Abstract
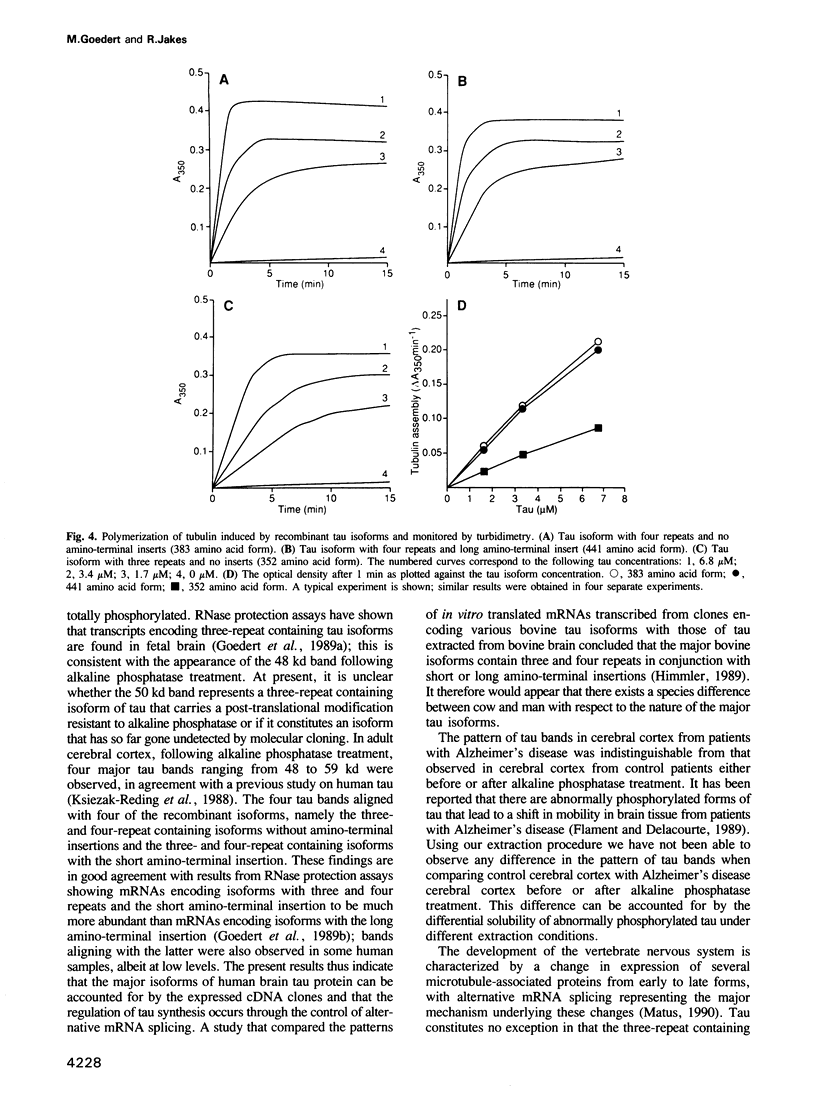
We have expressed six previously cloned isoforms of human microtubule-associated tau protein in Escherichia coli and purified them to homogeneity in a biologically active form. They range from 352 to 441 amino acids in length and differ from each other by the presence of three or four tandem repeats in the carboxy-terminal half and by the presence or absence of 29 or 58 amino acid inserts in the amino-terminus. When mixed together they gave a set of six bands on SDS-PAGE gels with apparent molecular weights of 48-67 kd and with a characteristic pattern of spacings. Four of these bands aligned with the major tau bands found in adult human cerebral cortex following perchloric acid extraction and alkaline phosphatase treatment. They consisted of isoforms with three repeats and no insertions, four repeats and no amino-terminal insertions and three- and four-repeat containing isoforms with the 29 amino acid insertion. In fetal human brain extracts treated with alkaline phosphatase one of the two major tau bands aligned with the three-repeat containing isoform with no insertions, whereas the molecular nature of the second major tau band remains to be established. The recombinant tau isoforms were biologically active at micromolar concentrations, as assessed by their ability to promote microtubule assembly. The rates of assembly were 2.5-3.0 times faster for isoforms containing four repeats when compared with three-repeat containing isoforms, with no significant contribution by the amino-terminal insertions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa H., Kawasaki H., Murofushi H., Kotani S., Suzuki K., Sakai H. A common amino acid sequence in 190-kDa microtubule-associated protein and tau for the promotion of microtubule assembly. J Biol Chem. 1989 Apr 5;264(10):5885–5890. [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareff W., Wischik C. M., Novak M., Amos W. B., Klug A., Roth M. Molecular analysis of neurofibrillary degeneration in Alzheimer's disease. An immunohistochemical study. Am J Pathol. 1990 Sep;137(3):711–723. [PMC free article] [PubMed] [Google Scholar]
- Caceres A., Kosik K. S. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990 Feb 1;343(6257):461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977 Oct 25;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977 Oct 25;116(2):207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Couchie D., Nunez J. Immunological characterization of microtubule-associated proteins specific for the immature brain. FEBS Lett. 1985 Sep 2;188(2):331–335. doi: 10.1016/0014-5793(85)80397-5. [DOI] [PubMed] [Google Scholar]
- Delacourte A., Defossez A. Alzheimer's disease: Tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. J Neurol Sci. 1986 Dec;76(2-3):173–186. doi: 10.1016/0022-510x(86)90167-x. [DOI] [PubMed] [Google Scholar]
- Ennulat D. J., Liem R. K., Hashim G. A., Shelanski M. L. Two separate 18-amino acid domains of tau promote the polymerization of tubulin. J Biol Chem. 1989 Apr 5;264(10):5327–5330. [PubMed] [Google Scholar]
- Flament S., Delacourte A. Abnormal tau species are produced during Alzheimer's disease neurodegenerating process. FEBS Lett. 1989 Apr 24;247(2):213–216. doi: 10.1016/0014-5793(89)81337-7. [DOI] [PubMed] [Google Scholar]
