Abstract
Objective
To explore whether subregional laminar femorotibial cartilage spin-spin relaxation time (T2) is associated with subsequent radiographic progression and cartilage loss and/or whether one-year change in subregional laminar femorotibial cartilage T2 is associated with concurrent progression in knees with established radiographic OA (ROA).
Methods
In this case-control study, Osteoarthritis Initiative (OAI) knees with medial femorotibial progression were selected based on one-year loss in both quantitative cartilage thickness (MRI) and radiographic JSW. Non-progressor knees were matched by sex, BMI, baseline Kellgren-Lawrence-grade (2/3), and pain. Baseline and 1-year follow-up superficial and deep cartilage T2 was analyzed in 16 femorotibial subregions using multi-echo spin-echo MRI.
Results
37 knees showed medial femorotibial progression whereas 37 matched controls had no medial or lateral compartment progression. No statistically significant baseline differences between progressor and non-progressor knees in medial femorotibial cartilage T2 were observed in the superficial (48.9±3.0ms; 95%CI:[47.9,49.9] vs. 47.8±3.6ms; 95%CI:[46.6,49.0], p=0.07) or deep cartilage layer (40.8±3.6ms; 95%CI:[39.5,42.0] vs. 40.1±4.7ms; 95%CI:[38.5,41.6], p=0.29). Concurrent T2 change was more pronounced in the deep than the superficial cartilage layer. In the medial femorotibial compartment, longitudinal change was greater in the deep layer of progressor than non-progressor knees (1.8±4.5ms; 95%CI:[0.3,3.3] vs. −0.2±1.9ms; 95%CI:[−0.8,0.5], p=0.02), whereas no difference was observed in the superficial layer.
Conclusion
Medial compartment cartilage T2 did not appear to be a strong prognostic factor for subsequent structural progression in the same compartment of knees with established ROA, when appropriately controlling for covariates. Yet, deep layer T2 change in the medial compartment occurred concurrent with medial femorotibial progression.
Keywords: Spin-spin (T2) relaxation time, cartilage, progression, osteoarthritis, knee
Introduction
Magnetic resonance imaging (MRI) spin-spin (transverse) relaxation time (T2) has been proposed as an imaging biomarker for the detection of alterations in cartilage composition before the onset of knee osteoarthritis (OA), to differentiate stages of OA, and to monitor or predict disease progression1–3. T2 is known to reflect cartilage composition (collagen integrity, orientation, and hydration)1,3,4, and to correlate with histological grading5,6 and mechanical properties1,7 of articular cartilage.
Several studies investigated the association between cartilage T2 times and incidence or progression of knee OA. In a nested case-control study of knees with Kellgren Lawrence grade (KLG) 0 at baseline, Liebl et al. reported baseline T2 times to be significantly greater in most knee compartments of KLG 0 knees that developed radiographic OA over 4 years than in non-incident control knees8, in particular in the superficial layer8. Joseph et al.9 reported prevalence of MRI structural pathology to increase over time in subjects with risk factors for OA and the authors also reported that greater baseline cartilage T2 predicted longitudinal change in cartilage, meniscus, and bone marrow lesion scores. Based on a cohort including 55 knees with KLG 0–3 at baseline, Prasad et al.10 reported significantly longer baseline T2 (and T1rho) times in the 27 case knees with progression over two years (incidence or worsening of existing cartilage lesions) than in 28 control knees without progression using a modified WORMS scoring system. No statistically significant differences were, however, found between progressor and non-progressor knees, when T2 times were compared separately in knees with and without radiographic OA10. It is well-known that the likelihood of progression is associated with radiographic disease stage11–13, and it may thus be that the differences in baseline radiographic disease stages were responsible for the observed differences in baseline T214–16 rather than progression per se. Prasad et al. did not report statistically significant differences in demographic data or baseline pain between progressor and non-progressor knees10, but a study on the relationship of T2 with structural progression should ideally rule out potential bias from risk factors of OA structural progression, since high BMI or knee pain have been associated with both progression11,17,18 and with cartilage T219–22. Also, previous studies on the relationship between cartilage T2 and OA progression have analyzed bulk cartilage T2 of entire cartilage plates, albeit T2 is known to vary strongly between superficial and deep laminae23, and subregional differences in cartilage T2 are to be expected based on local variations in collagen architecture24.
To our knowledge, no study to date has evaluated the cross-sectional and longitudinal relationship of cartilage T2 with structural progression as defined by quantitative radiographic and/or MRI outcomes (i.e. radiographic JSW or MRI-based cartilage thickness or volume) and no previous study has evaluated the relationship of cartilage T2 with structural progression separately for deep and superficial cartilage and/or for different femorotibial subregions. Because loss in radiographic JSW or cartilage thickness is typically observed in knees with established ROA (KLG ≥2) and because participants with established ROA are those typically enclosed in clinical trials25, we used a matched case-control design of participants with established (but without end-stage [KLG 4]) radiographic OA (KLG 2/3), to study whether (subregional) laminar medial femorotibial compartment cartilage T2 times
are associated with subsequent medial compartment structural progression
change concurrently in knees with subsequent medial compartment structural progression
show a greater concurrent change in knees with subsequent medial compartment structural progression than in knees without such progression.
Based on previous reports that T2 is limited in monitoring progression once an advanced disease stage is reached2, we performed sensitivity analyses with stratification for baseline KLG.
Methods
Study design and sample selection
The study participants were selected from the Osteoarthritis Initiative cohort (OAI; http://www.oai.ucsf.edu/, clinicaltrials.gov: NCT00080171)26, which was approved by the Committee on Human Research, the Institutional Review Board (IRB) for the University of California, San Francisco (UCSF). All OAI participants provided written informed consent and this study was carried out in accordance with the IRB-approved OAI data user agreement. OAI participants were 45–79 years old, with or at risk of symptomatic knee OA in at least one knee. General exclusion criteria were rheumatoid or inflammatory arthritis, bilateral end-stage knee OA, inability to walk without aids, and MRI contraindications.
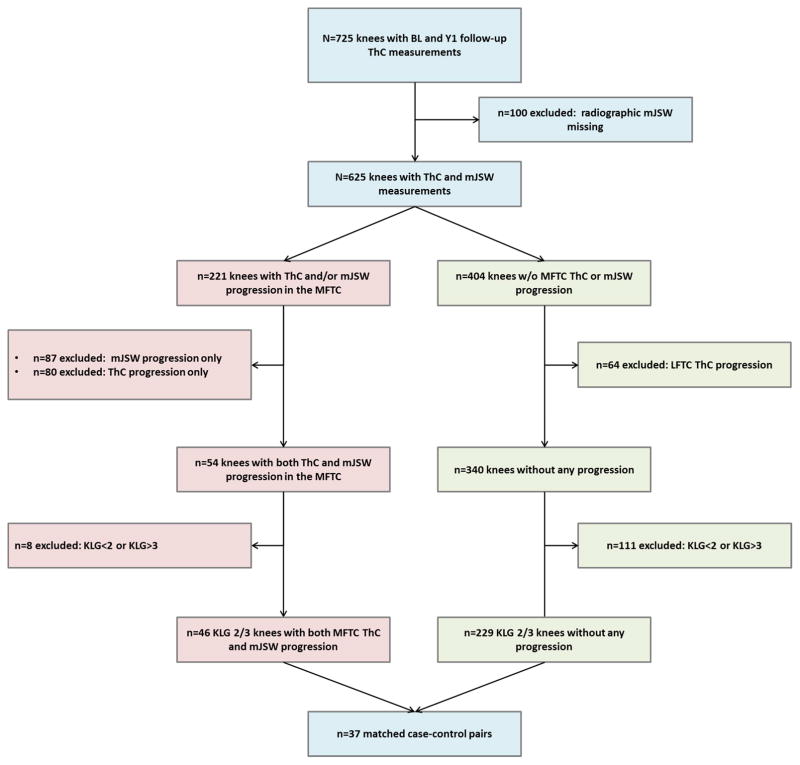
The inclusion criteria for the current nested case-control sample have been described previously: Knees with and without medial femorotibial progression were selected from a sample of 725 right knees from OAI participants, for which MRI-based cartilage thickness measurements were available for the baseline and the one-year follow-up visit (Fig. 1). At the baseline visit, these knees were KLG 2–4 12,17,25 according to the OAI clinical site radiographic readings17,25. Of these 725 right knees studied with MRI, 625 also had quantitative JSW measurements from fixed-flexion radiographs27 that we used here to ensure that apparent change in cartilage loss was not due to MRI-specific precision errors or artifacts, so that structural progression was confirmed by a second, independent method.
Fig. 1.
Flowchart illustrating the process of selecting case knees with structural progression exceeding the smallest detectable change (SDC) threshold for both minimum radiographic joint width (mJSW) and cartilage thickness (ThC) in the medial femorotibial compartment (MFTC) between the baseline (BL) and the year-one (Y1) follow-up visit and control knees without progression in mJSW, MFTC or lateral femorotibial compartment (LFTC) cartilage thickness. The case and control knees were matched by sex, BMI, baseline Kellgren-Lawrence grade (KLG 2/3), and pain.
