Abstract
Altered resting state functional connectivity (rsFC) and functional network connectivity (FNC), which is a measure of coherence between brain networks, may be associated with nicotine use disorder (NUD). We hypothesized that higher connectivity between insula and 1) dorsal anterior cingulate cortex (dACC) and 2) dorsolateral prefrontal cortex (dlPFC) would predict better treatment outcomes. We also performed an exploratory analysis of the associations between FNC values between additional key frontal and striatal regions and treatment outcomes. One hundred and forty four individuals with NUD underwent a resting state session during functional MRI prior to randomization to treatment with varenicline (n=82) or placebo. Group independent component analysis (ICA) was utilized to extract individual subject components and time series from intrinsic connectivity networks in aforementioned regions, and FNC between all possible pairs were calculated. Higher FNC between insula and dACC (rho=0.21) was significantly correlated with lower levels of baseline smoking quantity but did not predict treatment outcome upon controlling for baseline smoking. Higher FNC between putamen and dACC, caudate and dACC, and caudate and dlPFC significantly predicted worse treatment outcome in participants reporting high subjective withdrawal before the scan. FNC between key regions hold promise as biomarkers to predict outcome in NUD.
Keywords: resting state, functional network connectivity, nicotine dependence, treatment, treatment outcome, prediction
1. Introduction
Nicotine use disorder (NUD) is a major public health problem, and relapse rates remain high for individuals undergoing treatment; the vast majority relapse by 6 months (Richmond and Kehoe, 2007). Finding reliable markers of relapse vulnerability has the potential to improve treatment outcomes; it could both identify individuals who require higher treatment intensity (Leventhal et al., 2012) and improve treatment-matching efforts (Mann et al., 2014).
Functional neuroimaging can be used to measure the coherence in blood oxygen dependent (BOLD) signal during resting state between brain regions (resting state connectivity; rsFC) or networks (functional network connectivity; FNC). These measures have potential to be useful biomarkers of NUD severity and relapse risk (Fedota and Stein, 2015; Sutherland et al., 2012). In particular, networks such as the default mode network (DMN), executive control network (ECN), and salience network (SN) (Fedota and Stein, 2015), and regions within these networks including the rostral anterior cingulate cortex (rACC), ventromedial prefrontal cortex (vmPFC), precuneus and posterior cingulate cortex (PCC) (DMN), dorsolateral prefrontal cortex (dlPFC) and lateral parietal cortex (ECN), insula and dorsal anterior cingulate cortex (dACC)/dorsomedial prefrontal cortex (dmPFC) (SN), and caudate and putamen (striatum) are likely related to NUD severity and outcome during treatment (Fedota and Stein, 2015). RsFC and FNC would be feasible to obtain in clinical settings compared to other functional neuroimaging measures (i.e., task-based measures), given the ease of acquisition for resting state data, and thus lower cost (Fedota and Stein, 2015). More robust outcome predictors are needed as, so far, few self-report measures have proven to be reliable. For example, even self-reported nicotine dependence severity shows inconsistent predictive value (Berlin et al., 2016; McPherson et al., 2014), and recent work in the same subject sample examined in the present manuscript found that a variety of predictors previously identified (mood, impulsiveness, age) were not predictive of treatment outcome (Wilcox et al., 2017).
RsFC is altered in individuals with NUD. Although one study showed greater rsFC between fronto-parietal cortex and medial (mPFC) in smokers compared to controls (Janes et al., 2012), most studies demonstrate reduced rsFC (Bi et al., 2016; Fedota et al., 2016; Fedota and Stein, 2015; Fedota et al., 2015; Zanchi et al., 2015) or more negative rsFC (anti-correlation, negative coupling) between prefrontal cortical, cingulate, insular, and striatal regions in smokers relative to controls or in individuals with more severe dependence compared to those with less severe dependence (Stoeckel et al., 2016; Yuan et al., 2016). In line with the growing evidence that low rsFC between many of these key regions is a marker of the presence of NUD and/or the degree of dependence severity, high rsFC, especially between insula and dmPFC (Addicott et al., 2015; Janes et al., 2010) or dACC (Janes et al., 2010), and between insula and dlPFC (Janes et al., 2010) predicts better outcomes during treatment. Additionally, increases in connectivity between ventral striatum and PFC/ACC (Sweitzer et al., 2016) over 24 hours of abstinence also predicts better later outcomes during treatment, whereas decreases with abstinence predicts worse outcomes.
Varenicline is an established treatment for NUD (Hartmann-Boyce et al., 2014), and its efficacy is likely mediated, in part, by reductions in craving (Ashare et al., 2012; Hajek et al., 2011; Hitsman et al., 2013), although this has not been observed in all studies of varenicline (Jhanjee et al., 2015). Investigations into the effects of varenicline on withdrawal have been even more mixed with some showing improvements in withdrawal (Hitsman et al., 2013) and others not (Brandon et al., 2011; Jhanjee et al., 2015). Finally, there is some evidence to suggest that varenicline may be acting via improvements in inhibitory control and attention (and thus impulsiveness) as well (Austin et al., 2014; Rhodes et al., 2012). Varenicline has been observed to decrease connectivity between insula and rACC, parahippocampus, dACC, PCC (Sutherland et al., 2013a) but to what degree these changes in rsFC are related to improving craving, withdrawal or treatment outcomes is not known.
In this study, we chose to do an exploratory analysis in a dataset of 144 individuals who were randomized to varenicline or placebo, and who also underwent a baseline resting state scan. Six small-sized a priori networks (the word “networks” will hereafter be used interchangeably with “regions”) which fell primarily on 6 key brain regions [caudate and putamen (striatum), dlPFC (ECN), rACC (DMN), dACC and insula (SN)] were selected for this study based on the preceding literature review establishing the likely importance of rsFC between striatum ECN, DMN, and SN (Fedota and Stein, 2015; Lerman et al., 2014; Sutherland et al., 2012) in NUD severity. We chose an approach which could be replicated using pre-existing templates that are downloadable (Allen et al., 2014) (http://mialab.mrn.org/data/index.html) and measured FNC between all possible pairs of these 6 apriori regions (15 pairs). Our overall goal was to investigate the degree to which FNC values could serve as clinically-relevant biomarkers of disorder severity. We had 2 primary aims: 1) To investigate whether higher FNC between insula-dACC and insula-dlPFC was associated with better treatment outcomes, in support of previous literature (Addicott et al., 2015; Janes et al., 2010) 2) To explore whether FNC between 6 a priori regions could predict treatment outcomes in general, or differentially on varenicline versus placebo. We hoped these findings would further clarify the mixed results in the literature and lead us to identify clinically relevant markers of nicotine dependence severity.
