 and 14–16), suggesting an autoregressive (or Simplex) pattern.
Intra-pair twin correlations for ATT and ACT scores are presented in Table 2 (all twin-pair correlations and confidence intervals were calculated using Mx, a statistical package designed for use with data containing related individuals;. At all ages, MZ correlations were higher than DZ correlations, suggesting genetic influence on both traits across all time points. The DZ correlations for ACT were more than one-half of MZ correlations, suggesting that shared environmental influences might be important. However, the pattern for ATT was inconsistent, with the DZ correlation much less than one-half of MZ correlation at age 12, about half the MZ correlation at age 14, and more than half the MZ correlation at age 16, suggesting the potential for non-additive genetic influences in early adolescence and shared environmental influences in later adolescence. Although correlations can be used to test the significance of total familiality, structural equation modeling is required to test the significance of the specific contributions of genetic and shared environmental factors to total familiality.
Table 2.
Intra-pair twin correlations and proportions of variance for the best-fitting models for Attention and Activity (95% confidence intervals)
| rmz | rdz | a2 | c2 | e2 | % A transmitted from previous age | % C transmitted from previous age | |
|---|---|---|---|---|---|---|---|
| Attention | |||||||
| Age 12 | 0.74 (0.64 – 0.81)* | 0.08 (−0.12 – 0.27) | 0.73 (0.64–0.79)* | 0 | 0.27 (0.21–0.36)* | --- | --- |
| Age 14 | 0.77 (0.70–0.83)* | 0.36 (0.22–0.49)* | 0.54 (0.44–0.63)* | 0.22 (0.15–0.30)* | 0.24 (0.19–0.31)* | 100% | --- |
| Age 16 | 0.84 (0.77–0.89)* | 0.53 (0.37–0.66)* | 0.69 (0.54–0.80)* | 0.13 (0.04–0.26)* | 0.18 (0.13–0.25)* | 51.9% | 100% |
|
| |||||||
| Activity | |||||||
| Age 12 | 0.87 (0.82–0.91)* | 0.51 (0.35–0.64)* | 0.73 (0.58–0.84)* | 0.13 (0.02–0.28)* | 0.15 (0.11–0.19)* | --- | --- |
| Age 14 | 0.88 (0.84–0.91)* | 0.72 (0.63–0.79)* | 0.31 (0.19–0.46)* | 0.57 (0.41–0.68)* | 0.13 (0.10–0.16)* | 60.0% | 100% |
| Age 16 | 0.94 (0.91–0.96)* | 0.75 (0.65–0.83)* | 0.42 (0.28–0.61)* | 0.51 (0.33–0.65)* | 0.07 (0.05–0.09)* | 100% | 23.9% |
Note: All proportions of variance calculated controlling for gender. rMZ and rDZ: intra-pair correlations in monozygotic and dizygotic twin pairs; respectively;
indicates significance at the p<0.05 level;
A: additive genetic influences; C: shared environmental influences; E: non-shared (individually-unique) environmental influences; a2: proportion of total variance explained by additive genetic influences; c2: proportion of variance explained by shared environmental influences; e2: proportion of variance attributable to non-shared environmental influences.
Multivariate Model fitting
The results of model-fitting for ATT and ACT are presented in Table 3. The Cholesky models were used to test significance of the A, D (for ATT), and C components (E, which includes error as well as individual-specific effects is retained in all models, although specific paths may be eliminated), and as a reference for comparison with the more restrictive Simplex models.
Table 3.