Based on smallest detectable change (SDC) thresholds28,29 for MRI-based medial femorotibial compartment (MFTC) cartilage thickness (>102μm) and for radiographic medial minimum JSW (mJSW) loss (>328 μm), we selected progressor knees that exceeded both thresholds. Non-progressor knees were defined as knees that did not exceed the SDC thresholds for either cartilage thickness loss or mJSW loss in the MFTC and that also did not exceed the SDC threshold for lateral femorotibial compartment (LFTC) cartilage loss (92μm). Because the objective of this study was to determine, whether cartilage T2 is associated with subsequent structural progression in knees with established radiographic OA, the current study included knees with definite, but not end stage radiographic OA, whereas knees with KLG 1 and 4 at baseline were excluded. The radiographic inclusion/exclusion relied on the central KLG readings (release 0.5), which are deemed more reliable than the clinical site readings that were used to select the initial sample of 725 knees25.
Of the 625 knees for which both MRI-based cartilage thickness and radiography-based mJSW measurements were available, 404 knees did not exceed any SDC threshold, 80 exceeded only the MFTC SDC threshold for MRI-based cartilage loss, 87 exceeded only the SDC threshold for radiography-based mJSW loss, and 54 knees exceeded the MFTC SDC threshold for both MRI-based cartilage loss and radiography-based mJSW loss (Fig. 1). Cartilage loss exceeding the LFTC SDC threshold was observed in 64 of the 404 knees that did not display MFTC loss. After excluding knees without definite radiographic OA (KLG<2) or with end-stage radiographic OA (KLG 4) at baseline, 46 of the 54 knees with MFTC progression and 229 of the 340 knees without MFTC or LFTC progression qualified for inclusion in this study.
In order to reduce the potential bias introduced by covariates (e.g. sex, BMI, pain), knees with and without structural progression were matched 1:1 by the same sex, body height (±3cm), BMI (±5kg/m2), baseline KLG (2 or 3), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores (±5; scale from 0–20). An appropriate matching control was found for 37 of the 46 progressor knees. Because all knees fulfilling the selection criteria were included in this study and because no information about the expected effect size for laminar (and subregional) cartilage T2 was available, no apriori power analysis was performed.
T2 analysis of femorotibial cartilage
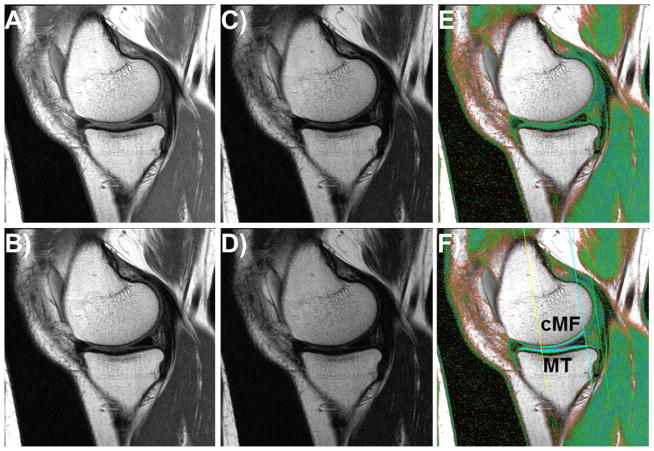
The right knees of the OAI participants had sagittal 3 Tesla multi-echo spin-echo (MESE) MR images acquired26,30 (Fig. 2). The repetition time was 2700ms and the echo times were 10, 20, 30, 40, 50, 60, and 70 ms (slice thickness 3.0 mm, in-plane resolution 0.3125 mm). T2 was computed for each voxel by fitting a mono-exponential decay curve to the measured signal intensities using a non-linear regression method minimizing with the proportionality constant k and the signal intensities SIi observed at the respective echo times TEi 31 (Fig. 2). The 1st echo (10 ms) was excluded to reduce the impact of stimulated echoes1. Segmentation of the cartilage of the medial tibia (MT) and medial weight-bearing femoral condyle (cMF) as well as the lateral tibia (LT) and lateral weight-bearing femoral condyle (cLF) was performed manually by one reader (P.B.) and underwent quality control and/or correction by an expert reader with >10 years of experience in cartilage segmentation (S.M.). The readers were provided with the ability to choose amongst images with different echoes or that of color-coded T2 values. The image with the shortest echo (Fig. 2) was generally used for segmenting the bone interface, and that with the longest echo (Fig. 2) for the cartilage surface32. Baseline and follow-up images were analyzed simultaneously, but with blinding to the acquisition dates. Because cartilage T2 is known to display spatial variation with tissue depth1,23, the cartilages were computationally divided into the top (superficial) and bottom (deep) 50% after segmentation was completed, based on the local distance between the segmented cartilage surface and bone interface32 (Fig. 2). To avoid contribution of T2 values from voxels with low image quality, voxels were excluded if the coefficient of determination for the curve fitting was below R2=0.66 32. This was observed in 7.5±0.6% of the femorotibial cartilage voxels. Within each knee, cartilage T2 times measured in the deep and superficial layer of the MT and the cMF were averaged to obtain the deep and superficial MFTC T2 times. Similarly, the deep and superficial layer cartilage T2 times observed for LT and cLF were averaged to obtain the deep and superficial lateral femorotibial compartment (LFTC) T2 times.
Fig. 2.
Sagittal multi-echo spin-echo (MESE) images showing the cartilages in the medial compartment; A) – D) MESE images acquired with echo times of 10, 30, 50, and 70 ms; E) Color-coded T2map; F) T2 map as in E), showing the femoral region of interest (ROI) and the segmentation of the medial tibia (MT) and the central medial femur (cMF).
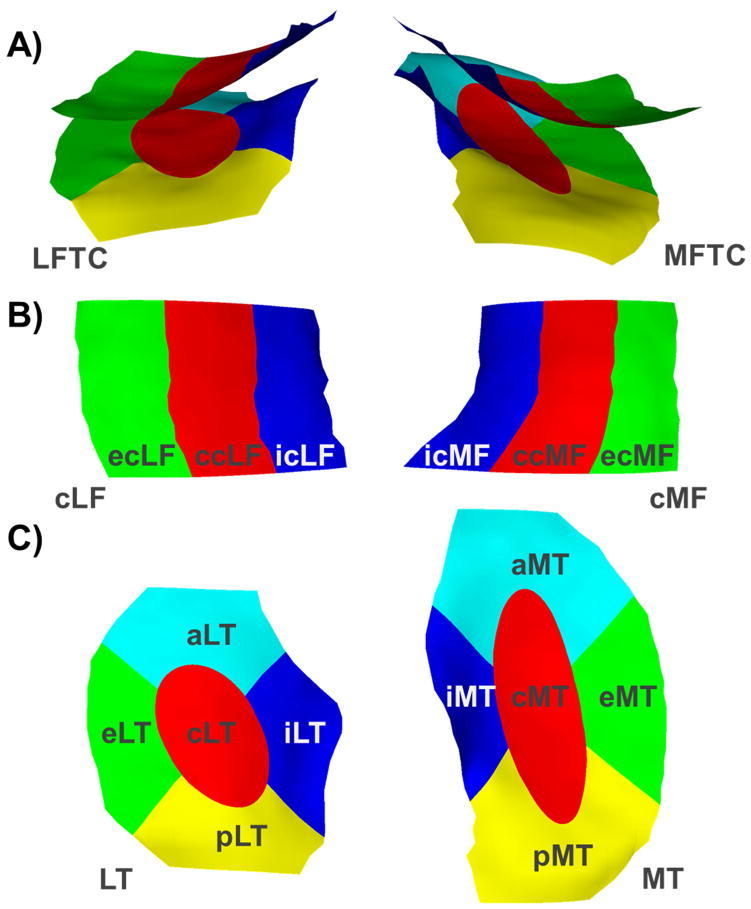
A subregional analysis approach (Fig. 3) that was previously developed for morphometric analyses33–35 was adapted for subregional analyses of laminar cartilage T2 times in the femorotibial joint36, using the same regional definition as for morphometric cartilage analyses33. To that end, the segmentation of the MT and LT were each divided into one central (cMT/cLT), external (eMT/eLT), internal (iMT/iLT), anterior (aMT/aLT), and posterior (pMT/pLT) subregion and the segmentation of the cMF and cLF were each divided into one central (ccMF/ccLF), external (ecMF/ecLF), and internal (icMF/icLF) subregion (Fig. 3). Subregional cartilage T2 times were then derived by attributing the cartilage T2 times measured in the voxels of the cartilage plates to the respective subregions. Deep and superficial cartilage T2 times in the central MFTC and central LFTC subregions were averaged to obtain central medial (cMFTC) and lateral femorotibial compartment (cLFTC) T2. The intra-observer precision (root mean square coefficient of variation determined from nine knees with repositioning between acquisitions) of the laminar T2 analyses was 2.5–4.4% for compartment measures and cartilage plates, and 2.2–7.1% for cartilage subregions.