2. Methods
2.1. Subjects
Subjects were treatment-seeking cigarette smokers between the ages of 18 and 55 recruited through newspapers and flyers and enrolled in a randomized, double-blind, placebo-controlled trial of varenicline. Inclusion criteria were that individuals smoke at least 10 cigarettes per day and that they had not previously taken varenicline. Participants were excluded if they were currently pregnant/nursing, used illicit drugs (excluding marijuana) in the past 60 days (confirmed by urine toxicology screen), had serious health concerns (cardiovascular disease, uncontrolled hypertension, had hepatic or renal disease, diabetes), or if they met DSM-IV criteria for psychotic, bipolar, or major depressive disorder in the past year. 205 individuals were treated for 12 weeks and, of these, 144 underwent a 6-minute resting state scan prior to initiation of medications (male n=91; varenicline n=82), and their data were used for the analyses that follow.
2.2. Clinical trial design
Consistent with previous trials (Gonzales et al., 2006) patients were titrated on varenicline to 1mg twice daily by day 7. All participants received a 30 minute baseline motivational enhancement session and brief (10 minute) counseling visits with their assigned therapist at each assessment (2, 6, 12 weeks) (Littlewood et al.). A target quit date was set for day 8. The manuscript summarizing clinical results is currently under review (Littlewood et al.), and results show that varenicline-treated participants were three times more likely to achieve prolonged abstinence compared to placebo.
Although abstinence is commonly used as a measure of success in smoking cessation trials, rates of complete abstinence were low in the placebo group (n=3 at 12 weeks for 30 day point prevalence), rendering logistic regression problematic as a method of analysis for measuring the interaction term (FNC*TrGrp), given small sample bias (G. King and Zeng, 2001). We therefore chose a continuous variable for our primary outcome measure which was a value for the total number of cigarettes smoked (NumCig) in the previous 28 days at the 6 week visit, and in the previous 30 days at the 12 week visit. NumCig at the screen visit for the previous 60 days (NumCig at Screen) was also calculated and used as the baseline smoking variable. Including NumCig at Screen as a predictor is analogous to (and the method often preferred over) predicting a change score (Vickers and Altman, 2001).
For the primary analyses (NumCig), missing data for outcomes (dropouts) were imputed to an adjusted screen visit value [e.g. (NumCig at screen visit)*28/60 = NumCig at 6 weeks; dropout numbers: n = 29 (15 varenicline)/144 by week 6 and 45 (24 varenicline)/144 by week 12] (Table 4). Imputing to baseline is a common approach for smoking cessation clinical trials (Ebbert et al., 2015; Higgins et al., 2008) even when rates of dropout approach ours. We also ran all analyses in the subgroup of subjects with complete data (n=99) who followed up at week 12, not using imputed outcomes (Table 5).
Table 4.
Outcome Prediction Results Using Generalized Estimating Equations: Whole Sample
| FNC | Coefficients for Predictor: Unstandardized Beta; Scr in model (No FTND) |
Coefficients for Predictor: Unstandardized Beta; (No Scr, No FNTD) |
Coefficients for Predictor: Unstandardized Beta; FTND in model (No Scr) |
|
|---|---|---|---|---|
| Hypothesized FNC Pairs | Insula-dACC | −0.5401 | −0.97 | −0.7722 |
| p value = 0.122 | p value = 0.008 | p value = 0.015 | ||
| Insula-dlPFC | −0.237 | −0.5793 | −0.233 | |
| p value = 0.477 | p value = 0.098 | p value = 0.513 |
FNC = functional network connectivity. dACC=dorsolateral anterior cingulate cortex, dlPFC = dorsolateral prefrontal cortex. FTND = Fagerstrom Test for Nicotine Dependence. Scr = number of cigarettes smoked during the 60 days prior to the screen visit.
No exploratory FNC pairs had significant coefficients using a Bonferroni correction (p<0.004); exploratory FNC pairs with sub-threshold significant findings (0.05>p>0.004) are in supplemental materials. Because the interaction term [FNC value * treatment group assignment (varenicline versus placebo; TrGrp)] was not significant for any of the models, results for models without the interaction term are presented.
TrGrp and head motion (rmsRot) were included in all models, but coefficients are not listed here for simplicity. TrGrp, FTND, and Scr were significant predictors in all models, and rmsRot was not a significant predictor in any of the models.
Standardized betas: Scr = 0.356(p<0.001), TrGrp = −0.180, rmsRot = −0.042, Insula-dACC=−0.105
Standardized betas: FTND = 0.327(p<0.001), TrGrp = −0.0211, rmsRot = −0.067, Insula-dACC = −0.151
Standardized betas: TrGrp = −0.194, rmsRot = −0.053, insula-dlPFC = −0.127
Table 5.
Outcome Prediction Results Using Generalized Estimating Equations: Complete Data Sample Only (n=99)
| FNC | Coefficients for Predictor: Unstandardized Beta; Scr in model |
Coefficients for Predictor: Unstandardized Beta (No Scr) |
|
|---|---|---|---|
| Hypothesized FNC Pairs | Insula-dACC | −1.2421 | −1.3652 |
| p value = 0.078 | p value = 0.043 | ||
| Insula-dlPFC | −0.050 | 0.009 | |
| p value = 0.910 | p value = 0.985 |
FNC = functional network connectivity. dACC=dorsolateral anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex. Varen = varenicline. Scr = number of cigarettes smoked during the 60 days prior to the screen visit.
No exploratory FNC pairs had significant coefficients using a Bonferroni correction (p<0.004); exploratory FNC pairs with sub-threshold significant findings (0.05>p>0.004) are in supplemental materials. Because the interaction term [FNC value * treatment group assignment (varenicline versus placebo; TrGrp)] was not significant for any of the models, results for models without the interaction term are presented.
TrGrp and head motion (rmsRot) were included in all models, but coefficients are not listed here for simplicity. TrGrp and Scr were significant predictors in all models, and rmsRot was not a significant predictor in any of the models.
Standardized betas: Scr = 0.266, TrGrp = −0.416, rmsRot=−0.180, Insula-dACC = −0.242
Standardized betas: TrGrp = −0.403, rmsRot = −0.137, Insula-dACC = −0.266
2.3. Measures
Withdrawal was measured with the Wisconsin Smoking Withdrawal Scale (WSWS) (Welsch et al., 1999) and craving with the Questionnaire of Smoking Urges (QSU) (Cox et al., 2001) 15–30 minutes prior to the scan. Participants were asked to be abstinent for 2 hours prior to the scan visit (there were only 8 participants who did not report being abstinent 2 hours prior to the scan). All participants were asked how many hours it had been since they last smoked at the time of the scan. Nicotine dependence severity was measured with the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton et al., 1991) at the screen visit. The time-line follow-back procedure (TLFB) (Sobell and Sobell, 1996) was used to record tobacco product, alcohol, and marijuana use in the 60 days prior to the screen and during the interim period between each follow-up assessment.
2.4. MRI acquisition and preprocessing
All fMRI scans were acquired on a Siemens 3T Trio scanner located at the Mind Research Network in Albuquerque NM. The resting state sessions were 6 minutes in length, during which participants were asked to stare at the cross on the screen, stay awake and alert, but to clear their mind and not think about anything in particular as if their brain was at rest. Further details on acquisition and preprocessing methods are available in Supplemental Materials.
