Alzheimer's disease (AD) is a chronic neurodegenerative disease and the most common cause of dementia. AD risk is foremost modified by allelic composition of the APOE gene encoding apolipoprotein (apo) E-brain's main lipid carrying protein. Emerging evidence suggests that APOE genotype also may modulate efficacy and safety of AD immunotherapy, which is under development as a potential disease-modifying treatment.
There are three allelic forms of the APOE gene ε2, ε3, and ε4. AD frequency reaches 91% and 47% in APOE ε4 homo- and heterozygous carriers compared to 20% in non-carriers, respectively; while the age of dementia onset averages 68 and 76 years in APOE ε4 homo- and heterozygous carriers compared to 84 years in non-carriers, respectively [1]. A protective effect of the APOE ε2 allele also can be seen among APOE ε4 non-carriers. APOE genotype effecting AD predisposition results from a combination of disease specific and constitutional biological effects differentially modulated by apo E isoforms encoded by various APOE alleles [2]. Apo E isoforms diversely affect rate of soluble β-amyloid (Aβ) clearance from the brain interstitial space and also promote formation of Aβ plaques and vascular deposits, what effects a down-stream neurodegenerative cascade involving neurofibrillary pathology, inflammatory microglia response, and synaptic and neuronal loss. APOE ε4 carriers typically show much greater load of Aβ deposits compared to non-carriers. Constitutional effects of apo E implicated in AD pathogenesis concern its involvement in microglia phagocytic function, synaptic plasticity and neuronal network repair. APOE ε4 carriers have an attenuated reparative response to the neurodegenerative cascade induced by Aβ accumulation they are more prone to develop.
Development of anti-Aβ immunotherapy as a disease-modifying treatment is being actively pursued. One tested approach concerns intravenous administration of monoclonal antibodies (mAbs) recognizing antigens exposed in brain deposited Aβ. A modest fraction of such mAbs permeates the blood-brain barrier and upon binding Aβ plaques facilitates their clearance by macrophage transformed microglia. Bapinezumab was the first humanized mAb having this modus operandi, which came to clinical development. Its phase 2 trial evidenced potential effect of APOE genotype on efficacy and safety. Significant treatment effects on cognitive and functional endpoints were found in APOE ε4 non-carriers but not in APOE ε4 carriers [3], while amyloid related imaging abnormalities (ARIA) (including vasogenic edema [ARIA-E] and microhemorrhages [ARIA-H]) occurred seven and three times more frequently in APOE ε4 homo- and heterozygous carriers compared to non-carriers, respectively [4]. Exact pathomechanism of ARIA remains elusive but it is linked to immune response against perivascular Aβ causing transient increase in vascular wall permeability. Roughly 20% of ARIA-E affected patients reported clinical signs and symptoms. Enrolment to subsequent phase III trials was prospectively segregated based on APOE ε4 carrier status and the maximal bapinezumab dose was reduced from 2 mg/kg tested in phase 2 trial to 0.5 mg/kg and 1 mg/kg in APOE ε4 carrier and non-carrier groups, respectively [5]. Although dose reduction was a rationale measure to manage ARIA risk, in retrospect it can be viewed as one of several reasons these trials failed to meet efficacy endpoints.
Aducanumab is a newer anti-Aβ mAb, which like bapinezumab binds deposited Aβ exerting effector microglia response but can be tolerated in significantly higher doses [6]. Its phase 1b clinical trial showed dose dependent reduction of Aβ deposits. The maximal tested dose was 10 mg/kg administered every four weeks, while bapinezumab was infused quarterly. Unlike previous AD clinical trials, aducanumab development focuses on prodromal AD patients in attempt to contain Aβ pathology early and attenuate down-stream neurodegenerative cascade. In aforementioned phase 1b trial, 10 mg/kg aducanumab dose showed strong effect on cognitive endpoints. ARIA remains main adverse effect of aducanumab and its risk is mediated by the APOE ε4 allele. Thus, for two enrolling phase 3 trials patients are prospectively segregated based on APOE ε4 status. APOE ε4 carriers are randomized to either placebo, 3 or 6 mg/kg aducanumab doses while non-carriers to either placebo, 6 or 10 mg/kg doses.