Fig. 3.
Illustration of A) the medial (MFTC) and lateral (LFTC) femorotibial compartment subregions, B) the central (c), external (e), and internal (i) subregions of the central part of the medial (cMF) and lateral (cLF) femur, and C) the central (c), external (e), internal (i), anterior (a), and posterior (p) subregions of the medial (MT) and lateral (LT) tibia.
Statistical analysis
All analyses were performed using SPSS 23 (IBM Corporation, Armonk, NY). The mean, standard deviation (SD) and 95% confidence intervals were computed for the baseline cartilage T2 times, for the change in cartilage T2 times, and for the differences between progressor and non-progressor knees. The 95% confidence intervals were computed using the bias-corrected and accelerated (BCa) bootstrapping method with 1000 replications. To determine whether changes from baseline to follow-up were statistically significant, Wilcoxon signed-rank tests were used, because the paired differences between the cartilage T2 times observed in progressor and non-progressor knees were not generally normally distributed (Online Fig. 1).
Wilcoxon signed-rank tests were also used to test whether baseline MFTC T2 times differed between knees with and without subsequent progression, and to test whether change in MFTC T2 times differed between knees with and without subsequent structural progression. The deep and superficial MFTC T2 times were considered the two primary outcome measures, given that the progressor knees showed MFTC cartilage and mJSW loss. The significance level was adjusted to account for two parallel comparisons (deep/superficial layer; p=0.05/2=0.025). Analyses in the lateral compartment, single cartilage plates and subregions were considered exploratory. Cohen’s D was used as a measure of the effect size for the between-group comparisons, the standardized response mean (SRM) was used as a measure of the sensitivity to change. Sensitivity analyses were performed for baseline KLG strata (2/3).
Results
Sample description and demographic data
The 37 participants (21 KLG 2, 16 KLG 3, 13 male, 24 female) in the progressor group had a similar age (64.7±8.0 years vs. 64.6±9.8 years, p=0.98) and BMI (30.2±4.6 kg/m2 vs. 30.2±4.4 kg/m2, p=0.94) but higher WOMAC pain scores (3.5±3.8 vs. 2.8±3.3, p=0.04) than the 37 matched participants in the non-progressor group (Table 1). The baseline mJSW and cartilage thickness in the medial compartment were similar in progressor and non-progressor knees (p≥0.36, Table 2), but the baseline lateral compartment cartilage thickness was statistically significantly greater in progressor than in non-progressor knees (p=0.01, Table 2). As expected by the inclusion criteria, a statistically significant loss (p<0.01) was observed in medial compartment mJSW and cartilage thickness of progressor knees over the one-year follow-up period, whereas no statistically significant change was observed in non-progressor knees (Table 3). Further, no statistically significant change occurred in lateral compartment cartilage thickness in either progressor or non-progressor knees (Table 3).
Table 1.
Demographic data for the 37 participants with and the 37 matched participants without medial compartment structural progression
| Progressor cases | Non-progressor controls | ||||||
|---|---|---|---|---|---|---|---|
| All (N=37) | KLG 2 (N=21) | KLG 3 (N=16) | All (N=37) | KLG 2 (N=21) | KLG 3 (N=16) | ||
| Mean ± SD | Mean | Mean | Mean | Mean | Mean | ||
| Age | [years] | 64.7 ± 8.0 | 65.0 ± 7.1 | 64.3 ± 9.3 | 64.6 ± 9.8 | 61.2 ± 7.8 | 69.1 ± 10.7 |
| Weight | [kg] | 82.8 ± 13.9 | 84.3 ± 14.8 | 80.8 ± 12.8 | 82.9 ± 13.9 | 86.4 ± 15.9 | 78.3 ± 9.3 |
| Height | [cm] | 165.6 ± 7.9 | 165.1 ± 7.6 | 166.4 ± 8.5 | 165.6 ± 7.7 | 165.3 ± 7.3 | 166.0 ± 8.5 |
| BMI | [kg/m2] | 30.2 ± 4.6 | 30.9 ± 4.9 | 29.2 ± 4.2 | 30.2 ± 4.4 | 31.5 ± 5.0 | 28.4 ± 2.9 |
| WOMAC | [1…20] | 3.5 ± 3.8 # | 3.4 ± 4.5 | 3.7 ± 2.8 | 2.8 ± 3.3 | 2.6 ± 3.9 | 3.0 ± 2.3 |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Sex | Male | 13 (35.1) | 6 (28.6) | 7 (43.8) | 13 (35.1) | 6 (28.6) | 7 (43.8) |
| Female | 24 (64.9) | 15 (71.4) | 9 (56.3) | 24 (64.9) | 15 (71.4) | 9 (56.3) | |
| KLG | 2 | 21 (56.8) | 21 (100.0) | 0 (0.0) | 21 (56.8) | 21 (100.0) | 0 (0.0) |
| 3 | 16 (43.2) | 0 (0.0) | 16 (100.0) | 16 (43.2) | 0 (0.0) | 16 (100.0) | |
| med JSN | 0 | 7 (18.9) | 5 (23.8) | 2 (12.5) | 14 (37.8) | 10 (47.6) | 4 (25.0) |
| 1 | 17 (45.9) | 16 (76.2) | 1 (6.3) | 11 (29.7) | 11 (52.4) | 0 (0.0) | |
| 2 | 13 (35.1) | 0 (0.0) | 13 (81.3) | 12 (32.4) | 0 (0.0) | 12 (75.0) | |
| lat JSN | 0 | 32 (86.5) | 19 (90.5) | 13 (81.3) | 28 (75.7) | 17 (81.0) | 11 (68.8) |
| 1 | 2 (5.4) | 2 (9.5) | 0 (0.0) | 4 (10.8) | 4 (19.0) | 0 (0.0) | |
| 2 | 3 (8.1) | 0 (0.0) | 3 (18.8) | 5 (13.5) | 0 (0.0) | 5 (31.3) | |
KLG: Kellgren and Lawrence grade; BMI: Body mass index; WOMAC: Western Ontario and McMasters Universities pain scale (range: 0…20); med/lat JSN: medial lateral OARSI joint space narrowing score;
p<0.05 for differences between progressor cases and non-progressor controls.
Table 2.