2.5. FNC analyses
After preprocessing, group independent components analysis (spatio-temporal regression) (Calhoun et al., 2001; Erhardt et al., 2011) was utilized to extract individual subject components (networks and time series) from 15 of 100 intrinsic connectivity networks identified from a large separate sample of controls which fell on regions previously implicated in craving, withdrawal, and nicotine dependence, namely the insula, rACC, caudate, putamen, DLPFC, dACC (Figure 1) (Allen et al., 2014). Although 30 components are more frequently used, 100 component ICA is increasingly common (Damaraju et al., 2014; Wu et al., 2015). The relevant time series were then filtered using a low-pass filter of > 0.15 Hz, and detrending to remove low frequency noise, despiked, and motion was regressed out on a per-subject basis using the six motion parameters. In addition, mean root mean squared translational (rmsTrans) and rotational (rmsRot) displacement over the run were calculated for each subject to measure head motion (Jenkinson, 2003), and were included as covariates in all subsequent analyses for which they were correlated at a significance (p value) of <0.05 with the FNC value or the outcome of interest.
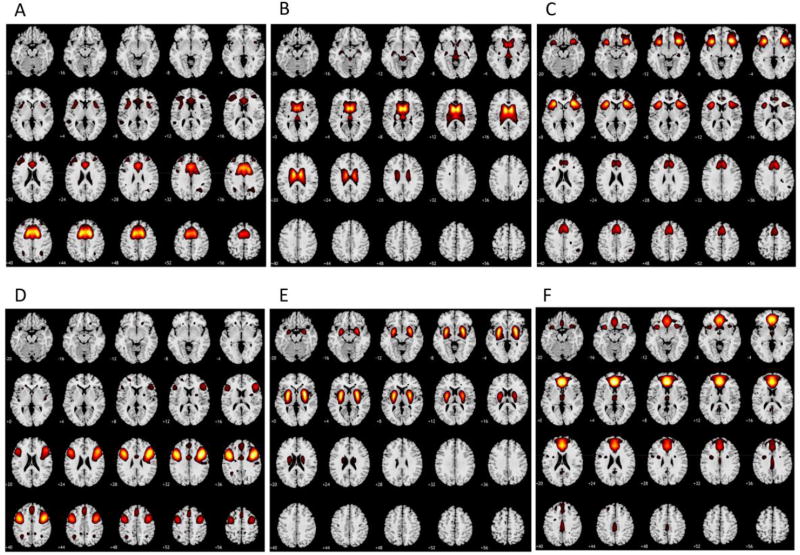
Figure 1.
This figure shows the six networks utilized in our study (A-dorsal anterior cingulate cortex B-putamen C-insula D-dorsolateral prefrontal cortex E-caudate F-rostral anterior cingulate cortex) in the original sample of 405 controls (Allen et al. 2014). These networks were used to extract the individual-subject time-series from our sample of participants from which functional network connectivity values were subsequently calculated. Each axial slice is marked with a number representing the MNI z coordinate at its lower left. Yellow/red indicates voxels which are more positively associated with the network (region) of interest (positive z scores with a value of ≥ 1 included, yellow is the peak value in that population for that component). Voxels which are more negatively associated with the network are excluded from the images for ease of interpretation.
The functional network connectivity toolbox (http://icatb.sourceforge.net/fnc/software/FncVer2.2.zip; icatb_corr) was used to calculate the degree of correlation between time courses. For all FNC analyses, correlations were transformed to z-scores using Fisher’s transformation (z = arctanh(r)).
First, in line with our initial hypotheses, all analyses for Aim 1 were performed on the following 2 pairs: insula-dACC, insula-dlPFC. Then an exploratory analysis of all possible pairs (13 additional) was performed to complete Aim 2. For our 2 hypothesized FNC, we did not correct for multiple comparisons, and simply report the findings. For the exploratory FNC we report the p values from the analyses directly as well, without correcting for multiple comparisons. However, we alert the reader to the fact that a Bonferroni-corrected p value for 13 tests (number of exploratory FNC) is 0.004, and correspondingly, the results are only highlighted in the discussion if they meet this threshold for a Bonferroni correction.
2.6. Relationship with NUD severity
First, we correlated FNC values with baseline smoking quantity and FTND. We felt this was especially important to establish first, as, in the previous work predicting treatment outcome with rsFC (Addicott et al., 2015; Janes et al., 2010) baseline smoking was not used as a covariate, and their results could also have therefore simply been markers of the quantity that the individual had recently been smoking.
2.7. Clinical outcome prediction
We tested a series of models with each FNC individually (first the 2 hypothesized FNC, and then the remaining 13 pairs). For these models, baseline smoking, treatment group assignment (TrGrp), FNC and rms were entered as predictors and smoking at 6 and 12 weeks as the predicted outcomes. In addition, an interaction term (FNC*TrGrp) was added as a predictor in all models to identify whether there was a differential predictive value of the FNC depending on what treatment the individual received. When the interaction term (FNC*TrGrp) was not significant, we reran the model without it, in order to measure the overall ability of the predictor to predict outcome (eg. not specifically to treatment group assignment).
The outcome variables (NumCig at 6 and 12 weeks) were count variables which appeared to have a Poisson distribution, but did not meet the assumption required for a Poisson distribution (mean equaling variance) so we chose a negative binomial with log link model to predict clinical outcomes in SPSS. In order to test a repeated measures outcome comprised of correlated variables we used generalized estimating equations with a robust covariance estimation method and assigned time as a within-subjects variable, and smoking as our outcome variable, which outputs a single test for the relationship between predictor and smoking outcome incorporating NumCig at 6 and 12 weeks. Maximum likelihood estimation (MLE) was used to estimate the negative binomial dispersion parameters and correlation matrix representing within subjects dependencies was assigned as AR(1). P values associated with the Wald Chi Square were used to test significance of predictors.
These analyses were repeated 1) adding FTND, WSWS, and QSU to the model as predictors, to determine whether or not FNC predicted smoking outcome above and beyond these additional easy-to-obtain clinical variables (because these variables were correlated with NumCig at Screen, which could result in multicollinearity, they are in Supplementary Materials) (Table S5) 2) using an abstinence-based binary outcome (logistic regression regression) without including a FNC*TrGrp interaction term due to small sample bias (discussed above) (Table S6, S7).
2.8. Binning by high and low withdrawal
Changes in resting state connectivity over 24 hours of abstinence were observed to predict treatment outcomes (Sweitzer et al., 2016), and a variety of studies have shown alterations in brain activity during subjective withdrawal or from satiation to abstinence in NUD (Bi et al., 2016; Cole et al., 2010; Fedota and Stein, 2015; Froeliger et al., 2015; Huang et al., 2014; Lerman et al., 2014; Moran-Santa Maria et al., 2015; Sutherland et al., 2013b; Sweitzer et al., 2016). Out of concern that the FNC values could have a different relationship with clinical outcome, depending on the withdrawal state of the individual at the time the measure was obtained, we felt it important to explore for interactions between FNC values and withdrawal in our outcome prediction models. In our study, because the time between the last cigarette and the scan was not controlled [participants were simply asked to not smoke for 2 hours before the scan, and number of hours since the last cigarette was negatively correlated with dependence severity and not correlated with subjective withdrawal (Table 2) indicating that this variable was more a reflection of their ability to remain abstinent for a period of time than of withdrawal severity], we chose to use a self-report measure of withdrawal (WSWS) to explore for these interactions.
