Abstract
BACKGROUND
Irreversible hemorrhagic shock is characterized by hyporesponsiveness to vasopressor and fluid therapy. Little is known, however, about the mechanisms that contribute to this phenomenon. Previous studies have shown that decreased intestinal perfusion in hemorrhagic shock leads to proteolytically-mediated increases in gut permeability, with subsequent egress of vasoactive substances systemically. Maintenance of blood pressure is achieved in part by α1 receptor modulation, which may be affected by vasoactive factors; we thus hypothesized that decreases in hemodynamic stability and vasopressor response in shock can be prevented by enteral protease inhibition.
METHODS
Rats were exposed to experimental hemorrhagic shock (35 mmHg mean arterial blood pressure for 2 hrs, followed by reperfusion for 2 hrs) and challenged with phenylephrine (2 μg/kg) at discrete intervals to measure vasopressor responsiveness. A second group of animals received enteral injections with the protease inhibitor tranexamic acid (TXA) (127 mM) along the small intestine and cecum one hour following induction of hemorrhagic shock.
RESULTS
Blood pressure response (duration and amplitude) to phenylephrine after reperfusion was significantly attenuated in animals subjected to hemorrhagic shock compared to baseline and control non-shocked animals, and was restored to near baseline by enteral TXA. Arteries from shocked animals also displayed decreased α1 receptor density with restoration to baseline following enteral TXA treatment. In vitro, rat shock plasma decreased α1 receptor density in smooth muscle cells, which was also abrogated by enteral TXA treatment.
CONCLUSIONS
Results from this study demonstrate that experimental hemorrhagic shock leads to decreased response to the α1-selective agonist phenylephrine and decreased α1 receptor density via circulating shock factors. These changes are mitigated by enteral TXA with correspondingly improved hemodynamics. Proteolytic inhibition in the lumen of the small intestine improves hemodynamics in hemorrhagic shock, possibly by restoring α1 adrenergic functionality necessary to maintain systemic blood pressure and perfusion.
Keywords: Hemorrhagic Shock, vasopressor resistance, tranexamic acid, Alpha-1 adrenergic receptor
BACKGROUND
One of the most challenging problems in the care of patients suffering from circulatory shock is vasopressor resistance, which is characterized by hyporesponsiveness to the infusion of vasoactive drugs required to maintain adequate hemodynamics. The mortality of patients in shock requiring vasopressors is high (> 50%) and hypotension characterizes the majority of deaths despite vasopressor support.1–3
While the cause of hypotension that typically accompanies and characterizes circulatory shock can be attributed to vasodilation generated by the strong inflammatory response occurring in shock, no consensus exists as to the mechanisms for vasopressor hyporeactivity. Several hypotheses have been postulated to account for vasopressor resistance. For instance, it is known that systemic vasopressin levels are reduced in shock4 and that nitric oxide production may be increased and contributes to vasodilation and cardiac dysfunction.5 However, clinical trials have proposed therapies interfering with these pathways without success in reducing mortality to vasopressor resistance-induced refractory shock.6, 7
One potential mechanism contributing to hyporesponsiveness to vasopressor administration in hemorrhagic shock may be altered function of the vascular α1 adrenergic receptor, a Gq-coupled receptor which is a fundamental controller of vascular tone and determinant of systemic vascular resistance (SVR). Activation of α1 increases intracellular calcium and subsequent smooth muscle contraction resulting in vasoconstriction of the vasculature. Although the α1 adrenergic receptor is also found in the heart, α1 appears to be almost exclusively involved in the maintenance of vascular tone.8, 9 SVR and thus blood pressure, when exogenously supported in the critical care setting, are with the exception of vasopressin, controlled by agents that act on the α1 adrenergic receptor (phenylephrine, epinephrine, norepinephrine, dopamine). Therefore, decreased responsiveness to this receptor has important implications for available treatment options and outcome.