Baseline values in cartilage T2 times in the superficial and deep layer of 37 knees with and 37 matched knees without medial compartment structural progression
| Progressor Cases | Non-progressor Controls | Cases vs. Controls | P | Cohen’s D | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | 95% CI | Mean±SD | 95% CI | Mean±SD | 95% CI | ||||
| Medial femorotibial compartment T2 (MFTC): | |||||||||
| MFTC | SL | 48.9±3.0 | [47.9, 49.9] | 47.8±3.6 | [46.7, 48.9] | 1.2±3.9 | [0.0, 2.4] | 0.07 | 0.35 |
| DL | 40.8±3.6 | [39.7, 41.8] | 40.1±4.7 | [38.7, 41.4] | 0.7±5.4 | [−1.2, 2.5] | 0.29 | 0.17 | |
| MT | SL | 44.3±3.0 | [43.5, 45.2] | 43.7±2.8 | [42.8, 44.6] | 0.6±3.7 | [−0.5, 1.8] | 0.45 | 0.19 |
| DL | 35.2±3.0 | [34.3, 36.1] | 35.2±3.6 | [34.2, 36.4] | 0.0±4.7 | [−1.7, 1.5] | 0.55 | −0.01 | |
| cMF | SL | 53.6±5.0 | [51.8, 55.2] | 51.8±5.8 | [50.1, 53.5] | 1.7±6.7 | [−0.3, 4.0] | 0.10 | 0.32 |
| DL | 46.3±5.5 | [44.7, 47.9] | 44.9±6.9 | [43.0, 46.7] | 1.4±8.5 | [−1.6, 4.3] | 0.17 | 0.23 | |
|
| |||||||||
| cMFTC | SL | 48.8±3.5 | [47.6, 49.8] | 48.9±5.4 | [47.3, 50.7] | −0.1±6.5 | [−2.1, 2.0] | 0.89 | −0.02 |
| DL | 37.0±4.9 | [35.6, 38.4] | 36.6±5.7 | [34.9, 38.5] | 0.4±7.4 | [−2.1, 2.9] | 0.44 | 0.08 | |
| cMT | SL | 43.6±5.9 | [42.1, 45.2] | 44.7±5.0 | [43.0, 46.3] | −1.0±8.5 | [−3.6, 2.0] | 0.42 | −0.19 |
| DL | 31.7±5.0 | [30.4, 33.2] | 32.3±6.5 | [30.8, 34.4] | −0.6±8.4 | [−3.6, 2.0] | 0.93 | −0.10 | |
| eMT | SL | 49.7±5.1 | [48.1, 51.5] | 48.0±5.8 | [46.0, 49.8] | 1.7±6.8 | [−0.7, 4.2] | 0.12 | 0.31 |
| DL | 42.1±10.0 | [39.2, 45.2] | 42.4±16.9 | [38.2, 48.2] | −0.3±19.3 | [−7.1, 5.3] | 0.67 | −0.02 | |
| iMT | SL | 37.1±7.3 | [33.9, 39.2] | 37.8±4.4 | [36.6, 39.1] | −0.7±8.6 | [−4.3, 2.2] | 0.93 | −0.12 |
| DL | 32.8±8.0 | [30.9, 35.2] | 31.9±3.8 | [30.9, 32.9] | 0.9±8.5 | [−1.4, 3.7] | 0.93 | 0.14 | |
| aMT | SL | 45.0±3.3 | [44.1, 46.0] | 43.6±3.4 | [42.5, 44.7] | 1.4±4.6 | [−0.1, 2.9] | 0.13 | 0.43 |
| DL | 35.8±3.0 | [34.9, 36.7] | 35.0±3.7 | [34.0, 36.0] | 0.7±4.8 | [−0.8, 2.3] | 0.21 | 0.22 | |
| pMT | SL | 45.1±4.5 | [43.8, 46.5] | 44.1±4.4 | [42.6, 45.6] | 1.0±5.0 | [−0.5, 2.5] | 0.17 | 0.22 |
| DL | 36.5±5.3 | [35.3, 38.5] | 36.5±2.8 | [35.6, 37.5] | 0.0±5.1 | [−1.4, 1.6] | 0.45 | 0.00 | |
| ccMF | SL | 53.9±5.6 | [52.1, 55.8] | 53.1±8.1 | [50.9, 55.4] | 0.9±9.0 | [−2.1, 3.8] | 0.41 | 0.12 |
| DL | 42.3±6.8 | [40.2, 44.4] | 40.9±8.5 | [38.7, 43.1] | 1.4±11.1 | [−2.2, 5.0] | 0.24 | 0.18 | |
| ecMF | SL | 48.5±5.4 | [46.8, 50.4] | 48.1±7.6 | [46.2, 50.3] | 0.4±8.7 | [−2.5, 3.1] | 0.28 | 0.06 |
| DL | 46.0±8.0 | [43.8, 48.6] | 45.6±6.3 | [43.7, 47.6] | 0.4±9.3 | [−2.6, 3.5] | 0.41 | 0.06 | |
| icMF | SL | 56.4±12.2 | [51.8, 60.1] | 54.3±6.7 | [52.1, 56.5] | 2.1±12.4 | [−1.7, 6.2] | 0.26 | 0.21 |
| DL | 50.4±8.7 | [47.8, 53.1] | 47.6±7.9 | [45.2, 49.8] | 2.8±12.6 | [−0.7, 6.3] | 0.23 | 0.33 | |
|
| |||||||||
| Lateral femorotibial compartment T2 (LFTC): | |||||||||
| LFTC | SL | 46.2±3.2 | [45.1, 47.4] | 46.3±3.5 | [45.3, 47.3] | −0.1±4.8 | [−1.7, 1.5] | 0.90 | −0.03 |
| DL | 37.2±2.6 | [36.4, 38.0] | 37.1±3.0 | [36.3, 38.0] | 0.1±3.8 | [−1.2, 1.3] | 0.73 | 0.02 | |
| LT | SL | 42.7±3.2 | [41.7, 43.9] | 43.2±3.3 | [42.3, 44.2] | −0.5±4.9 | [−2.0, 1.1] | 0.62 | −0.15 |
| DL | 32.5±2.4 | [31.7, 33.4] | 32.7±3.5 | [31.8, 33.8] | −0.2±4.2 | [−1.6, 1.1] | 0.98 | −0.06 | |
| cLF | SL | 49.7±4.3 | [48.1, 51.2] | 49.4±4.4 | [48.1, 50.7] | 0.3±6.4 | [−2.0, 2.6] | 0.71 | 0.07 |
| DL | 41.8±3.7 | [40.6, 43.1] | 41.5±3.4 | [40.4, 42.5] | 0.3±4.8 | [−1.3, 2.0] | 0.75 | 0.08 | |
|
| |||||||||
| cLFTC | SL | 44.9±4.0 | [43.6, 46.1] | 46.0±4.1 | [44.8, 47.2] | −1.2±5.7 | [−3.0, 0.7] | 0.26 | −0.29 |
| DL | 33.6±2.9 | [32.6, 34.5] | 34.6±6.0 | [33.2, 36.4] | −1.1±6.1 | [−3.3, 0.8] | 0.70 | −0.22 | |
| cLT | SL | 39.6±3.9 | [38.4, 40.8] | 41.7±4.2 | [40.5, 43.0] | −2.1±5.6 | [−4.0, −0.4] | 0.04 | −0.53 |
| DL | 28.4±2.7 | [27.4, 29.3] | 30.2±9.1 | [28.2, 33.2] | −1.9±8.3 | [−5.0, 0.3] | 0.25 | −0.28 | |
| eLT | SL | 47.7±5.0 | [46.0, 49.4] | 47.0±5.1 | [45.4, 48.5] | 0.6±6.5 | [−1.6, 2.9] | 0.35 | 0.13 |
| DL | 37.9±4.0 | [36.6, 39.2] | 38.4±5.7 | [36.6, 40.3] | −0.5±8.0 | [−3.0, 1.9] | 0.98 | −0.10 | |
| iLT | SL | 39.7±7.0 | [37.5, 42.1] | 40.2±7.9 | [37.9, 42.2] | −0.4±10.8 | [−3.7, 3.0] | 0.73 | −0.06 |
| DL | 30.8±5.0 | [29.4, 32.4] | 30.2±4.6 | [28.9, 31.6] | 0.6±7.1 | [−1.7, 2.7] | 0.67 | 0.12 | |
| aLT | SL | 44.1±3.6 | [43.0, 45.3] | 43.8±4.3 | [42.5, 44.9] | 0.3±5.6 | [−1.5, 2.0] | 0.70 | 0.09 |
| DL | 32.7±3.4 | [31.6, 33.8] | 32.1±3.2 | [31.2, 33.0] | 0.6±4.3 | [−0.8, 1.9] | 0.43 | 0.17 | |
| pLT | SL | 44.2±4.5 | [42.8, 45.7] | 44.8±6.0 | [43.1, 46.7] | −0.6±7.9 | [−3.1, 1.9] | 0.84 | −0.12 |
| DL | 34.9±3.4 | [33.8, 35.9] | 35.1±5.0 | [33.7, 37.0] | −0.3±7.0 | [−2.6, 1.9] | 0.91 | −0.07 | |
| ccLF | SL | 50.1±5.6 | [48.2, 52.0] | 50.3±5.4 | [48.7, 51.8] | −0.2±8.1 | [−3.0, 2.5] | 0.92 | −0.03 |
| DL | 38.8±4.1 | [37.4, 40.1] | 39.0±4.9 | [37.5, 40.7] | −0.3±6.0 | [−2.3, 1.7] | 0.91 | −0.06 | |
| ecLF | SL | 48.5±4.9 | [46.8, 50.1] | 48.0±5.5 | [46.1, 49.7] | 0.5±7.6 | [−2.1, 3.1] | 0.72 | 0.09 |
| DL | 44.7±4.0 | [43.5, 46.1] | 45.4±4.5 | [44.2, 46.8] | −0.6±5.9 | [−2.6, 1.2] | 0.50 | −0.15 | |
| icLF | SL | 50.2±4.7 | [48.6, 51.9] | 49.4±5.1 | [47.9, 51.0] | 0.7±6.6 | [−1.5, 2.7] | 0.41 | 0.15 |
| DL | 42.4±5.2 | [40.7, 44.0] | 40.9±4.3 | [39.6, 42.3] | 1.4±7.6 | [−1.0, 3.9] | 0.18 | 0.30 | |
|
| |||||||||
| Minimum joint space width (mJSW) and cartilage thickness: | |||||||||
| mJSW | mm | 3.9±1.4 | [3.4, 4.3] | 3.8±1.3 | [3.3, 4.3] | 0.1±1.5 | [−0.4, 0.6] | 0.62 | 0.07 |
| MFTC | mm | 3.2±0.6 | [3.0, 3.4] | 3.3±0.6 | [3.1, 3.5] | −0.1±0.7 | [−0.3, 0.1] | 0.36 | −0.14 |
| LFTC | mm | 3.8±0.5 | [3.7, 4.0] | 3.6±0.5 | [3.4, 3.7] | 0.3±0.6 | [0.1, 0.5] | 0.01 | 0.56 |
SL: superficial layer (in ms); DL: deep layer (in ms); MFTC/LFTC: medial/lateral femorotibial compartment; cMFTC/cLFTC: combined central subregion of the MFTC/LFTC; MT/LT: medial/lateral tibia; cMF/cLF: central medial/lateral femur; mJSW: minimum radiographic joint space width in fixed-flexion x-rays; SD: standard deviation; 95% CI: 95% confidence intervals computed using the bias-corrected and accelerated (BCa) bootstrapping method with 1000 replications; c/e/i/a/p MT/LT: central/external/internal/anterior/posterior subregion of the MT/LT; c/e/i cMF/cLF: central/external/internal subregion of the cMF/cLF. P-values were computed using non-parametric Wilcoxon signed-rank tests.