Table 2.
Bivariate Correlations (Spearman’s rho) Between Clinical Variables
| FTND | WSWS | QSU | NumCig 6Wk | NumCig 12Wk | Hrs | |
|---|---|---|---|---|---|---|
| Scr | 0.471** | 0.0001 | 0.311** | 0.250** | 0.284** | −0.276**1 |
| FTND | 0.255**1 | 0.489** | 0.254** | 0.324** | −0.200**1 | |
| WSWS | 0.309**1 | 0.0951 | 0.0341 | −0.0222 | ||
| QSU | 0.169* | 0.208* | 0.0211 | |||
| NumCig 6Wk | 0.841** | −0.0151 | ||||
| NumCig 12Wk | −0.0271 |
Significant at p < 0.05
Significant at p < 0.01
n=143
n=142
Scr = total number of cigarettes smoked during the past 60 days at the screen visit, FTND = Fagerstrom Test for Nicotine Dependence, WSWS = Wisconsin Smoking Withdrawal Scale, QSU = Questionnaire of Smoking Urges, Hrs = Hours since the last cigarette at the time of the scan, NumCig 6Wk = total number of cigarettes smoked in the previous 28 days at the 6 week visit, NumCig 12Wk = total number of cigarettes smoked in the previous 30 days at the 12 week visit.
With this in mind, we performed a final exploratory analysis for each FNC pair individually by first adding both 3 way (WSWS*FNC*TrGrp) and a 2 way (WSWS*FNC) interaction term into the model as predictors, and then just a 2 way interaction term (WSWS*FNC) along with WSWS. To explore significant interaction terms, we then stratified the sample into 2 equally sized groups - high withdrawal (n=72) and low withdrawal (n=71) based on WSWS scores - and then reran the outcome prediction models for each individual FNC using the following predictors in each group separately: NumCig at Screen, TrGrp, the interaction term (FNC*TrGrp), rms, as well as the FNC.
3. Results
3.1. Sample characteristics and relationships between variables used in subsequent analyses
Table 1 presents baseline demographic characteristics and Table 2 presents correlations between the clinical variables. Table 3 presents the correlations between the FNC and baseline clinical variables. Notably, there were no significant differences in key variables between treatment groups, NumCig at Screen was highly correlated with QSU and FTND (ps < 0.001), but not WSWS, and insula-dACC FNC was correlated with NumCig at Screen.
Table 1.
| a. Baseline Characteristics by Treatment Group
| ||
|---|---|---|
| Varenicline (n=82) | Placebo (n=62) | |
| Number of males (percent) | 54 (66%) | 37 (60%) |
| Age years (SD) | 34.3 (10.4) | 33.4 (10.1) |
| Total number of cigarettes smoked past 60 days at screen (SD) | 936.9 (395.2) | 917.3 (325.9) |
| Fagerstrom Test for Nicotine Dependence (SD) | 4.6 (2.0) | 4.6 (2.2) |
| Wisconsin Smoking Withdrawal Scale (SD, max, min) | 11.2 (3.8, 3, 22)1 | 12.0 (3.4, 13, 68) |
| Questionnaire of Smoking Urges (SD, max, min) | 41.2 (14.1, 10, 68) | 42.3 (14.1, 5, 21) |
| rmsRot | 0.23 (0.1) | 0.24 (0.1) |
| rmsTrans | 87.08 (281.4) | 42.1 (182.6) |
| Hours since the last cigarette at the time of the scan | 6.13 (5.0) | 5.17 (3.9)2 |
| Number standard alcohol drinks past 60 days | 41.71(81.98) | 45.07 (72.54) |
| Number marijuana smoking days past 60 days | 9.55 (19.45) | 8.16 (19.95) |
| b. Baseline Substance Use by Treatment Group
| |||||
|---|---|---|---|---|---|
| Never | Once or twice | Monthly | Weekly | Daily/ Almost Daily | |
| Percent used cocaine in last 3 months | 97.9 | 2.1 | 0 | 0 | 0 |
| Percent used methamphetamine in last 3 months | 99.3 | 0.7 | 0 | 0 | 0 |
| Percent used street opioids | 0 | 0 | 0 | 0 | 0 |
rmsRot = mean root mean squared rotational displacement over the resting state run, rmsTrans = mean root mean squared translational displacement over the resting state run, max = maximum value, min = minimum value.
Chi square tests for dichotomous variables and independent samples t tests for continuous variables demonstrated no significant differences (ps>0.05) on any measures between varenicline and placebo groups. All measures were obtained prior to initiation of treatment.
n=81.
n=61.
Table 3.
Bivariate Correlations (Spearman’s rho) Between FNC and Baseline Smoking Variables
| Scr | FTND | WSWS1 | ||
|---|---|---|---|---|
| Hypothesized FNC Pairs | Insula-dACC | −0.205* | −0.057 | 0.057 |
| Insula-dlPFC | −0.153 | −0.115 | 0.158 | |
| Exploratory FNC Pairs | Putamen-Caudate | −0.182* | ||
| Putamen-dlPFC | 0.165* | 0.219** | ||
| Insula-rACC | 0.170* |
Significant at p < 0.05
Significant at p < 0.01
Scr = total number of cigarettes smoked during the past 60 days at the screen visit, FTND = Fagerstrom Test for Nicotine Dependence, WSWS = Wisconsin Smoking Withdrawal Scale, QSU = Questionnaire of Smoking Urges, FNC = functional network connectivity. dACC= dorsolateral anterior cingulate cortex, dlPFC = dorsolateral prefrontal cortex, rACC = rostral anterior cingulate cortex.
Only results for exploratory FNC pairs with significant findings presented here; exploratory FNC pairs with no significant findings are not listed.
n=143
Table S1 presents the correlations between head motion (rmsTrans, rmsRot) and the FNC and behavioral measures. RmsTrans and/or rmsRot were significantly correlated with caudate-dlPFC FNC, dACC-dlPFC FNC, caudate-rACC FNC, rACC-DLPFC FNC, rACC-dACC, putamen-insula FNC, putamen-rACC FNC, putamen-dACC FNC, and insula-rACC FNC and NumCig at 12 weeks. RmsRot was not correlated with baseline smoking, FTND, withdrawal, or treatment group assignment. RmsTrans and rmsRot were highly correlated with one another (rho=0.916, p<0.001) and we used rmsRot as a covariate for any analyses in which one of them was correlated with the FNC or outcome of interest.
