Abstract
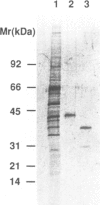
A frog 'peptidylglycine alpha-amidating monooxygenase (PAM, EC 1.14.17.3)' was expressed in cultured insect cells by using the baculovirus expression vector system. The enzyme, recovered in the culture medium, was purified to homogeneity. Its apparent molecular mass (43 kd), estimated by both SDS-PAGE and molecular sieving, was higher than the value (39 kd) for the 'PAM' (AE-I) purified from frog skin. N-terminal sequence analysis indicated that cleavage of signal sequence had occurred but the propeptide still remained at the N terminus. The glycine-extended model peptide X-Gly (mean = Ala-Ile-Gly-Val-Gly-Ala-Pro) was used as substrate for the purified enzyme. The reaction product formed at pH 5.4 was isolated and characterized by amino acid sequence analysis, FAB-MASS and 1H-NMR. It was shown that the purified enzyme had converted the model peptide to the C-terminal alpha-hydroxyglycine-extended peptide [X-Gly(OH)] instead of the amidated product (X-NH2), indicating that the enzyme widely known as 'PAM' should be called 'peptidylglycine alpha-hydroxylating monooxygenase'. A novel enzyme, present in the insect cell culture medium and separable from the expressed monooxygenase, could convert the alpha-hydroxyglycine-extended peptide to the amidated product at physiological pH values. It is concluded that the alpha-amidation of glycine-extended peptides is a two-step process catalyzed by the monooxygenase and the novel enzyme.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudry G. A., Bertelsen A. H. Secreted alpha amidating enzymes are generated by specific posttranslational processing of precursors containing transmembrane domains. Biochem Biophys Res Commun. 1989 Sep 15;163(2):959–966. doi: 10.1016/0006-291x(89)92315-2. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Mistry J., Roos B. A., Smyth D. G. 4-Phenyl-3-butenoic acid, an in vivo inhibitor of peptidylglycine hydroxylase (peptide amidating enzyme). Eur J Biochem. 1990 Apr 30;189(2):363–368. doi: 10.1111/j.1432-1033.1990.tb15497.x. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Biosynthesis of the C-terminal amide in peptide hormones. Biosci Rep. 1987 Dec;7(12):907–916. doi: 10.1007/BF01122123. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Enzyme-catalysed peptide amidation. Isolation of a stable intermediate formed by reaction of the amidating enzyme with an imino acid. Eur J Biochem. 1987 Dec 15;169(3):579–584. doi: 10.1111/j.1432-1033.1987.tb13648.x. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Substrate specificity of an amidating enzyme in porcine pituitary. Biochem Biophys Res Commun. 1983 Apr 29;112(2):372–377. doi: 10.1016/0006-291x(83)91473-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Glembotski C. C. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Park L. P., Dickerson I. M., Keutmann H. T., Thiele E. A., Rodriguez H., Schofield P. R., Mains R. E. Structure of the precursor to an enzyme mediating COOH-terminal amidation in peptide biosynthesis. Mol Endocrinol. 1987 Nov;1(11):777–790. doi: 10.1210/mend-1-11-777. [DOI] [PubMed] [Google Scholar]
- Fisher J. M., Scheller R. H. Prohormone processing and the secretory pathway. J Biol Chem. 1988 Nov 15;263(32):16515–16518. [PubMed] [Google Scholar]
- Glauder J., Ragg H., Rauch J., Engels J. W. Human peptidylglycine alpha-amidating monooxygenase: cDNA, cloning and functional expression of a truncated form in COS cells. Biochem Biophys Res Commun. 1990 Jun 15;169(2):551–558. doi: 10.1016/0006-291x(90)90366-u. [DOI] [PubMed] [Google Scholar]