- Francon J., Lennon A. M., Fellous A., Mareck A., Pierre M., Nunez J. Heterogeneity of microtubule-associated proteins and brain development. Eur J Biochem. 1982 Dec 15;129(2):465–471. doi: 10.1111/j.1432-1033.1982.tb07072.x. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Potier M. C., Ulrich J., Crowther R. A. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989 Feb;8(2):393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Wischik C. M., Crowther R. A., Walker J. E., Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Himmler A., Drechsel D., Kirschner M. W., Martin D. W., Jr Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol. 1989 Apr;9(4):1381–1388. doi: 10.1128/mcb.9.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A. Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol. 1989 Apr;9(4):1389–1396. doi: 10.1128/mcb.9.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel P. H., Schmitter M. J., Dessen P., Fayat G., Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T., Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986 Jun 5;321(6070):605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Nukina N., Miura R., Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem. 1986 Jun;99(6):1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Takemura R., Oshima T., Mori H., Ihara Y., Yanagisawa M., Masaki T., Hirokawa N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989 Sep;109(3):1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo J., Honda T., Mori H., Hamada Y., Miura R., Ogawara M., Ihara Y. The carboxyl third of tau is tightly bound to paired helical filaments. Neuron. 1988 Nov;1(9):827–834. doi: 10.1016/0896-6273(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Bakalis S., Neve R. L. Developmentally regulated expression of specific tau sequences. Neuron. 1989 Apr;2(4):1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Binder L. I., Yen S. H. Immunochemical and biochemical characterization of tau proteins in normal and Alzheimer's disease brains with Alz 50 and Tau-1. J Biol Chem. 1988 Jun 15;263(17):7948–7953. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lee G., Neve R. L., Kosik K. S. The microtubule binding domain of tau protein. Neuron. 1989 Jun;2(6):1615–1624. doi: 10.1016/0896-6273(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Lichtenberg B., Mandelkow E. M., Hagestedt T., Mandelkow E. Structure and elasticity of microtubule-associated protein tau. Nature. 1988 Jul 28;334(6180):359–362. doi: 10.1038/334359a0. [DOI] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984 Apr 25;259(8):5301–5305. [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. The purification of tau protein and the occurrence of two phosphorylation states of tau in brain. J Biol Chem. 1984 Oct 10;259(19):12241–12245. [PubMed] [Google Scholar]
- Mandelkow E. M., Herrmann M., Rühl U. Tubulin domains probed by limited proteolysis and subunit-specific antibodies. J Mol Biol. 1985 Sep 20;185(2):311–327. doi: 10.1016/0022-2836(85)90406-1. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Matus A. Microtubule-associated proteins. Curr Opin Cell Biol. 1990 Feb;2(1):10–14. doi: 10.1016/s0955-0674(05)80024-9. [DOI] [PubMed] [Google Scholar]
- McLeod M., Stein M., Beach D. The product of the mei3+ gene, expressed under control of the mating-type locus, induces meiosis and sporulation in fission yeast. EMBO J. 1987 Mar;6(3):729–736. doi: 10.1002/j.1460-2075.1987.tb04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Hamada Y., Kawaguchi M., Honda T., Kondo J., Ihara Y. A distinct form of tau is selectively incorporated into Alzheimer's paired helical filaments. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1221–1226. doi: 10.1016/0006-291x(89)92240-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Edwards P. C., Klug A., Tichelaar W., Crowther R. A. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]