3.2. Relationships with baseline smoking and FTND
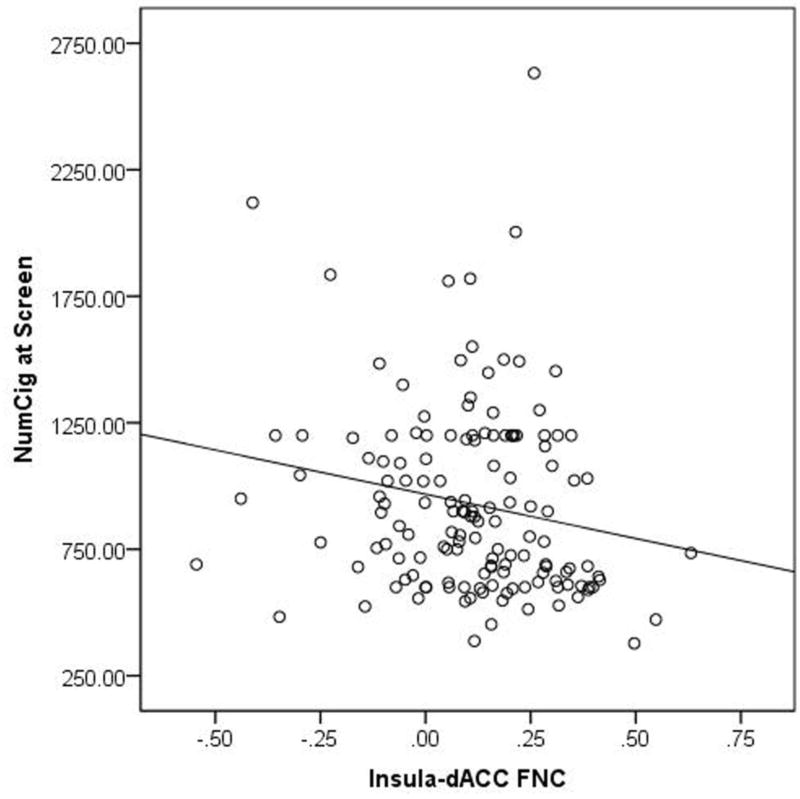
Our primary hypothesis was that greater FNC between 2 pairs of regions, insula-dACC FNC and insula-dlPFC FNC, would be associated with worse outcome to treatment. However, given that this was based on findings from 2 studies which had controlled for FTND but had not controlled for baseline smoking quantities, we first looked at correlations between FNC with NumCig at Screen and FTND. We observed that for insula-dACC FNC, higher NumCig at Screen was associated with lower FNC, (rho=−0.205, p=0.014) (Figure 2), but not with FTND (p>0.1). For insula-dLPFC FNC there was a relationship significant at a trend level, and in the same direction as that observed for insula-dACC, with NumCig at Screen (rho=−0.153, p=0.068), but not with FTND (p>0.1) (Table 3).
Figure 2.
This figure demonstrates a simple scatter plot showing the relationship between the insula to dorsal anterior cingulate functional connectivity value (Insula-dACC FNC) and the total number of cigarettes smoked in the 60 days prior to the screen visit (NumCig at Screen).
For the exploratory analyses, there were no significant relationships between the FNC on an individual basis, a few significant (p<0.05) relationships emerged including a negative correlation between putamen-caudate and NumCig at Screen (rho=-0.182, p=0.029), and a positive correlation between putamen-dlPFC FNC (rho=0.165, p=0.048) and FTND (Table 3).
3.3. Outcome prediction
As stated previously, rmsRot was negatively correlated with both NumCig at 6 and 12 weeks and was therefore entered as a covariate in all outcome prediction analyses.
For our primary analyses (NumCig as outcome, full sample with missing imputed to baseline; Table 4) of the hypothesized pairs of regions (insula-dACC, insula-dlPFC), neither FNC significantly predicted outcome [unstandardized beta(B)=−0.54, p=0.122; B=−0.237, p=0.477, respectively]. Given the significant negative correlation between insula-dACC and baseline smoking, and since the previous work that showed a relationship between connectivity and treatment outcome upon which our hypotheses based utilized FTND instead of baseline smoking as a covariate (Addicott et al., 2015; Janes et al., 2010), we reran the prediction models with baseline smoking removed from the model, and observed that insula-dACC FNC significantly predicted outcome (B=− 0.978, and p=0.008) and insula-dlPFC FNC at a trend level (B=−.579, p=0.098) (Table 4). If we replaced NumCig at Screen with FTND, insula-dACC significantly predicted outcome in the expected direction (B=−0.772, p=0.015) but insula-dlPFC did not (B=− 0.233, p=0.513).
Rerunning the models to obtain standardized betas demonstrated that both NumCig at Screen and FTND were equally robust at predicting outcome (standardized betas for both were 0.3, p values <0.001 for the insula-dACC analyses) (Table 4) whereas the correlation between insula-dACC FNC and NumCig at Screen was larger than with FTND (Table 3), indicating that the latter was driving the difference between the two models for insula-dACC. In all models, neither the interaction terms nor rmsRot were significant predictors of outcome, whereas TrGrp and NumCig at Screen were always significant predictors of outcome.
When only subjects that followed up at 12 weeks were analyzed (n=99), results for the insula-dACC FNC were similar to the results for the whole sample (Table 5). For the exploratory analyses, none of the exploratory FNC significantly (Bonferroni threshold p<0.004) predicted the NumCig outcome, correcting for baseline smoking, but a few were significant before applying a Bonferonni correction (Table S2, S3).
Additional supporting analyses were performed, as described in the methods, and results are presented in Supplemental Materials. Insula-dACC FNC was a significant predictor of the NumCig outcome when additional clinical variables were added to the model (FTND, QSU, WSWS) (Table S4, S5). Neither insula-dACC nor insula-dlPFC FNC were significant predictors of outcome using a binary outcome measure (Table S6, S7).
3.4. Binning by high and low withdrawal
When we reran the predictive models adding 2- and 3- way interaction terms for WSWS for all 15 FNC pairs separately, we found no significant 3-way (WSWS*FNC*TrGrp) interaction terms for any of the FNC, so we dropped this term. Two significant (ps<0.004) 2-way (WSWS*FNC) interaction terms were identified as follows: caudate-dlPFC (p <0.001), caudate-dACC (p < 0.001) (Table 6) and a few sub-threshold interaction terms (Table S4).
Table 6.