Table 3.
One-year change in cartilage T2 times in the superficial and deep layer of 37 knees with and 37 matched knees without medial compartment structural progression
| Progressor Cases | Non-progressor Controls | Cases vs. Controls | P | Cohen’s D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | 95% CI | SRM | Mean±SD | 95% CI | SRM | Mean±SD | 95% CI | ||||
| Medial femorotibial compartment T2 (MFTC): | |||||||||||
| MFTC | SL | 0.1±3.4 | [−1.1, 1.3] | 0.02 | 0.0±1.9 | [−0.6, 0.6] | 0.02 | 0.0±3.7 | [−1.2, 1.3] | 0.97 | 0.01 |
| DL | 1.8±4.5 | [0.4, 3.6] | 0.39 | −0.2±1.9 | [−0.7, 0.4] | −0.08 | 1.9±4.8 | [0.4, 3.8] | 0.02 | 0.56 | |
| MT | SL | −0.3±3.0 | [−1.2, 0.7] | −0.09 | 0.2±2.0 | [−0.5, 0.9] | 0.11 | −0.5±3.2 | [−1.5, 0.7] | 0.24 | −0.18 |
| DL | 1.5±3.7 | [0.4, 2.9] | 0.41 | −0.4±1.3 | [−0.8, 0.0] | −0.30 | 1.9±4.3 | [0.6, 3.6] | 0.01 | 0.69 | |
| cMF | SL | 0.4±5.5 | [−1.4, 2.0] | 0.07 | −0.1±2.9 | [−1.1, 0.7] | −0.05 | 0.5±6.4 | [−1.4, 2.5] | 0.51 | 0.12 |
| DL | 2.1±6.4 | [0.2, 4.5] | 0.32 | 0.1±3.6 | [−0.8, 1.1] | 0.02 | 2.0±6.3 | [−0.3, 4.2] | 0.06 | 0.38 | |
|
| |||||||||||
| cMFTC | SL | −0.2±3.8 | [−1.4, 1.1] | −0.05 | −0.7±3.6 | [−1.9, 0.5] | −0.19 | 0.5±5.3 | [−1.1, 2.2] | 0.71 | 0.13 |
| DL | 3.4±8.1 | [1.2, 6.0] | 0.43 | −0.5±2.6 | [−1.2, 0.3] | −0.19 | 3.9±8.2 | [1.3, 7.0] | <0.01 | 0.65 | |
| cMT | SL | −0.7±6.2 | [−2.7, 1.2] | −0.12 | −0.5±4.4 | [−1.8, 0.8] | −0.11 | −0.2±7.1 | [−2.2, 2.1] | 0.32 | −0.05 |
| DL | 2.6±7.7 | [0.0, 5.4] | 0.33 | 0.0±2.7 | [−0.7, 1.1] | 0.02 | 2.5±8.5 | [−0.3, 5.6] | 0.08 | 0.44 | |
| eMT | SL | 1.8±8.1 | [−1.0, 5.4] | 0.22 | 1.5±4.5 | [0.1, 2.9] | 0.33 | 0.3±8.5 | [−2.5, 4.0] | 0.98 | 0.04 |
| DL | 6.3±15.7 | [1.3, 12.5] | 0.40 | 0.5±7.7 | [−2.1, 2.9] | 0.07 | 5.8±18.7 | [0.0, 13.5] | 0.39 | 0.47 | |
| iMT | SL | 0.1±6.5 | [−1.6, 2.0] | 0.02 | 0.6±4.3 | [−0.6, 2.4] | 0.13 | −0.4±8.3 | [−2.8, 2.0] | 0.45 | −0.08 |
| DL | 0.2±3.6 | [−0.8, 1.4] | 0.07 | −0.3±3.0 | [−1.3, 0.6] | −0.10 | 0.5±4.9 | [−0.9, 2.3] | 0.85 | 0.17 | |
| aMT | SL | 0.3±3.1 | [−0.6, 1.2] | 0.10 | 0.6±2.7 | [−0.3, 1.5] | 0.20 | −0.2±3.5 | [−1.4, 0.9] | 0.47 | −0.08 |
| DL | 1.1±3.3 | [0.2, 2.2] | 0.33 | −0.7±1.7 | [−1.2, −0.1] | −0.39 | 1.7±4.2 | [0.5, 3.1] | 0.01 | 0.67 | |
| pMT | SL | −0.9±3.8 | [−2.1, 0.5] | −0.23 | −0.1±2.4 | [−0.9, 0.6] | −0.04 | −0.8±3.9 | [−2.1, 0.5] | 0.36 | −0.24 |
| DL | 0.7±2.9 | [−0.2, 1.7] | 0.24 | −0.5±2.0 | [−1.1, 0.1] | −0.27 | 1.2±4.0 | [0.0, 2.6] | 0.05 | 0.49 | |
| ccMF | SL | 0.3±6.2 | [−1.7, 2.2] | 0.05 | −0.9±5.7 | [−2.6, 0.7] | −0.15 | 1.2±8.1 | [−1.1, 3.7] | 0.20 | 0.20 |
| DL | 4.3±10.1 | [1.3, 7.8] | 0.42 | −1.0±4.8 | [−2.3, 0.3] | −0.21 | 5.3±9.2 | [2.5, 8.5] | <0.01 | 0.67 | |
| ecMF | SL | 1.1±7.7 | [−1.1, 3.6] | 0.15 | 1.3±8.0 | [−0.6, 3.9] | 0.17 | −0.2±11.2 | [−4.0, 3.4] | 0.98 | −0.02 |
| DL | 0.4±6.8 | [−1.7, 2.8] | 0.06 | 0.7±6.2 | [−1.1, 2.7] | 0.11 | −0.3±8.5 | [−2.9, 2.6] | 0.59 | −0.04 | |
| icMF | SL | 0.2±9.4 | [−3.1, 3.4] | 0.02 | 0.2±4.1 | [−1.2, 1.6] | 0.05 | 0.0±10.9 | [−3.5, 3.5] | 0.62 | 0.00 |
| DL | 1.7±8.1 | [−0.6, 4.7] | 0.21 | 1.1±5.0 | [−0.3, 2.5] | 0.21 | 0.6±8.6 | [−2.3, 3.8] | 0.52 | 0.10 | |
|
| |||||||||||
| Lateral femorotibial compartment T2 (LFTC): | |||||||||||
| LFTC | SL | −0.2±2.3 | [−0.9, 0.6] | −0.09 | −0.2±1.5 | [−0.8, 0.3] | −0.14 | 0.0±2.7 | [−0.9, 1.1] | 0.63 | 0.01 |
| DL | −0.1±1.6 | [−0.6, 0.4] | −0.07 | 0.1±1.4 | [−0.4, 0.5] | 0.04 | −0.2±1.9 | [−0.7, 0.4] | 0.91 | −0.11 | |
| LT | SL | 0.4±2.4 | [−0.2, 1.2] | 0.19 | −0.1±1.7 | [−0.7, 0.4] | −0.07 | 0.6±2.8 | [−0.3, 1.4] | 0.32 | 0.27 |
| DL | 0.1±1.6 | [−0.3, 0.7] | 0.09 | 0.4±1.6 | [−0.1, 1.0] | 0.25 | −0.3±2.3 | [−1.0, 0.4] | 0.68 | −0.16 | |
| cLF | SL | −0.8±2.5 | [−1.7, 0.2] | −0.33 | −0.3±2.1 | [−1.0, 0.3] | −0.16 | −0.5±3.5 | [−1.5, 0.5] | 0.20 | −0.22 |
| DL | −0.4±2.2 | [−1.0, 0.3] | −0.17 | −0.3±1.8 | [−0.8, 0.3] | −0.16 | −0.1±2.3 | [−0.8, 0.5] | 0.93 | −0.04 | |
|
| |||||||||||
| cLFTC | SL | 0.5±3.3 | [−0.5, 1.6] | 0.14 | −0.1±2.2 | [−0.7, 0.7] | −0.03 | 0.5±3.9 | [−0.7, 1.9] | 0.80 | 0.19 |
| DL | 0.3±2.1 | [−0.4, 1.0] | 0.14 | −0.1±1.5 | [−0.6, 0.3] | −0.10 | 0.5±2.2 | [−0.2, 1.1] | 0.24 | 0.24 | |
| cLT | SL | 1.3±4.7 | [0.1, 2.6] | 0.27 | 0.3±3.9 | [−0.9, 1.7] | 0.09 | 1.0±6.1 | [−0.8, 3.0] | 0.37 | 0.22 |
| DL | 0.4±1.8 | [−0.2, 0.9] | 0.20 | 0.5±2.2 | [−0.1, 1.2] | 0.22 | −0.1±2.8 | [−1.1, 0.6] | 0.83 | −0.07 | |
| eLT | SL | −0.4±3.0 | [−1.4, 0.4] | −0.12 | 0.2±3.4 | [−0.9, 1.3] | 0.05 | −0.5±4.9 | [−2.0, 1.2] | 0.42 | −0.16 |
| DL | −0.7±3.3 | [−1.8, 0.2] | −0.23 | 0.2±2.7 | [−0.7, 1.2] | 0.08 | −1.0±4.7 | [−2.6, 0.7] | 0.27 | −0.32 | |
| iLT | SL | 1.0±7.2 | [−1.1, 3.8] | 0.13 | −0.3±4.8 | [−1.8, 1.2] | −0.05 | 1.2±7.3 | [−0.8, 3.3] | 0.56 | 0.20 |
| DL | 1.0±4.7 | [−0.5, 2.9] | 0.21 | 0.4±3.0 | [−0.6, 1.4] | 0.14 | 0.5±5.5 | [−1.2, 2.3] | 0.72 | 0.14 | |
| aLT | SL | −0.2±2.0 | [−0.8, 0.5] | −0.08 | −0.1±2.2 | [−0.8, 0.6] | −0.04 | −0.1±2.9 | [−1.0, 0.7] | 0.82 | −0.03 |
| DL | 0.0±2.3 | [−0.8, 0.6] | −0.01 | 0.4±2.4 | [−0.3, 1.1] | 0.18 | −0.4±3.1 | [−1.4, 0.4] | 0.71 | −0.19 | |
| pLT | SL | 0.3±3.2 | [−0.6, 1.4] | 0.11 | −0.6±2.9 | [−1.6, 0.3] | −0.21 | 1.0±4.6 | [−0.3, 2.2] | 0.31 | 0.31 |
| DL | 0.1±1.6 | [−0.4, 0.7] | 0.06 | 0.9±4.8 | [−0.2, 2.5] | 0.19 | −0.8±5.0 | [−2.8, 0.4] | 0.72 | −0.23 | |
| ccLF | SL | −0.3±3.3 | [−1.5, 0.9] | −0.10 | −0.5±3.1 | [−1.4, 0.4] | −0.15 | 0.1±5.0 | [−1.4, 1.6] | 0.56 | 0.04 |
| DL | 0.3±3.2 | [−0.7, 1.2] | 0.08 | −0.8±2.4 | [−1.5, −0.1] | −0.33 | 1.0±3.2 | [0.1, 1.9] | 0.08 | 0.37 | |
| ecLF | SL | −0.6±2.8 | [−1.5, 0.6] | −0.21 | −0.2±3.3 | [−1.2, 0.9] | −0.06 | −0.4±4.1 | [−1.7, 0.7] | 0.44 | −0.13 |