It has been previously shown that hemorrhagic shock may reduce the surface density of important transmembrane receptors, such as the insulin receptor,10 thus reducing the response to insulin (insulin resistance) in shock. We hypothesize that decreased transmembrane receptor density in shock may result, either directly or indirectly, from uncontrolled proteolytic activity, possibly by digestive pancreatic enzymes from the small intestine.11 Integrity of the intestinal mucous layer is compromised in circulatory shock,12–14 and a consequence of the breakdown of this barrier may be leakage of digestive enzymes and inflammatory products from the intestinal lumen into the bloodstream.15 Once in the systemic circulation, these mediators may lead to remote organ failure and mortality (autodigestion).11
The objective of this study was to investigate a new hypothesis for vasopressor resistance in shock, by analyzing alterations in α1 adrenergic receptor expression in a rat model of hemorrhagic shock. We also tested a therapeutic intervention aimed at maintaining vasopressor responsiveness in hemorrhagic shock, by the administration of tranexamic acid (TXA) in the intestinal lumen. Used intravenously to decrease bleeding predominantly due to its actions as a plasminogen inhibitor,16 TXA also can function as a trypsin inhibitor and has been shown to improve outcomes in shock when given enterally,15 in part by protecting the gut mucosal barrier from proteolytic degradation.17, 18
METHODS
A. Experimental Protocol
All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Diego and conform to the Guide for the Care and Use of Laboratory Animals, 8th edition, by the National Institutes of Health (2011). Twenty-four non-fasted male Wistar rats (300–450 g, Harlan Laboratories, Inc., Indianapolis, IN) were randomly assigned to either Control (no shock) (n=6), hemorrhagic shock (HS) (n=6), hemorrhagic shock with enteral tranexamic acid treatment (HS + TXA) (n=6), or hemorrhagic shock with enteral Golytely® (HS + Vehicle) as a hemorrhagic shock control (n=6). All rats were anesthetized (xylazine, 4 mg/kg; ketamine 75 mg/kg IM) with supplemental anesthesia administered intravenously as needed (xylazine, 4 mg/kg; ketamine 7.5 mg/kg IV). The right femoral vein and artery were cannulated for blood withdrawal and intravenous supplemental anesthesia and continuous monitoring of arterial pressure, respectively. Body temperature was maintained at 37°C via water-heated support and heat blanket.
Control animals were monitored for 4 hours under anesthesia without other interventions. HS and HS + TXA animals were allowed 5 minutes for hemodynamic stabilization after induction of anesthesia and vascular line placement. All animals were heparinized (1 unit heparin/cc total blood volume, estimated at 6% body weight, intravenously) to allow for blood withdrawal. Hemorrhage was induced by blood withdrawal through the femoral vein (0.5 cc/min) to a target mean arterial blood pressure (MABP) of 35 mmHg. MABP was maintained between 30 and 40 mmHg for 2 hours, after which time the withdrawn blood was returned to the animals (0.5 cc/min). Maintenance of MABP during the ischemic period was accomplished by the removal or return of small aliquots of blood as necessary to ensure maintenance of the targeted pressure. The shed blood was maintained at room temperature (22°C) during hypovolemia, and warmed to 37°C prior to return to the animals. Animals were monitored for an additional two hours upon completion of blood return.
To test for vascular responsiveness the selective α1 adrenergic agonist phenylephrine (2 μg/kg) was administered as intravenous bolus (0.1 ml) once before hemorrhage to obtain baseline response and again serially at 30 minutes, 90 minutes, and 120 minutes after the start of reperfusion. Total deflection of MABP from baseline (ΔMABP), i.e. difference between maximum value of MABP after challenge and value before challenge, as well as duration of response (DOR) (time to return to within 3% of baseline) were measured as indices of vascular response to phenylephrine. In addition, % changes in the area-under-the-curve (ΔAUC) from baseline were calculated for all animals and all groups as another method for understanding and interpreting the data.