Outcome Prediction Results Using Generalized Estimating Equations: High WSWS and Low WSWS Analyzed Separately; Whole Sample
| FNC | Interaction Term (TrGrp by FNC) p value |
Coefficients for Predictor: Unstandardized Beta |
||
|---|---|---|---|---|
| High WSWS | Hypothesized FNC Pairs | Insula-dACC | 0.758 | −0.822 |
| p value = 0.133 | ||||
| Insula-dlPFC | 0.840 | −0.522 | ||
| p value = 0.339 | ||||
| Exploratory FNC Pairs with Significant WSWS * FNC Interaction Terms | Caudate-dACC | 0.818 | 2.2841 | |
| p value = 0.001 | ||||
| Putamen-dACC | 0.372 | 1.6682 | ||
| p value < 0.001 | ||||
| Low WSWS | Hypothesized FNC Pairs | Insula-dACC | 0.173 | −0.598 |
| p value = 0.231 | ||||
| Insula-dlPFC | 0.837 | −0.266 | ||
| p value = 0.590 | ||||
| Exploratory FNC Pairs with Significant WSWS * FNC Interaction Terms | Caudate-dACC | 0.045 | Varen 0.912 | |
| p value = 0.258 | ||||
| Placebo −0.608 | ||||
| p value = 0.286 | ||||
| Putamen-dACC | 0.088 | −0.618 | ||
| p value = 0.274 |
WSWS = Wisconsin Withdrawal Scale obtained at time of scan, FNC = functional network connectivity. dACC=dorsolateral anterior cingulate cortex, dlPFC = dorsolateral prefrontal cortex, rACC = rostral anterior cingulate cortex. Varen = varenicline. TrGrp = treatment group assignment (varenicline versus placebo).
Only results for exploratory FNC pairs with significant interaction terms (WSWS * FNC) using a Bonferroni correction (p value < .004) listed here. Exploratory FNC pairs with sub-threshold significant findings (0.05>p>0.004) are in supplemental materials, and FNC pairs with no significant findings are not reported. When the interaction terms [FNC value * TrGrp] were significant (p<0.05) models were run in varenicline and placebo groups separately and coefficients for each are presented.
TrGrp, head motion (rmsRot) and number of cigarettes smoked during the 60 days prior to the screen visit (Scr) were included in all models, but coefficients are not generally listed here for simplicity. TrGrp was a significant predictor in all models, Scr was a significant predictor for all models run in the Low WSWS subgroup but not for any of the models run in the High WSWS subgroup, and rmsRot was a significant predictor in individuals with low WSWS on varenicline for rACC-dlPFC (beta = −7.818) and caudate-dACC (beta = −8.344), and in all individuals for putamen-dACC (beta = −3.701).
Standardized betas: Scr = 0.179, TrGrp = −0.352, rmsRot = 0.048, Caudate-dACC = 0.426.
Standardized betas: Scr = 0.144, TrGrp = −0.329, rmsRot = 0.128, Putamen-dACC = 0.371.
We then split participants into either a high withdrawal (WSWS mean=14.00 SD=2.24) or a low withdrawal (WSWS mean=8.69, SD=2.13) subgroup to explore interactions. For those reporting low withdrawal symptomatology, there was a significant FNC*TxGrp interaction term for the caudate-dACC FNC but no significant effects of either caudate-dACC or putamen-dACC on outcome. For individuals reporting high withdrawal symptomatology, by contrast, caudate-dACC (B=2.284, p=0.001), and putamen-dACC (B = 1.668, p<0.001) were highly significant predictors of treatment outcome such that higher connectivity at the time of the scan predicted greater later smoking. In addition, caudate-dlPFC (sub-threshold by Bonferroni-corrected p value) was a predictor of outcome in the high withdrawal group of individuals (B=1.366, p=0.022) (Table 6).
We also examined relationships between WSWS grouping (high vs. low) and Gender (Chi square), age, FTND, and FD values using independent samples t-tests. High WSWS participants had significantly higher head motion by rmsRot (p=0.017, t=2.419), and higher FTND scores (p=0.019, t=2.383), but did not otherwise differ from Low WSWS participants. Further analyses (Supplemental Materials) indicated that it was the withdrawal state which was driving interactions.
4. Discussion
In summary, in this analysis of the association of FNC between key regions there were some notable findings. First, greater insula-dACC was negatively related to recent smoking quantities, and greater connectivity between these regions predicted better treatment outcomes, but only when FTND was used instead of current smoking quantities as a covariate. These findings were actually in line with the previous work from which we had derived our hypotheses showing that lower connectivity between these regions predicts increased rates of relapse, and which used FTND as a covariate (Addicott et al., 2015; Janes et al., 2010). It was also somewhat supported by previous work showing a positive relationship between insula-dACC FNC and nicotine dependence severity in schizophrenia (Moran et al., 2012). However, since this marker was not a significant predictor of outcome when baseline smoking quantity was in the model, it remains to be seen whether or not insula-dACC rsFC/FNC contribute any additional value in outcome prediction which is not already provided by baseline smoking quantities. In short, connectivity between these regions may be important biomarker of current smoking quantities which is a robust predictor of overall treatment outcome (Addicott et al., 2015; Janes et al., 2010).
Although these findings do not necessarily support the use of insula-dACC FNC in a clinical setting to identify individuals at risk for poorer treatment outcome, requiring more intensive intervention (asking about smoking quantities is easier than obtaining a fMRI scan), it could be studied for use as a probe during pharmacotherapeutic trials (eg. as a biological target for treatments under-development) to get at medication efficacy more quickly and efficiently than behavioral measures – medications which increase insula-dACC FNC may prove to be more likely to promote abstinence.
In a post-hoc exploratory analysis, when the sample was divided into a low and high withdrawal group, FNC differentially predicted clinical outcome, depending on the group to which they were assigned. Specifically, in individuals who were experiencing higher withdrawal, higher FNC between striatum and dPFC (particularly dACC) was associated with worse treatment outcomes. This would indicate that higher striatal-dPFC FNC when measured during the subjective experience of withdrawal may be a biomarker for relapse vulnerability. Although some studies have found a negative correlation between fronto-striatal connectivity and nicotine dependence (Fedota and Stein, 2015; Hong et al., 2009) and that an increase in fronto-striatal connectivity occurs concurrently with a reduction in withdrawal symptoms (Froeliger et al., 2015), the literature regarding the neural signatures of craving and withdrawal in fronto-striatal circuits is decidedly mixed; in support of our findings, other studies have found an association between higher craving and higher connectivity between striatum and PFC (Fedota and Stein, 2015; Huang et al., 2014; Sweitzer et al., 2016). Top-down fronto-striatal excitatory projections are important for cue-induced drug-seeking behavior (Everitt and Robbins, 2005; Kalivas and Volkow, 2005). Although speculative, it is possible that greater connectivity between PFC and striatum in individuals who are experiencing higher withdrawal-related discomfort may be a marker of greater mobilization of this circuit in anticipation of smoking, which thereby predicts a worse treatment outcome.
Additionally, higher dlPFC-dACC FNC predicted better outcomes in the low subjective withdrawal group of individuals (p=0.010, Table S4). Although this finding did not meet significance after correcting for multiple comparisons it is still notable in that in other SUD, high within PFC may relate to better emotion regulation (Seo et al., 2016) and longer-term abstinence (Camchong et al., 2013).
Unfortunately, our study did not shed much light on the predictors of outcome during treatment with varenicline, or its mechanisms of action. Varenicline has been found to be associated with decreases in connectivity between insula and dACC/rACC/parahippocampal gyrus/PCC (Sutherland et al., 2013a), and we therefore would have expected that individuals who have higher connectivity between these regions would have a larger treatment response to varenicline (indicating that the effect of dampening connectivity was a therapeutic one). However, there were no significant FNC*TrGrp interaction terms after correcting for multiple comparisons. Genetic variations and adherence could affect response to varenicline (D. P. King et al., 2012); had we had a more robust measure of treatment compliance or included genetic markers to identify optimal responders, we may have had more power to detect an effect.