| DL | −0.4±3.2 | [−1.3, 0.6] | −0.11 | 0.5±3.1 | [−0.4, 1.6] | 0.17 | −0.9±4.0 | [−2.1, 0.4] | 0.29 | −0.28 | |
| icLF | SL | −1.4±3.3 | [−2.5, −0.2] | −0.42 | −0.4±3.4 | [−1.6, 0.7] | −0.11 | −1.0±5.3 | [−2.8, 0.7] | 0.23 | −0.31 |
| DL | −0.8±3.5 | [−1.9, 0.5] | −0.22 | −0.3±3.1 | [−1.3, 0.7] | −0.09 | −0.5±4.2 | [−1.7, 0.8] | 0.56 | −0.14 | |
|
| |||||||||||
| Minimum joint space width (mJSW) and cartilage thickness: | |||||||||||
| mJSW | mm | −1052±788 | [−1323, −803] | −1.33 | 88±259 | [6, 181] | 0.34 | −1140±795 | [−1445, −841] | <0.01 | −1.94 |
| MFTC | mm | −254±165 | [−313, −201] | −1.54 | 21±78 | [−1, 47] | 0.27 | −275±192 | [−346, −209] | <0.01 | −2.13 |
| LFTC | mm | −39±144 | [−81, 5] | −0.27 | 19±59 | [−1, 38] | 0.32 | −57±163 | [−112, 0] | 0.02 | −0.52 |
SL: superficial layer (in ms); DL: deep layer (in ms); MFTC/LFTC: medial/lateral femorotibial compartment; cMFTC/cLFTC: combined central subregion of the MFTC/LFTC; MT/LT: medial/lateral tibia; cMF/cLF: central medial/lateral femur; mJSW: minimum radiographic joint space width in fixed-flexion x-rays; SD: standard deviation; 95% CI: 95% confidence intervals computed using the bias-corrected and accelerated (BCa) bootstrapping method with 1000 replications; SRM: standardized response mean; c/e/i/a/p MT/LT: central/external/internal/anterior/posterior subregion of the MT/LT; c/e/i cMF/cLF: central/external/internal subregion of the cMF/cLF. P-values were computed using non-parametric Wilcoxon signed-rank tests. Measures with statistically significant changes from baseline to year-one follow-up were highlighted using italic (p<0.025) or bold (p<0.01) face.
Association of baseline cartilage T2 times with subsequent progression
The superficial layer cartilage T2 times in the MFTC were slightly longer in progressor knees (48.9±3.0 ms, 95% CI: [47.9, 49.9] ms) than in non-progressor knees (47.8±3.6 ms, 95% CI: [46.7, 48.9] ms), but the difference did not reach statistical significance (p=0.07, Cohen’s D: 0.35, Table 2). In the deep layer, the cartilage T2 times in the MFTC also did not differ statistically significantly between progressor (40.8±3.6 ms, 95% CI: [39.7, 41.8] ms) and non-progressor knees (40.1±4.7 ms, 95% CI: [38.7, 41.4] ms, p=0.29, Cohen’s D: 0.17, Table 2). Exploratory analyses of laminar T2 times in cMFTC/cLFTC, cartilage plates, and in cartilage subregions revealed no statistically significant differences between progressor and non-progressor knees (Table 2).
In the stratum of KLG2 knees, the superficial MFTC T2 times tended to be longer in progressor (49.1±3.4 ms, 95% CI: [47.7, 50.6] ms) than in non-progressor knees (46.8±3.7 ms, 95% CI: [45.4, 48.3] ms), but the difference failed to reach the adjusted significance level (p=0.03, Cohen’s D: 0.64, Online Table 1). No statistically significant difference was observed in the deep MFTC layer (40.0±3.0 ms, 95% CI: [38.7, 41.2] ms vs. 38.7±3.4 ms, 95% CI: [37.6, 39.9] ms, p=0.11, Cohen’s D: 0.39, Online Table 1). Exploratory analyses revealed longer T2 times in the superficial layer of the cMF, and in the deep layer of the LFTC, the cLF, and the icLF of progressor knees when compared to non-progressor knees (p≤0.02, Online Table 1).
In the stratum of KLG3 knees, no statistically significant differences were observed in superficial or deep MFTC T2 times between progressor and non-progressor knees (p≥0.84, Online Table 3). Exploratory analyses showed statistically significantly shorter T2 times in the deep layer of cLF and in the superficial layer of cLT of progressor knees when compared to non-progressor knees (p≤0.02, Online Table 3).
Longitudinal change in cartilage T2 in knees with and without concurrent progression
No statistically significant longitudinal change over the 1 year follow-up period was observed in the superficial layer of the MFTC of either progressor and non-progressor knees (Table 3). In the deep layer, an increase in MFTC T2 times was observed in progressor knees (1.8±4.0 ms, 95% CI: [0.4, 3.6] ms, SRM=0.39, p=0.04) that was statistically significantly greater (p=0.02, Cohen’s D: 0.56) than the change observed in non-progressor knees (−0.2±1.9 ms, 95% CI: [−0.7, 0.4] ms, SRM=−0.08, p=0.21).
Exploratory analyses showed statistically significant increases in cartilage T2 times in progressor knees in the deep layer of MT, cMFTC, and cMT (Table 3), with the changes in MT and cMFTC being statistically significantly greater than the changes observed in matched non-progressor knees (p≤0.01, Table 3). Statistically significant differences between changes observed in progressor and non-progressor knees were also observed in the deep layer of both aMT and ccMF (p≤0.01, Table 3). A statistically significant decrease in cartilage T2 times was observed in the superficial layer of cLF and icLF in progressor knees (p≤0.025, Table 3), but these changes did not differ from the changes observed in non-progressor knees.
In the 21 knees with baseline KLG 2, no statistically significant increase or decrease in laminar T2 was observed in both progressor and non-progressor knees (Online Table 2). The change in the deep layer of the cMFTC and ccMF was, however, statistically significantly greater in progressor than non-progressor knees (p≤0.01, Online Table 2).
In the 16 knees with baseline KLG 3, we observed a statistically significant decrease in cartilage T2 times in the superficial layer of the pMT in progressor knees and in the deep layer of the aMT, ccMF, cLF, and icLF of non-progressor knees (Online Table 4). These longitudinal changes did not differ statistically significantly between progressor and non-progressor knees (Online Table 4).