Multivariate Model fitting for Attention and Activity subscales at ages 12, 14 and 16 controlling for gender
| ATT
|
ACT
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compare to models | Compare to models | ||||||||||||||
| Model | para | −2LL | df | Model | Δx2 | Δdf | p | Model | para | −2LL | df | Model | Δx2 | Δ df | p |
| 1. ADE Cholesky* | 27 | 9309.015 | 1364 | --- | --- | --- | --- | ||||||||
| 2. ACE Cholesky | 27 | 9303.734 | 1364 | --- | --- | --- | --- | 1. ACE Cholesky | 27 | 9091.150 | 1364 | --- | --- | --- | --- |
| 3 ACE reduced Cholesky, no C on attention at age 12 | 24 | 9303.733 | 1367 | 2 | −.001 | 3 | 1.00 | 2. AE Cholesky | 21 | 9148.177 | 1370 | 1 | 57.027 | 6 | <0.001 |
| 4. AE Cholesky, no C on attention | 21 | 9314.898 | 1370 | 3 | 11.164 | 3 | 0.01 | 3. CE Cholesky | 21 | 9180.042 | 1370 | 1 | 88.892 | 6 | <0.001 |
| 5. CE Cholesky, no A on attention | 21 | 9369.747 | 1370 | 2 | 66.013 | 6 | <0.001 | ||||||||
| 6. ACE Simplex * | 24 | 9320.667 | 1367 | 2 | 16.933 | 3 | <0.001 | 4. ACE Simplex | 24 | 9094.411 | 1367 | 1 | 3.261 | 3 | 0.35 |
| 7. ACE: A (Simplex) C & E (Cholesky) |
26 | 9305.081 | 1365 | 2 | 1.347 | 1 | 0.25 | 5. ACE (reduced Simplex, no new A@16) | 23 | 9094.411 | 1368 | 4 | 0 | 1 | 1.00 |
| 8. ACE:C (Simplex) A&E (Cholesky) |
26 | 9303.733 | 1365 | 2 | −0.001 | 1 | 1.00 | 6b. ACE (Reduced Simplex) | 20 | 9096.537 | 1371 | 5 | 2.126 | 3 | 0.55 |
| 9. ACE: A&C (Simplex) E (Cholesky) |
25 | 9305.080 | 1366 | 2 | 1.346 | 2 | 0.25 | ||||||||
|
10a. ACE: A&C (reduced Simplex) E (Cholesky) |
21 | 9308.435 | 1370 | 9 | 3.355 | 4 | 0.56 | ||||||||
Note: A=additive Genetic variance; C=shared environmental variance; E=non-shared environmental variance; −2LL=−2 log likelihood.
the model was rejected by comparing with the full ACE Cholesky model;
best fitting model for ATT; no C@12 (and thus no transmission to age 14), no new A@14, no new C@16;
best fitting model for ACT; no new A@16; no new C@14; No E transmission from 12–14 or 14–16); significant p-values indicate poor model fit.
For ATT, the ACE Cholesky model fit slightly better overall than the ADE model (Models 2 and 1 respectively), and thus was used as the base model for ATT. There was no evidence of C at age 12 for ATT (Model 3), but significant C was found at ages 14 and 16 (Model 4). A model which eliminated shared environmental influences while retaining genetic effects (Model 4) and one which eliminated genetic effects while retaining shared environmental effects (Model 5) were both rejected, suggesting significant genetic and shared environmental influences for ATT. Thus, the model ACE was selected as the final Cholesky model, and used as the reference model for the Simplex model. The Simplex ACE model (Model 6) fit significantly less well than the full Cholesky model, indicating that the more restrictive Simplex pattern did not describe the data as well as the full Cholesky model. However, models specifying a Simplex pattern for A (Model 7), or for C (Model 8) individual, or for A and C simultaneously (Model 9) did not result in a decrement in fit, indicating that they described the data well. Additional testing confirmed no C at age 12 (and thus no transmission from 12 to 14), no new C at age 16, and no new A at age 14 (Model 10). Thus the final model for ATT (shown in Figure 1) included a Simplex pattern for A and C, with E left as a Cholesky parameterization.
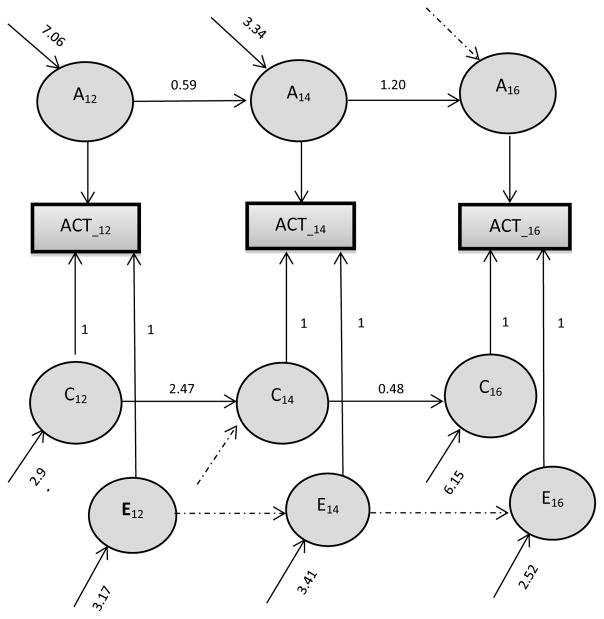
For ACT, neither A nor C could be removed from the Cholesky model without a significant decrement in fit (models 3 and 2 respectively), indicating significant genetic and shared environmental contributions to ATT. For ACT, the Simplex model did not have a significantly poorer fit than the Cholesky model (Model 4), suggesting that the more parsimonious Simplex model described the data well. Additional testing indicated that there was no new A at age 16 (Model 5), no new C at age 14, and no E transmitted over time (either from 12 to 14 or from 14 to 16). The final model, Model 6, is shown in Figure 2.