Finally, we mention an apparently contradictory study which demonstrated that insula- dACC coupling at rest was positively correlated with enhanced smoking cue-reactivity in brain areas associated with attention and motor preparation, including the right vlPFC and the dorsal striatum (Janes et al., 2015). Moreover, greater cue reactivity has repeatedly been shown to predict worse outcome in NUD and SUD at large (Janes et al., 2010; Mann et al., 2014; Versace et al., 2014). However, at closer inspection, this study may not be contradictory to our findings: previous work in NUD showed higher cue reactivity in insula and dACC predicts worse outcome (Janes et al., 2010) whereas higher cue reactivity in vlPFC and dorsal striatum have not been linked to worse treatment outcome. Activation in areas such as the vlPFC which are involved in inhibition and cognitive control (Levy and Wagner, 2011; Wilcox et al., 2014), may actually be protective.
There are a few notable limitations to our work. For one, in order to minimize the concerns for multiple comparisons, we chose to explore a limited number of brain regions, based a comprehensive review on the topic that highlighted DMN, ECN, SN and striatum (Fedota and Stein, 2015). However, there has been growing awareness that other key areas in the DMN like PCC and precuneus, and outside of these networks like the vlPFC, and hippocampus may be playing important roles in NUD (Cole et al., 2010; Froeliger et al., 2015; Huang et al., 2014; Moran-Santa Maria et al., 2015; Sutherland et al., 2013a). Second, our select set of 15 components was derived from 100 component ICA instead of a lower order model, which is more commonly done. This was done because the 100 component atlas is easily downloadable and because it allowed for more fine-tuned exploration in cases where significant results existed. Also it allowed us to test our main hypotheses about the insula-dACC and dlPFC. But we were not able to test hypotheses about the larger DMN, ECN or SN networks commonly discussed in the rsFC nicotine dependence literature (Lerman et al., 2014). Third, replication of our results in an independent sample would be important. However, we do highlight that we replicated some findings (greater insula-dACC/dlPFC FNC and worse treatment outcome) from the broader literature. Finally, when we used logistic regression to predict a binary abstinence-based outcome with FNC there was not a significant effect of insula-dACC FNC on outcome. This was likely because we had very low numbers of abstinent individuals (only 24 out of 144) and so, in our sample, a reduction in number of cigarettes smoked may have been a more sensitive outcome measure. At any rate, reduction is likely a valid outcome measure, as it predicts later cessation (Begh et al., 2015). We therefore believe that dACC-insula FNC deserves continued attention.
In summary, our results indicate that lower connectivity between dACC and insula may be a biomarker of greater current levels of smoking, that greater connectivity between striatum and PFC predict worse treatment outcome when these measures are obtained during a state of high subjective withdrawal, and that it is important to account for self-reported withdrawal symptom severity during measurement of brain rsFC and FNC, and during attempts to identify biomarkers of treatment outcome. RsFC and FNC have potential to be useful biomarkers of nicotine dependence severity (current smoking levels), states associated with smoking relapse (craving and withdrawal) and of particular clinical import, treatment outcome.
Supplementary Material
Highlights.
Resting state functional network connectivity (FNC) between insula and dorsal anterior cingulate cortex (dACC) is negatively correlated with current smoking quantities in individuals seeking treatment for nicotine use disorder (NUD).
Higher FNC between insula and dACC predicts better treatment outcome in NUD but only when current smoking quantity is not included as a covariate.
Higher FNC between striatum and dorsal prefrontal cortex (dACC and dorsolateral prefrontal cortex) predicts worse treatment outcome when these measures are obtained from individuals reporting higher subjective withdrawal at the time of the MRI scan.
Acknowledgments
Funding Sources:
This work was supported by the National Institutes of Health grant number 1R01-DA025074 awarded to Kent Hutchison and grant number K23-AA021156 awarded to Claire Wilcox
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ. Increased functional connectivity in an insula-based network is associated with improved smoking cessation outcomes. Neuropsychopharmacology. 2015;40(11):2648–2656. doi: 10.1038/npp.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24(3):663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Tang KZ, Mesaros AC, Blair IA, Leone F, Strasser AA. Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol. 2012;26(10):1383–1390. doi: 10.1177/0269881112449397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin AJ, Duka T, Rusted J, Jackson A. Effect of varenicline on aspects of inhibitory control in smokers. Psychopharmacology (Berl) 2014;231(18):3771–3785. doi: 10.1007/s00213-014-3512-7. [DOI] [PubMed] [Google Scholar]
- Begh R, Lindson-Hawley N, Aveyard P. Does reduced smoking if you can’t stop make any difference? BMC Med. 2015;13:257. doi: 10.1186/s12916-015-0505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Singleton EG, Heishman SJ. A comparison of the fagerstrom test for cigarette dependence and cigarette dependence scale in a treatment-seeking sample of pregnant smokers. Nicotine Tob Res. 2016;18(4):477–483. doi: 10.1093/ntr/ntv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Yuan K, Guan Y, Cheng J, Zhang Y, Li Y, Yu D, Qin W, Tian J. Altered resting state functional connectivity of anterior insula in young smokers. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9511-z. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 2011;218(2):391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional mri data using independent component analysis. Hum Brain Mapp. 2001;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2013;37(1):75–85. doi: 10.1111/j.1530-0277.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52(2):590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (qsu-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Caprihan A, Lowe JR, Allen EA, Calhoun VD, Phillips JP. Functional connectivity in the developing brain: A longitudinal study from 4 to 9months of age. Neuroimage. 2014;84:169–180. doi: 10.1016/j.neuroimage.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, Treadow J, Yu CR, Dutro MP, Park PW. Effect of varenicline on smoking cessation through smoking reduction: A randomized clinical trial. JAMA. 2015;313(7):687–694. doi: 10.1001/jama.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ica methods for analysis of fmri data. Hum Brain Mapp. 2011;32(12):2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fedota JR, Matous AL, Salmeron BJ, Gu H, Ross TJ, Stein EA. Insula demonstrates a non-linear response to varying demand for cognitive control and weaker resting connectivity with the executive control network in smokers. Neuropsychopharmacology. 2016;41(10):2557–2565. doi: 10.1038/npp.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: Prospects for biomarker development. Ann N Y Acad Sci. 2015;1349:64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Sutherland MT, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Reward anticipation is differentially modulated by varenicline and nicotine in smokers. Neuropsychopharmacology. 2015;40(8):2038–2046. doi: 10.1038/npp.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger B, McConnell PA, Stankeviciute N, McClure EA, Kalivas PW, Gray KM. The effects of n-acetylcysteine on fronto-striatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fmri pilot study. Drug Alcohol Depend. 2015;156:234–242. doi: 10.1016/j.drugalcdep.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard S, Nides M, Oncken C, Azoulay S, Billing C, Watsky E, Gong J, Williams K, Reeves K. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Jama. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: Decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171(8):770–777. doi: 10.1001/archinternmed.2011.138. [DOI] [PubMed] [Google Scholar]
- Hartmann-Boyce J, Stead LF, Cahill K, Lancaster T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2013 reviews. Addiction. 2014;109(9):1414–1425. doi: 10.1111/add.12633. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. British Journal of Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins JP, White IR, Wood AM. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clin Trials. 2008;5(3):225–239. doi: 10.1177/1740774508091600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitsman B, Hogarth L, Tseng LJ, Teige JC, Shadel WG, DiBenedetti DB, Danto S, Lee TC, Price LH, Niaura R. Dissociable effect of acute varenicline on tonic versus cue-provoked craving in non-treatment-motivated heavy smokers. Drug Alcohol Depend. 2013;130(1-3):135–141. doi: 10.1016/j.drugalcdep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66(4):431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, King JA, Ursprung WW, Zheng S, Zhang N, Kennedy DN, Ziedonis D, DiFranza JR. The development and expression of physical nicotine dependence corresponds to structural and functional alterations in the anterior cingulate-precuneus pathway. Brain Behav. 2014;4(3):408–417. doi: 10.1002/brb3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, Frederick Bde B, Lukas SE. Insula-dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology. 2015;40(7):1561–1568. doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick Bde B, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125(3):252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Measuring transformation error by rms deviation. 2003 from http://www.fmrib.ox.ac.uk/analysis/techrep/tr99mj1/tr99mj1/index.html.