In HS + TXA animals, tranexamic acid (127 mM TXA, Cyclokapron, Pfizer) was administered at 1 hour into the hypovolemic period into the lumen of the small intestine via sequential injections (BD Sub-Q 26G 5/8 PrecisionGlide Needle, Becton Dickinson & Co.) spaced approximately 5 cm apart for an average of 8 injections longitudinally along its length. GoLytely® (0.14 g/ml 0.9% sterile water) was used as carrier solution. A total fluid volume of 15 ml was injected into the lumen of the small intestine. An additional 2 ml of TXA in vehicle was injected into the cecum. The dosage of the TXA was based on previously determined effective concentrations.15 A second group of animals (HS + Vehicle) undergoing hemorrhagic shock was instrumented as above and enteral injections of carrier solution only (GoLytely®) were performed as an additional hemorrhagic shock control. Hemodynamics from this group were indistinguishable from those of the HS group, and subsequent analyses were carried out on the HS animals (Figure 1). HS, HS + TXA, and HS + Vehicle groups were subjected to phenylephrine challenge (2 μg/kg) as described above once before hemorrhage and at 30, 90, and 120 minutes after the start of the reperfusion period.
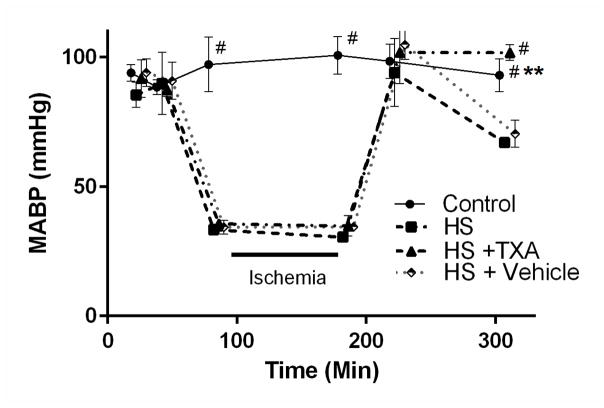
Figure 1.
Mean arterial blood pressure (MABP) of Control, Hemorrhagic Shock (HS), Hemorrhagic Shock + Enteral Tranexamic Acid (HS + TXA), and Hemorrhagic Shock + Vehicle (HS + Vehicle) groups. MABP was decreased to a mean of 35 mmHg over several minutes by the serial withdrawal of small aliquots of blood (0.5 ml/min). Reperfusion was carried out in analogous fashion by slow reinfusion of shed blood (0.5 ml/min). **p < 0.01 Control vs. HS + TXA and #p < 0.0001 HS, HS + Vehicle vs. HS + TXA and Control; #p < 0.0001 Control vs. HS, HS + Vehicle, HS + TXA during ischemia period as per experimental design. There were no statistical differences between HS and HS + Vehicle groups at any time points. Results shown as Mean ± SD.
Following a two-hour observation period after return of shed blood (Reperfusion), animals were euthanized with B-Euthanasia (120 mg/kg). Death was confirmed by loss of signal on blood pressure monitor followed by bilateral thoracotomy.
B. Tissue Collection
Renal arteries were harvested following laparotomy, gently rinsed to remove residual blood and homogenized in lysis buffer (ThermoScientific). Homogenates were treated with the addition of a protease inhibitor cocktail (1:25, Roche) for Western blot analysis. Arterial homogenates were stored at −80°C for later use. Right femoral arteries were gently excised, washed four times in 10% formalin for 15 minutes each, and placed directly into 10% formalin and stored at room temperature for immunohistochemical analysis (IHC).
C. Western Blot Detection of Arterial α1 Adrenergic Receptor
Renal artery homogenate samples from each animal (60 μl/sample; 0.5 μg/μl) were separated by SDS-PAGE. For Western blotting, proteins were transferred to a 0.45 μm pore size nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA; #162-0097). Following blockade with 5% nonfat dry milk in buffer (Tris-Buffered Saline and Tween 20, TBST), primary antibodies against α1D adrenergic receptor (α1R) (H-142, 1:300, sc-10721, Santa Cruz Biotechnology, Santa Cruz, CA) were applied. β-actin (C4, 1:100, sc-47778, Santa Cruz Biotechnology, Santa Cruz, CA) was used as loading control. Corresponding secondary antibodies were applied at 1:5000 dilution. Antibodies were diluted in TBST. Supersignal West Pico Chemiluminescent substrate (ThermoFisher Scientific, Waltham, MA; #34096) was used for imaging. Molecular weights were estimated by use of an electrophoresis marker (Sigma Aldrich, C1992). Gels were digitized and bands were analyzed by densitometry in ImageJ, and results are reported as ratios of α1R/β-actin.