Figure 2. Best fitting structural equation model for genetic and environmental determinants of activity score, controlling for gender.
Note: Rectangles represent the observed variance for each age, and circles represent the latent factors. For Additive genetic influences (A12, A14 and A16), shared environmental influences (C12, C14 and C16) and non-shared environmental effects (E12, E14 and E16), a simplex model is shown.
The proportions of variance explained by the three components based on best-fitting models are listed in Table 2. At each age, the contribution of A was significant and substantial, accounting for 54%–73% of the variance in ATT, and 31 %–73% of the variance of ACT. Although no C was found at age 12 for ATT, significant C were found at ages 14 (22%) and 16 (13%) for ATT, and at all ages for ACT (13–57%). As shown in Table 2, these familial influences were highly stable. For ATT, 100% and 52% of A at ages 14 and 16 were transmitted from the prior assessments respectively, and 100% of Cat age 16 was transmitted from age 14. For ACT, at ages 14 and 16, 60% and 100% of A, and 100% and 20% of C were transmitted from the prior age respectively.
DISCUSSION
This is the first longitudinal twin study to assess the stability of and change in genetic and environmental influences on the full range of variability of attention and activity, two behavioral dimensions relevant to ADHD. Similar to existing studies that focused on symptoms, we found very high levels of familiality on both the attention and activity levels of adolescents as rated by their mothers. In contrast to most previous research, our study suggests that shared environmental factors contribute to twin resemblance, in addition to the genetic influences typically found. In a meta-analysis of childhood and adolescent behavioral disorders, Burt (2009) found that ADHD was the sole disorder that showed no evidence of shared environmental influence. Although not observed consistently, several studies have shown evidence of shared environmental influence on attention and activity (Greven et al., 2011b; Hay et al., 2007; McLoughlin et al., 2007; Saudino & Zapfe, 2008; Wood et al., 2007). In a response to Burt’s (2009) meta-analysis, Wood and colleagues (Wood et al., 2010) not ed that shared environmental influences were observed in 16% of studies on ADHD symptoms, and accounted for 27% of the variability in the studies in which they were observed. Research using the SWAN has shown mixed results, with Polderman and colleagues (Polderman et al., 2007) finding familiality attributable entirely to genetic factors, and Hay and colleagues (Hay et al., 2007) finding evidence for significant genetic and shared environmental influence for both inattention and hyperactivity/impulsivity in children (6–9 years of age) and for hyperactivity/impulsivity in adolescents (12–20 years of age). Our hyperactivity/impulsivity genetic (31% and 42%) and shared environmental (57% and 51%) components at ages 14 and 16 respectively were highly consistent with Hay and colleagues’ 31% genetic and 66% shared environment in adolescents.
There are several possible explanations for the inconsistent findings regarding shared environmental influences on attention and activity. Hay and colleagues (Hay et al., 2007) suggested that studying the full range of variability of behavior might partially explain the finding of shared environmental influences on these two traits during adolescence. Wood and colleagues (Wood et al., 2010) suggested several additional reasons why studies of ADHD might not show evidence of shared environment. In addition to low power and the potential presence of both non-additive genetic and shared environmental influences (which cannot be disentangled using only twin pairs who grew up together), Wood and colleagues also suggested the possibility that the absence of shared environment could stem from the highly skewed nature of traditional diagnostic measures of ADHD. In the present study, both the range of variability (going from “far better than age-mates” to “far below age-mages”) and the normal distribution in the general population (and our sample) might have enhanced our ability to detect shared environmental influences. Neither suggestion would explain the absence of shared environment in Polderman and colleagues’ study (Polderman et al., 2007) examining the SWAN, although cultural differences between the studies might be important to consider (our sample is from the USA, Hay and colleagues’ was Australian, and Polderman and colleagues’ was Dutch). In particular, the Midwestern USA population from which the sample was drawn may have a broader range of variability in family-level environmental factors (e.g. socioeconomic status, ethnic background, neighborhood and school characteristics) than both Australian and Dutch populations.