- Jhanjee S, Jain R, Jain V, Gupta T, Mittal S, Goelz P, Schnoll RA. Evaluating the effects of varenicline on craving, withdrawal, and affect in a randomized, double-blind, placebo-controlled clinical trial of varenicline for smokeless tobacco dependence in india. J Psychoactive Drugs. 2015;47(4):325–330. doi: 10.1080/02791072.2015.1075092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, Kaprio J, Lerman C, Park PW. Smoking cessation pharmacogenetics: Analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2012;37(3):641–650. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G, Zeng L. Logistic regression in rare events data. Political Analysis. 2001;9:137–163. [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71(5):523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Japuntich SJ, Piper ME, Jorenby DE, Schlam TR, Baker TB. Isolating the role of psychological dysfunction in smoking cessation: Relations of personality and psychopathology to attaining cessation milestones. Psychol Addict Behav. 2012;26(4):838–849. doi: 10.1037/a0028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood RA, Claus ED, Wilcox CE, Mickey J, Arenella P, Bryan AD, Hutchison KE. Moderators of smoking cessation and reduction in a randomized-controlled trial of varenicline versus placebo (submitted) doi: 10.1007/s00213-017-4721-7. [DOI] [PubMed] [Google Scholar]
- Mann K, Vollstadt-Klein S, Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN. Predicting naltrexone response in alcohol-dependent patients: The contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res. 2014;38(11):2754–2762. doi: 10.1111/acer.12546. [DOI] [PubMed] [Google Scholar]
- McPherson S, Packer RR, Cameron JM, Howell DN, Roll JM. Biochemical marker of use is a better predictor of outcomes than self-report metrics in a contingency management smoking cessation analog study. Am J Addict. 2014;23(1):15–20. doi: 10.1111/j.1521-0391.2013.12059.x. [DOI] [PubMed] [Google Scholar]
- Moran LV, Sampath H, Stein EA, Hong LE. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142(1-3):223–229. doi: 10.1016/j.schres.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Hartwell KJ, Hanlon CA, Canterberry M, Lematty T, Owens M, Brady KT, George MS. Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addict Biol. 2015;20(2):407–414. doi: 10.1111/adb.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JD, Hawk LW, Jr, Ashare RL, Schlienz NJ, Mahoney MC. The effects of varenicline on attention and inhibitory control among treatment-seeking smokers. Psychopharmacology (Berl) 2012;223(2):131–138. doi: 10.1007/s00213-012-2700-6. [DOI] [PubMed] [Google Scholar]
- Richmond RL, Kehoe L. Ten-year survival outcome of the nicotine transdermal patch with cognitive behavioural therapy. Aust N Z J Public Health. 2007;31(3):282–285. doi: 10.1111/j.1467-842x.2007.00062.x. [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Sinha R. Neural correlates and connectivity underlying stress-related impulse control difficulties in alcoholism. Alcohol Clin Exp Res. 2016;40(9):1884–1894. doi: 10.1111/acer.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback user’s guide: A calendar method for assessing alcohol and drug use. Toronto, Ontario, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- Stoeckel LE, Chai XJ, Zhang J, Whitfield-Gabrieli S, Evins AE. Lower gray matter density and functional connectivity in the anterior insula in smokers compared with never smokers. Addict Biol. 2016;21(4):972–981. doi: 10.1111/adb.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013a;74(7):538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Stein EA. Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl) 2013b;228(1):143–155. doi: 10.1007/s00213-013-3018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62(4):2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Addicott MA, Denlinger R, Raiff BR, Dallery J, McClernon FJ, Donny EC. Smoking abstinence-induced changes in resting state functional connectivity with ventral striatum predict lapse during a quit attempt. Neuropsychopharmacology. 2016;41(10):2521–2529. doi: 10.1038/npp.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY, Minnix JA, Karam-Hage MA, Brown VL, Wetter DW, Cinciripini PM. Prequit fmri responses to pleasant cues and cigarette-related cues predict smoking cessation outcome. Nicotine Tob Res. 2014;16(6):697–708. doi: 10.1093/ntr/ntt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323(7321):1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the wisconsin smoking withdrawal scale. Experimental and Clinical Psychopharmacology. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Claus ED, Calhoun VD, Rachakonda S, Littlewood RA, Mickey J, Arenella PB, Goodreau N, Hutchison KE. Default mode network deactivation to smoking cue relative to food cue predicts treatment outcome in nicotine use disorder. Addict Biol. 2017 doi: 10.1111/adb.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA. Cognitive control in alcohol use disorder: Deficits and clinical relevance. Rev Neurosci. 2014:1–24. doi: 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Calhoun VD, Jung RE, Caprihan A. Connectivity-based whole brain dual parcellation by group ica reveals tract structures and decreased connectivity in schizophrenia. Hum Brain Mapp. 2015;36(11):4681–4701. doi: 10.1002/hbm.22945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Yu D, Bi Y, Li Y, Guan Y, Liu J, Zhang Y, Qin W, Lu X, Tian J. The implication of frontostriatal circuits in young smokers: A resting-state study. Hum Brain Mapp. 2016;37(6):2013–2026. doi: 10.1002/hbm.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi D, Brody AL, Montandon ML, Kopel R, Emmert K, Preti MG, Van De Ville D, Haller S. Cigarette smoking leads to persistent and dose-dependent alterations of brain activity and connectivity in anterior insula and anterior cingulate. Addict Biol. 2015;20(6):1033–1041. doi: 10.1111/adb.12292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.