- Jones B. N., Tamburini P. P., Consalvo A. P., Young S. D., Lovato S. J., Gilligan J. P., Jeng A. Y., Wennogle L. P. A fluorometric assay for peptidyl alpha-amidation activity using high-performance liquid chromatography. Anal Biochem. 1988 Feb 1;168(2):272–279. doi: 10.1016/0003-2697(88)90318-1. [DOI] [PubMed] [Google Scholar]
- Kreil G., Mollay C., Kaschnitz R., Haiml L., Vilas U. Prepromelittin: specific cleavage of the pre- and the propeptide in vitro. Ann N Y Acad Sci. 1980;343:338–346. doi: 10.1111/j.1749-6632.1980.tb47262.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- May V., Cullen E. I., Braas K. M., Eipper B. A. Membrane-associated forms of peptidylglycine alpha-amidating monooxygenase activity in rat pituitary. Tissue specificity. J Biol Chem. 1988 Jun 5;263(16):7550–7554. [PubMed] [Google Scholar]
- May V., Cullen E. I., Braas K. M., Eipper B. A. Membrane-associated forms of peptidylglycine alpha-amidating monooxygenase activity in rat pituitary. Tissue specificity. J Biol Chem. 1988 Jun 5;263(16):7550–7554. [PubMed] [Google Scholar]
- Mizuno K., Ohsuye K., Wada Y., Fuchimura K., Tanaka S., Matsuo H. Cloning and sequence of cDNA encoding a peptide C-terminal alpha-amidating enzyme from Xenopus laevis. Biochem Biophys Res Commun. 1987 Oct 29;148(2):546–552. doi: 10.1016/0006-291x(87)90911-9. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Sakata J., Kojima M., Kangawa K., Matsuo H. Peptide C-terminal alpha-amidating enzyme purified to homogeneity from Xenopus laevis skin. Biochem Biophys Res Commun. 1986 Jun 30;137(3):984–991. doi: 10.1016/0006-291x(86)90322-0. [DOI] [PubMed] [Google Scholar]
- Murthy A. S., Keutmann H. T., Eipper B. A. Further characterization of peptidylglycine alpha-amidating monooxygenase from bovine neurointermediate pituitary. Mol Endocrinol. 1987 Apr;1(4):290–299. doi: 10.1210/mend-1-4-290. [DOI] [PubMed] [Google Scholar]
- Murthy A. S., Mains R. E., Eipper B. A. Purification and characterization of peptidylglycine alpha-amidating monooxygenase from bovine neurointermediate pituitary. J Biol Chem. 1986 Feb 5;261(4):1815–1822. [PubMed] [Google Scholar]
- Noguchi M., Takahashi K., Okamoto H. Rat peptidylglycine alpha-amidating enzyme: the relation between activities at neutral and alkaline pH Values. Arch Biochem Biophys. 1989 Dec;275(2):505–513. doi: 10.1016/0003-9861(89)90397-4. [DOI] [PubMed] [Google Scholar]
- Ohsuye K., Kitano K., Wada Y., Fuchimura K., Tanaka S., Mizuno K., Matsuo H. Cloning of cDNA encoding a new peptide C-terminal alpha-amidating enzyme having a putative membrane-spanning domain from Xenopus laevis skin. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1275–1281. doi: 10.1016/0006-291x(88)90767-x. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S. N., Eipper B. A., Mains R. E. Stable expression of full-length and truncated bovine peptidylglycine alpha-amidating monooxygenase complementary DNAs in cultured cells. Mol Endocrinol. 1990 Jan;4(1):132–139. doi: 10.1210/mend-4-1-132. [DOI] [PubMed] [Google Scholar]
- Stoffers D. A., Green C. B., Eipper B. A. Alternative mRNA splicing generates multiple forms of peptidyl-glycine alpha-amidating monooxygenase in rat atrium. Proc Natl Acad Sci U S A. 1989 Jan;86(2):735–739. doi: 10.1073/pnas.86.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M., Iida T., Yoshida S., Komatsu K., Namba R., Yanagi M., Noguchi M., Okamoto H. The reaction product of peptidylglycine alpha-amidating enzyme is a hydroxyl derivative at alpha-carbon of the carboxyl-terminal glycine. J Biol Chem. 1990 Jun 15;265(17):9602–9605. [PubMed] [Google Scholar]
- Takahashi K., Okamoto H., Seino H., Noguchi M. Peptidylglycine alpha-amidating reaction: evidence for a two-step mechanism involving a stable intermediate at neutral pH. Biochem Biophys Res Commun. 1990 Jun 15;169(2):524–530. doi: 10.1016/0006-291x(90)90362-q. [DOI] [PubMed] [Google Scholar]