Given emerging evidence concerning effects of APOE genotype on efficacy and safety of AD immunotherapy we took “bedside-to-bench” approach re-testing outcomes of passive immunization in APPSWE/PS1dE9 AD transgenic mice made homozygous for each of human APOE alleles. We used 10D5 anti-Aβ mAb with similar modus operandi to that of bapinezumab or aducanumab. The same 10D5 mAb dose (10 mg/kg/week) was used across all APOE genotypes. APOE ε4 mice showed the greatest reduction in Aβ deposits and the most robust microglia activation adjusted for Aβ plaque load, which for the first time evidenced that the APOE ε4 allele mediates stronger microglia response to anti-Aβ immunotherapy and enhances microglia phagocytic effect [7] (Figure 1). As aducanumab phase III clinical trials employ Aβ PET imaging, it would be interesting to see whether reduction in Aβ load with comparable dose is higher in APOE ε4 carriers than non-carrier, counteracting typically higher Aβ plaque load of the former.
Figure 1. Interaction between APOE genotype, Aβ deposition, and microglia.
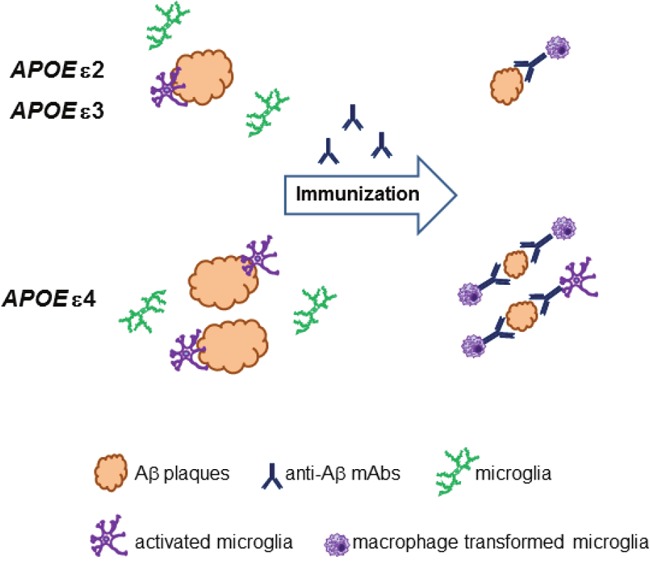
The APOE ε4 allele is associated with greater β-amyloid (Aβ) deposition than APOE ε2 and ε3 alleles. Aβ parenchymal plaques attract microglia cells and cause their peri-plaque activation, which is proportional to Aβ plaque load across all APOE genotypes. Therapeutic anti-Aβ monoclonal antibodies (mAbs) bind deposited Aβ and activate microglia cells to clear Aβ through Fc receptor-mediated phagocytosis. The APOE ε4 allele is associated with greater microglia activation than APOE alleles ε2 and ε3 resulting in greater clearance of Aβ plaque load during Aβ-directed passive immunization.
We also investigated effects of APOE genotype on vascular complications of anti-Aβ immunotherapy. Using μMRI we showed occurrence of new microhemorrhages (ARIA-H) in APOE ε4 mice undergoing 10D5 mAb treatment but we found no evidence of “vasogenic edema” (ARIA-E) [8]. Though ARIA-E remains the most troublesome and dose-limiting adverse effect of anti-Aβ immunotherapy its nature remains obscure largely due to absence of animal models allowing to dissect its pathogenesis. Postmortem analysis of perivascular microhemorrhages across APOE genotypes showed their greatest incidence in APOE ε2 mice evidencing for the first time the APOE ε2 allele as a risk factor for Aβ immunotherapy related microhemorrhages [7]. When translating these observations from transgenic mice back to humans one needs to be mindful that ε2/ε2 genotype is rare among AD patients, nevertheless careful monitoring of APOE ε2 carriers during clinical trials of anti-Aβ immunotherapy may effect a reduction in ARIA-H events.
REFERENCES
- 1.Corder EH, et al. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 2.Liu CC, et al. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salloway S, et al. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling R, et al. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salloway S, et al. N Engl J Med. 2014;370:322–33. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevigny J, et al. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 7.Pankiewicz JE, et al. Mol Neurodegener. 2017;12:12. doi: 10.1186/s13024-017-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pankiewicz JE, et al. Alzheimer and Dementia. 2016;12:P381–P382. [Google Scholar]