Our longitudinal analyses paralleled prior studies in finding that attention and activity levels remained largely stable (with significant, but not dramatic improvement) during adolescence even when examining behavior from the adaptive end of the spectrum. Cross-age correlations were also consistent with those previously reported for inattention and hyperactivity/impulsivity (Larsson et al., 2004), and stability over time was mainly accounted for by familial factors, which is also in keeping with previous ADHD research (Larsson et al., 2004; Nadder et al., 2002; Price et al., 2005). However, our familial contributions were again a combination of genetic and familial environmental factors, with most previous research finding genetic factors were the sole familial contributor to stability.
Although much of stability over time in attention and activity is attributable to genetic factors, the contribution of genetics to this stability varies within age group (Larsson et al., 2006; McLoughlin et al., 2007; Nadder et al., 2002; Nikolas & Burt, 2010). Our results suggested that stability over time was primarily attributable to familial influences. For attention, 43% of stability from ages 12 to 14 was attributable to non-shared (individual-specific) influences, and this dropped to only 13% when examining stability from ages 14 to 16. For activity, all stability was attributable to familial influences, since we were able to remove the non-shared environmental transmission paths from the model entirely (see Figure 2). More detailed examination of the transmission effects for attention indicated that genetic influences at age 14 were entirely overlapping with those from age 12, but that new genetic effects emerged at age 16, with about 50% of genetic influences at 16 being transmitted from age 14. For activity, there were new genetic influences at age 14 (with about 60% of genetic variance being transmitted from at 12) but no additional new genetic influences at age 16. We again found that some of the stability over time in activity was attributable to shared environmental influences. For attention, there was no evidence of shared environmental influence at age 12, with shared environmental influences apparent at age 14 and carrying through to age 16 with no additional shared environmental contributions arising at age 16. For activity, the shared environmental influences observed at age 12 carried through to age 14 (with no new effects observed), but shared environmental influences from age 14 explained 24% of the total C component at age 16. It is not surprising that genetic effects involve both stability and innovation during adolescence, as puberty is likely associated with both new genetic factors arising and some genes ceasing to be active. However, the development of novel shared environmental influences during adolescence seems somewhat counter-intuitive. Given that these are mother ratings of both attention and activity, one possible explanation is that the maternal reports are reflecting changes in the influence of peers as the twins progress from primary school into middle and high school, with new peer networks developing and parental supervision decreasing.
Studying attention and activity across the full range of variability is a more accurate reflection of the entire behavioral spectrum than the conventional symptomatic scales, and has several advantages. First of all, it has clinical implications to help identify both genetic and environmental risk and protective factors that contribute to the development of attention and activity, so that the risk and protective pathways that lead to adverse outcomes or resilience from ADHD can be elucidated. Second, the role of shared environmental influence on attention and activity suggested in the present study may facilitate the development of effective ADHD risk-reduction strategies. Third, it overcomes several major limitations that are commonly seen in the field of behavior genetics: such as skewness, rater contrast effect, and truncation. In keeping with this possibility, the data collected on a full range produces more normally distributed data. Without data-transforming, which could result in biased parameter estimates, the power to detect genes associated with attention and activity related to ADHD at varying degrees of expression increases (Arnett et al., 2013). It has also been suggested that the more detailed measure is more resistant to rater contrast effects than instruments focusing on the symptomatic end only by opening up a wider range of positive and negative response options (Kuntsi et al., 2005; Kuntsi & Stevenson, 2001). Additionally, in molecular genetic studies, the ability to define concordant unaffected pairs and extremely discordant pairs will be strengthened if the well -adapted range of behaviors is not truncated at “0” (Swanson, Wigal & Lakes, 2009). Importantly, the present study highlights the potential value of a full range dimensional approach on studying the common features of psychopathology in mental disorders (Casey, Oliveri & Insel, 2014).