Discussion
Based on a matched case-control design, this study is the first to test whether baseline values or one-year change in subregional laminar cartilage T2 in the medial compartment of knees with definite radiographic knee OA are associated with structural OA progression, defined as loss in both MRI-based cartilage thickness and radiographic JSW in the medial femorotibial compartment. The results of the cross-sectional analysis of medial compartment T2 times at baseline showed a small, but statistically not significant difference between matched knees with and without subsequent progression. In the longitudinal analysis of the one-year changes in cartilage T2 times, we found a statistically significantly greater concurrent increase in deep layer T2 times in the medial compartment of knees with progression than in matched knees without progression. Sensitivity analyses showed that baseline T2 times in the medial compartment tended to differ between progressor and non-progressor knees in the KLG 2 but not in the KLG 3 stratum, whereas the longitudinal changes did not differ significantly between progression and non-progressor knees in both the KLG2 and KLG3 stratum.
The cross-sectional analysis using the baseline T2 data is in agreement with a previous analysis of bulk femorotibial T2 that did not identify baseline differences between knees prospectively experiencing cartilage loss versus those that did not37. The current results, however, contrast those of several previous studies2,9,10 that reported statistically significantly greater cartilage T2 times in knees with subsequent progression in cartilage lesion scores than in knees without such progression. In our study, no statistically significant differences were observed between knees with and without subsequent progression. Possible explanations for this discrepancy include the differences in study designs (cohort studies vs. matched case-control study), different inclusion criteria (knees with and/or without radiographic OA2,9,10 vs. knees with established radiographic OA [KLG 2/3]), and different definitions of progression (incidence or worsening of semi-quantitative cartilage lesion scores vs. loss in both MRI-based cartilage thickness and radiographic JSW in the medial femorotibial compartment). To avoid potential bias from disease-related covariates such as BMI20,21, the presence or absence of radiographic OA14,15, or pain19, a matched case-control design controlling for these factors was chosen for the current study. It is of note that a sensitivity analysis in the study by Prasad et al.10 found the association with progressor status to disappear, once knees with and without definite radiographic knee OA were analyzed separately, which accords with the findings in the current study.
In our study, the baseline T2 times in the medial compartment were slightly (but not statistically significantly) longer in knees with subsequent progression than in knees without progression, with this effect being more pronounced in the superficial than in the deep layer. However, the differences in the medial compartment did not reach statistical significance, and this did not change when confining the analysis to KLG2 or KLG3 knees. Explorative analyses showed statistically significant differences in the superficial cMF layer and also in the lateral compartment, but such differences were only observed in the subsample of KLG 2 knees. Even when assuming that these differences may become statistically significant in a larger sample, cartilage T2 does not appear to be strongly associated with prospective structural progression in knees with established femorotibial radiographic OA, particularly when compared with other risk factors that are more easily measured using radiography, such as low baseline mJSW and JSN scores13,37.
Concurrent with the structural progression between baseline and year-one follow-up, we observed a longitudinal change in medial compartment deep layer T2 times of progressor knees that was significantly greater than that in non-progressor knees. No such increase was, however, observed in the superficial cartilage laminae. These deep layer T2 changes in progressor knees were statistically significant in MT, cMFTC, ccMF, and aMT, which have been shown to be also sensitive to change in cartilage thickness26. The longitudinal changes in deep layer T2 of the MFTC concurrent with progression were very similar in KLG2 and KLG3 knees, but the sensitivity to change in the MFTC of KLG3 knees was slightly greater (SRM = 0.42) than in KLG 2 knees (SRM=0.37) or the entire cohort (SRM=0.39). These results suggest that (subregional) deep cartilage T2 can be monitored with reasonable sensitivity in knees with structural progression, even over a relatively short period of 1 year, and even in knees with advanced radiographic OA (KLG 3). This increase in deep cartilage T2 appears to be specific to structural progression, as no such increase was observed in non-progressor knees or in the lateral femorotibial cartilage compartment. In contrast, a longitudinal decrease in cartilage T2 times was observed in the central lateral femur, which reached significance in knees with concurrent structural progression in the medial compartment. Because no significant cartilage loss was observed in the lateral femorotibial compartment in these knees, it is unlikely that this change is associated with cartilage loss (e.g. loss of superficial cartilage with long relaxation times). Instead, this change may potentially be attributed to a shift towards more varus induced by narrowing of the medial joint space38.
Although the statistical power of the present analysis was limited due to a relatively small sample size, it was sufficient to identify statistically significant longitudinal change in (subregional) deep medial femorotibial cartilage T2 with structural progression. The strength of the approach was that two established39 and mutually independent imaging methods were used to identify knees with (and without) structural progression40. The rates of cartilage thickness and mJSW loss obtained using the smallest detectable change (SDC) method28 were well above thresholds assumed to be clinically relevant41, well above those observed in KLG 2–3 knees in the OAI12, and even exceeded the change observed in knees prior to knee replacement surgery42–44. This highlights the effectiveness of a combined MRI and radiographic SDC threshold in identifying knees with structural progression and also reassures that the weakness of the association between cartilage T2 times and subsequent progression is not due to an insufficient magnitude of structural progression. Further, relying on MRI in addition to radiography involved the additional benefit of being able to rule out participants with lateral femorotibial progression as controls. A potential limitation of the current study is the use of less powerful non-parametric tests for the statistical comparisons, because the paired differences between progressor and non-progressor knees were not normally distributed for some of the analyzed parameters. However, when using parametric t-tests on those differences that were normally distributed, the results were similar to those obtained with non-parametric tests. Another limitation of the current study is that the femoropatellar compartment of the knee was not analyzed, although we cannot rule out that femoropatellar cartilage T2 may be associated with femorotibial progression. Previous studies have, however, shown that local risk factors such as joint space narrowing, meniscus or cartilage lesions are most relevant when being observed in the same compartment13,45.
In conclusion, the current study suggests that baseline laminar cartilage T2 in the medial femorotibial compartment is not a strong prognostic factor for subsequent structural progression in the same compartment of knees with established (but without end-stage) radiographic OA as defined by quantitative outcome measures, such as radiographic mJSW and MRI-based cartilage thickness. The statistically significant progression in both radiographic JSW and MRI-based cartilage thickness was, however, accompanied by a statistically significantly greater concurrent increase in deep layer T2 times in the medial compartment of knees with progression than in matched knees without progression. These findings indicate that compositional changes as identified by T2 occur in the deep cartilage layer whilst structural progression is ongoing.
Supplementary Material
Acknowledgments
We would like to thank the OAI participants, OAI investigators, OAI clinical and technical staff, the OAI coordinating center and the OAI funders for providing this unique public data base. We would also like to than Dr. Adam Culvenor for proof-reading the manuscript.
FUNDING SOURCES
The OAI, a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262), was funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners of the OAI include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. The sponsors were not involved in the design and conduct of this particular study, in the analysis and interpretation of the data, and in the preparation, review, or approval of the manuscript. The image analysis of this study was partly supported by a grant from the Paracelsus Medial University research fund (PMU FFF) E-13/17/090-WIR.
Footnotes
CONTRIBUTIONS
W.W. and F.E. were responsible for (1) the conception and design of the study. All authors were involved in (1) data acquisition, analysis and interpretation; (2) drafting the article or revising it critically and (3) final approval of the version to be submitted.
- Wolfgang Wirth is co-owner and has a part time employment with Chondrometrics GmbH (Ainring, Germany), a company providing medical image analysis service to academic researchers and to industry.
- Susanne Maschek is co-owner and has a part time employment with Chondrometrics GmbH.
- Felix Eckstein is CEO and co-owner of Chondrometrics GmbH. He provides consulting services to MerckSerono, Samumed, and Bioclinica, and has held educational lectures for Medtronic. He has received funding support (for studies not related to the current one) from Pfizer, Eli Lilly, Stryker, Novartis, MerckSerono, Glaxo Smith Kline, Wyeth, Contocor, Abbvie, Kolon, Synarc, Ampio, Nordic Bioscience, and Orthotrophix.