Although, to our knowledge, this study is the first to explore the stability and change of genetic and environmental influences on the full range of variability of attention and activity in a longitudinal twin design, there are limitations to bear in mind. The first one is the reliance on maternal ratings. Teachers’ ratings would have been an especially useful addition, but were not available. In addition, due to statistical power limitations, we could not perform gender-specific analyses. Importantly, these results need to be replicated and refined with larger samples of genetically related subjects. With more objective neuropsychological measures available in the present longitudinal genetics study, the developmental genetic architecture of these two traits could be further investigated in the near future.
References
- American Psychiatric Association, editor. Diagnostic and Statistical Mannula of Mental Disorders, 4th edition (DSM-IV) 1994. [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and Statistical Mannula of Mental Disorders, 5th edition (DSM-5) Washington D.C: 2013. [Google Scholar]
- Arnett AB, Pennington BF, Friend A, Willcutt EG, Byrne B, Samuelsson S, Olson RK. The SWAN captures variance at the negative and positive ends of the ADHD symptom dimension. Journal of attention disorders. 2013;17(2):152–62. doi: 10.1177/1087054711427399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Attention Deficit Hyperactivity Disorder. New York: Guiford Press; 2003. [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29(4):546–57. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone S, Milberger S, Curtis S, Chen L, Marrs A, Ouellette C, Moore P, Spencer T. Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(3):343–51. doi: 10.1097/00004583-199603000-00016. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Molenaar PC. The genetic analysis of repeated measures. I. Simplex models. Behavior genetics. 1987;17(2):111–23. doi: 10.1007/BF01065991. [DOI] [PubMed] [Google Scholar]
- Brikell I, Kuja-Halkola R, Larsson H. Heritability of attention-deficit hyperactivity disorder in adults. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2015 doi: 10.1002/ajmg.b.32335. [DOI] [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychological bulletin. 2009;135(4):608–37. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Burt SA, Larsson H, Lichtenstein P, Klump KL. Additional evidence against shared environmental contributions to attention-deficit/hyperactivity problems. Behavior genetics. 2012;42(5):711–21. doi: 10.1007/s10519-012-9545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biological psychiatry. 2014;76(5):350–3. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Mental health in the United States. Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder--United States, 2003. MMWR. Morbidity and mortality weekly report. 2005;54(34):842–7. [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Asherson PJ, Larsson H. Developmental twin study of attention problems: high heritabilities throughout development. JAMA psychiatry. 2013;70(3):311–8. doi: 10.1001/jamapsychiatry.2013.287. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Copeland W, Angold A. Trends in psychopathology across the adolescent years: what changes when children become adolescents, and when adolescents become adults? Journal of child psychology and psychiatry, and allied disciplines. 2011;52(10):1015–25. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World psychiatry: official journal of the World Psychiatric Association. 2014;13(1):28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Long J, Heath AC. A theory of developmental change in quantitative phenotypes applied to cognitive development. Behavior genetics. 1986;16(1):143–62. doi: 10.1007/BF01065484. [DOI] [PubMed] [Google Scholar]
- Flint J. Behavioral phenotypes: conceptual and methodological issues. American journal of medical genetics. 1998;81(3):235–40. [PubMed] [Google Scholar]
- Freitag CM, Rohde LA, Lempp T, Romanos M. Phenotypic and measurement influences on heritability estimates in childhood ADHD. European child & adolescent psychiatry. 2010;19(3):311–23. doi: 10.1007/s00787-010-0097-5. [DOI] [PubMed] [Google Scholar]
- Greven CU, Asherson P, Rijsdijk FV, Plomin R. A longitudinal twin study on the association between inattentive and hyperactive-impulsive ADHD symptoms. Journal of abnormal child psychology. 2011a;39(5):623–32. doi: 10.1007/s10802-011-9513-7. [DOI] [PubMed] [Google Scholar]
- Greven CU, Rijsdijk FV, Plomin R. A twin study of ADHD symptoms in early adolescence: hyperactivity-impulsivity and inattentiveness show substantial genetic overlap but also genetic specificity. Journal of abnormal child psychology. 2011b;39(2):265–75. doi: 10.1007/s10802-010-9451-9. [DOI] [PubMed] [Google Scholar]
- Hay DA, Bennett KS, Levy F, Sergeant J, Swanson J. A twin study of attention-deficit/hyperactivity disorder dimensions rated by the strengths and weaknesses of ADHD-symptoms and normal-behavior (SWAN) scale. Biological psychiatry. 2007;61(5):700–5. doi: 10.1016/j.biopsych.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Kan KJ, Dolan CV, Nivard MG, Middeldorp CM, van Beijsterveldt CE, Willemsen G, Boomsma DI. Genetic and environmental stability in attention problems across the lifespan: evidence from the Netherlands twin register. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(1):12–25. doi: 10.1016/j.jaac.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Rijsdijk F, Ronald A, Asherson P, Plomin R. Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biological psychiatry. 2005;57(6):647–54. doi: 10.1016/j.biopsych.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Stevenson J. Psychological mechanisms in hyperactivity: II. The role of genetic factors. Journal of child psychology and psychiatry, and allied disciplines. 2001;42(2):211–9. [PubMed] [Google Scholar]
- Lakes KD, Swanson JM, Riggs M. The reliability and validity of the English and Spanish Strengths and Weaknesses of ADHD and Normal behavior rating scales in a preschool sample: continuum measures of hyperactivity and inattention. Journal of attention disorders. 2012;16(6):510–6. doi: 10.1177/1087054711413550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K, Westlake J, Spence MA. Extensions to pedigree analysis. III. Variance components by the scoring method. Annals of human genetics. 1976;39(4):485–91. doi: 10.1111/j.1469-1809.1976.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Larsson H, Lichtenstein P, Larsson JO. Genetic contributions to the development of ADHD subtypes from childhood to adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(8):973–81. doi: 10.1097/01.chi.0000222787.57100.d8. [DOI] [PubMed] [Google Scholar]
- Larsson JO, Larsson H, Lichtenstein P. Genetic and environmental contributions to stability and change of ADHD symptoms between 8 and 13 years of age: a longitudinal twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(10):1267–75. doi: 10.1097/01.chi.0000135622.05219.bf. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(6):737–44. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Ronald A, Kuntsi J, Asherson P, Plomin R. Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive-impulsive components. Journal of abnormal child psychology. 2007;35(6):999–1008. doi: 10.1007/s10802-007-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. MPlus version 7. Los Angeles, CA: 2012. [Google Scholar]
- Nadder TS, Rutter M, Silberg JL, Maes HH, Eaves LJ. Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (Odd/CD) symptomatologies across informant and occasion of measurement. Psychological medicine. 2002;32(1):39–53. doi: 10.1017/s0033291701004792. [DOI] [PubMed] [Google Scholar]
- Neale MC. Statistical Modeling with Mx. Department of Psychiatry; Richmond VA: 2004. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Nikolas MA, Burt SA. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. Journal of abnormal psychology. 2010;119(1):1–17. doi: 10.1037/a0018010. [DOI] [PubMed] [Google Scholar]
- Pingault JB, Viding E, Galera C, Greven CU, Zheng Y, Plomin R, Rijsdijk F. Genetic and Environmental Influences on the Developmental Course of Attention-Deficit/Hyperactivity Disorder Symptoms From Childhood to Adolescence. JAMA psychiatry. 2015;72(7):651–8. doi: 10.1001/jamapsychiatry.2015.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Knopik VS, Neiderhiser JM, editors. Behavioral Genetics. New York: Worth Publishers; 2013. [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of child psychology and psychiatry, and allied disciplines. 2015;56(3):345–65. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Derks EM, Hudziak JJ, Verhulst FC, Posthuma D, Boomsma DI. Across the continuum of attention skills: a twin study of the SWAN ADHD rating scale. Journal of child psychology and psychiatry, and allied disciplines. 2007;48(11):1080–7. doi: 10.1111/j.1469-7610.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Posthuma D, De Sonneville LM, Verhulst FC, Boomsma DI. Genetic analyses of teacher ratings of problem behavior in 5-year-old twins. Twin research and human genetics: the official journal of the International Society for Twin Studies. 2006;9(1):122–30. doi: 10.1375/183242706776402975. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Polderman TJ. What have we learned from recent twin studies about the etiology of neurodevelopmental disorders? Curr Opin Neurol. 2013;26(2):111–21. doi: 10.1097/WCO.0b013e32835f19c3. [DOI] [PubMed] [Google Scholar]