- Paula Beringer has no competing interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8(4):355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 2.Jungmann PM, Kraus MS, Nardo L, Liebl H, Alizai H, Joseph GB, et al. T2 relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur: Longitudinal data from the osteoarthritis initiative. J Magn Reson. 2013;38(6):1415–1424. doi: 10.1002/jmri.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr Cart. 2013;21(10):1474–1484. doi: 10.1016/j.joca.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liess C, Luesse S, Karger N, Heller M, Glueer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 5.Kim T, Min BH, Yoon SH, Kim H, Park S, Lee HY, et al. An in vitro comparative study of T2 and T2* mappings of human articular cartilage at 3-Tesla MRI using histology as the standard of reference. Skelet Radiol. 2014;43(7):947–954. doi: 10.1007/s00256-014-1872-z. [DOI] [PubMed] [Google Scholar]
- 6.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22(5):673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 7.Lammentausta E, Kiviranta P, Nissi MJ, Laasanen MS, Kiviranta I, Nieminen MT, et al. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: Relationships with tissue mechanical properties. J Orthop Res. 2006;24(3):366–374. doi: 10.1002/jor.20041. [DOI] [PubMed] [Google Scholar]
- 8.Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis. 2015;74(7):1353–1359. doi: 10.1136/annrheumdis-2013-204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T(2) are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3years - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20(7):727–735. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T(1)rho and T(2) relaxation times predict progression of knee osteoarthritis. Osteoarthr Cart. 2013;21(1):69–76. doi: 10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders J, Ding C, Cicuttini F, Jones G. Radiographic osteoarthritis and pain are independent predictors of knee cartilage loss: a prospective study. Intern Med J. 2011 Feb;:10–5994. doi: 10.1111/j.1445-5994.2011.02438.x. [DOI] [PubMed] [Google Scholar]
- 12.Maschek S, Wirth W, Ladel C, Hellio Le Graverand M-PP, Eckstein F. Rates and sensitivity of knee cartilage thickness loss in specific central reading radiographic strata from the osteoarthritis initiative. Osteoarthr Cartil. 2014;22(10):1550–1553. doi: 10.1016/j.joca.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirth W, Nevitt M, Hellio Le Graverand MP, Lynch J, Maschek S, Hudelmaier M, et al. Lateral and Medial Joint Space Narrowing Predict Subsequent Cartilage Loss in the Narrowed, but not in the Non-narrowed Femorotibial Compartment - Data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;22(1):63–70. doi: 10.1016/j.joca.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahl R, Blumenkrantz G, Carballido-Gamio J, Zhao S, Munoz T, MPHLG-G, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage. 2007;15(11):1225–1234. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson. 2009;61(6):1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckstein F, Cotofana S, Wirth W, Nevitt M, John MR, Dreher D, et al. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the osteoarthritis initiative. Arthritis Rheum. 2011;63(8):2257–2267. doi: 10.1002/art.30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: Data from the Osteoarthritis Initiative. Arthritis Care Res(Hoboken) 2012;64(2):248–255. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis Care Res(Hoboken) 2013;65(1):23–33. doi: 10.1002/acr.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serebrakian AT, Poulos T, Liebl H, Joseph GB, Lai A, Nevitt MC, et al. Weight loss over 48 months is associated with reduced progression of cartilage T2 relaxation time values: data from the osteoarthritis initiative. J Magn Reson. 2015;41(5):1272–1280. doi: 10.1002/jmri.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong H, Miller DJ, Urish KL. T2 map signal variation predicts symptomatic osteoarthritis progression: data from the Osteoarthritis Initiative. Skeletal Radiol. 2016;45(7):909–913. doi: 10.1007/s00256-016-2360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dardzinski BJ, Schneider E. Radiofrequency (RF) coil impacts the value and reproducibility of cartilage spin-spin (T2) relaxation time measurements. Osteoarthritis Cartilage. 2013;21(5):710–720. doi: 10.1016/j.joca.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser C, Putz R. Functional anatomy of articular cartilage under compressive loading Quantitative aspects of global, local and zonal reactions of the collagenous network with respect to the surface integrity. Osteoarthritis Cartilage. 2002;10(2):83–99. doi: 10.1053/joca.2001.0484. [DOI] [PubMed] [Google Scholar]
- 25.Eckstein F, Nevitt M, Gimona A, Picha K, Lee JH, Davies RY, et al. Rates of Change and Sensitivity to Change in Cartilage Morphology in Healthy Knees and in Knees With Mild, Moderate, and End-Stage Radiographic Osteoarthritis3: Results From 831 Participants From the Osteoarthritis Initiative. Arthritis Care Res(Hoboken) 2011;63(3):311–319. doi: 10.1002/acr.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging-the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012;8(May):622–630. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duryea J, Neumann G, Niu J, Totterman S, Tamez J, Dabrowski C, et al. Comparison of radiographic joint space width with magnetic resonance imaging cartilage morphometry: analysis of longitudinal data from the Osteoarthritis Initiative. Arthritis Care Res(Hoboken) 2010;62(7):932–937. doi: 10.1002/acr.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruynesteyn K, Boers M, Kostense P, van der LS, van der HD, van der Linden S, et al. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis. 2005;64(2):179–182. doi: 10.1136/ard.2003.018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckstein F, Kunz M, Schutzer M, Hudelmaier M, Jackson RD, Yu J, et al. Brief report Two year longitudinal change and testeretest-precision of knee cartilage morphology in a pilot study for the osteoarthritis initiative 1, 2. Osteoarthr Cart. 2007;15(11):1326–1332. doi: 10.1016/j.joca.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Hornak Joseph P. T2 Calculations in MRI: Linear versus Nonlinear Methods. J Imaging Sci Technol. 1994;38(2):154–157. [Google Scholar]
- 32.Wirth W, Eckstein F, Boeth H, Diederichs G, Hudelmaier M, Duda GNN. Longitudinal analysis of MR spin–spin relaxation times (T2) in medial femorotibial cartilage of adolescent vs mature athletes: dependence of deep and superficial zone properties on sex and age. Osteoarthr Cartil. 2014;22(10):1554–1558. doi: 10.1016/j.joca.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27(6):737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 34.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17(3):291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckstein F, Collins JE, Nevitt MC, Lynch JA, Kraus V, Katz JN, et al. Cartilage thickness change as an imaging biomarker of knee osteoarthritis progression - data from the fnih OA biomarkers consortium. Arthritis Rheumatol (Hoboken, NJ) 2015;67(12):3184–3189. doi: 10.1002/art.39324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wirth W, Maschek S, Eckstein F. Sex- and age-dependence of region- and layer-specific knee cartilage composition (spin–spin–relaxation time) in healthy reference subjects. Ann Anat - Anat Anzeiger. 2017;210:1–8. doi: 10.1016/j.aanat.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckstein F, Le Graverand MP, Charles HC, Hunter DJ, Kraus VB, Sunyer T, et al. Clinical, radiographic, molecular and MRI-based predictors of cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2011;70(7):1223–1230. doi: 10.1136/ard.2010.141382. [DOI] [PubMed] [Google Scholar]
- 38.Friedrich KM, Shepard T, Chang G, Wang L, Babb JS, Schweitzer M, et al. Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur Radiol. 2010;20(6):1532–1538. doi: 10.1007/s00330-009-1689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckstein F, Guermazi A, Gold G, Duryea J, Hellio Le Graverand M-P, Wirth W, et al. Imaging of cartilage and bone: promises and pitfalls in clinical trials of osteoarthritis. Osteoarthr Cartil. 2014;22(10):1516–1532. doi: 10.1016/j.joca.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dannhauer T, Sattler M, Wirth W, Hunter DJ, Kwoh CK, Eckstein F. Longitudinal sensitivity to change of MRI-based muscle cross-sectional area versus isometric strength analysis in osteoarthritic knees with and without structural progression: pilot data from the Osteoarthritis Initiative. Magn Reson Mater Phy. 2014;27(4):339–347. doi: 10.1007/s10334-013-0418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ornetti P, Brandt K, Hellio-Le Graverand MP, Hochberg M, Hunter DJ, Kloppenburg M, et al. OARSI-OMERACT definition of relevant radiological progression in hip/knee osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):856–863. doi: 10.1016/j.joca.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eckstein F, Kwoh CK, Boudreau R, Wang Z, Hannon M, Cotofana S, et al. Quantitative magnetic resonance imaging measures of cartilage predict knee replacement - a case-control study from the Osteoarthritis Initiative. Ann Rheum Dis. 2013;(72):707–714. doi: 10.1136/annrheumdis-2011-201164. [DOI] [PubMed] [Google Scholar]
- 43.Eckstein F, Boudreau RM, Wang Z, Hannon MJ, Wirth W, Cotofana S, et al. Trajectory of cartilage loss within 4 years of knee replacement - a nested case-control study from the Osteoarthritis Initiative. Osteoarthr Cartil. 2014;22(10):1542–1549. doi: 10.1016/j.joca.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckstein F, Boudreau R, Wang Z, Hannon MJ, Duryea J, Wirth W, et al. Comparison of radiographic joint space width and magnetic resonance imaging for prediction of knee replacement: A longitudinal case-control study from the Osteoarthritis Initiative. Eur Radiol. 2016;26(6):1942–1951. doi: 10.1007/s00330-015-3977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guermazi A, Eckstein F, Hayashi D, Roemer FW, Wirth W, Yang T, et al. Baseline radiographic osteoarthritis and semi-quantitatively assessed meniscal damage and extrusion and cartilage damage on MRI is related to quantitatively defined cartilage thickness loss in knee osteoarthritis: the Multicenter Osteoarthritis Study. Osteoarthr Cartil. 2015;23(12):2191–2198. doi: 10.1016/j.joca.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.