- Price TS, Simonoff E, Asherson P, Curran S, Kuntsi J, Waldman I, Plomin R. Continuity and change in preschool ADHD symptoms: longitudinal genetic analysis with contrast effects. Behavior genetics. 2005;35(2):121–32. doi: 10.1007/s10519-004-1013-x. [DOI] [PubMed] [Google Scholar]
- Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3):217–28. e1–3. [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM. Twin study of the longitudinal course of ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(7):625–6. doi: 10.1097/01.chi.0000162576.17579.9a. author reply 6–7. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Todorov AA. Exploration of ADHD Subtype Definitions and Co-Occurring Psychopathology in a Missouri Population-Based Large Sibship Sample. Scandinavian journal of child and adolescent psychiatry and psychology. 2013;1(1):3–13. doi: 10.21307/sjcapp-2013-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Briefings in bioinformatics. 2002;3(2):119–33. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS 9.2 User’s Guide. Cary, NC: SAS Institute; 2008. [Google Scholar]
- Saudino KJ, Cherny SS. Infancy to early childhood: Genetic and environmental influences on developmental change. Oxford: Oxford University Press; 2001. Parental ratings of temperament in twins; pp. 89–110. [Google Scholar]
- Saudino KJ, Zapfe JA. Genetic influences on activity level in early childhood: do situations matter? Child development. 2008;79(4):930–43. doi: 10.1111/j.1467-8624.2008.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: Release 9.2. College Station, TX: Author; 2005. [Google Scholar]
- Swanson J, Posner M, Fusella J, Wasdell M, Sommer T, Fan J. Genes and attention deficit hyperactivity disorder. Current psychiatry reports. 2001;3(2):92–100. doi: 10.1007/s11920-001-0005-2. [DOI] [PubMed] [Google Scholar]
- Swanson J, Schuck S, Mann M, Carlson C, Hartman K, Sergeant J, Clevernger W, Wasdell M, McCleary R. Catergorical and dimensional definitions and evaluations of symptoms of ADHD: The SNAP and the SWAN Ratings Scale. 2005 [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Wigal T, Lakes K. DSM-V and the future diagnosis of attention-deficit/hyperactivity disorder. Current psychiatry reports. 2009;11(5):399–406. doi: 10.1007/s11920-009-0060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Harrington R, Ross K, McGuffin P. Does the definition of ADHD affect heritability? Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(12):1528–36. doi: 10.1097/00004583-200012000-00015. [DOI] [PubMed] [Google Scholar]
- Thapar A, Langley K, Asherson P, Gill M. Gene-environment interplay in attention-deficit hyperactivity disorder and the importance of a developmental perspective. The British journal of psychiatry: the journal of mental science. 2007;190:1–3. doi: 10.1192/bjp.bp.106.027003. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Todorov AA, Neuman RJ, Reiersen AM, Henderson CA, Reich WC. Predictors of stability of attention-deficit/hyperactivity disorder subtypes from childhood to young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(1):76–85. doi: 10.1097/chi.0b013e31815a6aca. [DOI] [PubMed] [Google Scholar]
- van der Sluis S, Posthuma D, Nivard MG, Verhage M, Dolan CV. Power in GWAS: lifting the curse of the clinical cut-off. Mol Psychiatry. 2013;18(1):2–3. doi: 10.1038/mp.2012.65. [DOI] [PubMed] [Google Scholar]
- Wood AC, Buitelaar J, Rijsdijk F, Asherson P, Kuntsi J. Rethinking shared environment as a source of variance underlying attention-deficit/hyperactivity disorder symptoms: comment on Burt (2009) Psychological bulletin. 2010;136(3):331–40. doi: 10.1037/a0019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Saudino KJ, Rogers H, Asherson P, Kuntsi J. Genetic influences on mechanically-assessed activity level in children. Journal of child psychology and psychiatry, and allied disciplines. 2007;48(7):695–702. doi: 10.1111/j.1469-7610.2007.01739.x. [DOI] [PubMed] [Google Scholar]