D. Immunohistochemistry (IHC) Detection of Arterial α1 Adrenergic Receptor
Femoral arteries were resected, formalin fixed, and mounted longitudinally for sectioning. 30 μm sections were made along the length of the artery and placed in 10% formalin overnight. Sections were rinsed in distilled water to remove residual formalin. Endogenous peroxidase activity was removed with Bloxall (Vector Laboratories, Burlingame, CA, SP-6000). 2.5% normal horse serum (Vector Laboratories, Burlingame, CA, S-2012) was then used as a blocking reagent followed by primary antibody incubation with α1D antibody (1:200, sc-10721, Santa Cruz Biotechnology, Santa Cruz, CA) for 90 minutes. Sections were incubated for 30 minutes in ImmPRESS anti-rabbit Ig (Vector Laboratories, Burlingame, CA, MP-7401) and ImmPACT DAB (Vector Laboratories, Burlingame, CA, SK-4105) was used for staining. Sections were mounted using Vectamount (Vector Laboratories, Burlingame, CA, H-5000). Bright-field imaging was carried out at 10X objective magnification. Images were digitized to 8-bit format and measurements were taken of smooth muscle regions in each section (6 measurements/image, 10 images/animal). All analysis was performed on ImageJ. Precautions were taken to assure that all IHC steps were carried out under standard conditions to allow quantitative comparison with digital image analysis.
E. Immunocytochemistry of Smooth Muscle Cells Following Rat Plasma Incubation (ICC)
Blood samples from Control (n = 6), HS (n = 6), HS + TXA (n = 6), and HS + Vehicle (n = 6) rats were drawn and blood was centrifuged at 600g for 10 minutes to achieve plasma separation. Human carotid smooth muscle cells (SMC) (HCtASMC; Cell Applications, San Diego, CA) were cultured in growth medium (311D-250; Cell Applications, San Diego, CA) at 37 °C in an atmosphere of 5% CO2 and allowed to reach approximately 100% confluence before being exposed to either starvation medium (310–500: Cell Applications, San Diego, CA), or plasma from Control, HS, HS +TXA, or HS + Vehicle animals. SMCs were incubated in the presence of plasma variant or starvation medium for 3 hours at 37°C. Cells were starved in serum free media for 12 h prior to experimentation and plasma samples were diluted in a 1:5 ratio in starvation medium immediately prior to incubation. Added plasma was normalized to volume rather than protein content. Cells were stained as described above for rat femoral artery sections. Images were acquired at 10X objective magnification, digitized to 8-bit format and measurements were taken as the average intensity for individual cells. All analysis was performed on ImageJ. Precautions were taken to assure that all ICC steps were carried out under standard conditions to allow quantitative comparison with digital image analysis.
F. Statistical Analysis
Data are presented as mean ± standard deviation (SD) unless otherwise noted. One-way analysis of variance (ANOVA) was used where appropriate to evaluate the differences between groups with a post-hoc Tukey correction. All analyses were performed using Graphpad (Graphpad Software Inc., La Jolla, CA). A value of p<0.05 was considered statistically significant for all tests. Sample-size calculations were made based on a preselected α=0.05, 1-β = 0.8, with an expected difference in the primary outcome of interest (MAP) of 30 mmHg (SD = 10) as being clinically important, based on preliminary studies.
RESULTS
A. Blood Pressure Response to Enteral TXA Treatment
All groups subjected to HS were maintained at similar systemic blood pressures during hypovolemia. After return of the shed blood, pressure was transiently restored, before dropping significantly in the HS and HS + Vehicle groups (Figure 1). The animal group treated enterally with TXA (HS + TXA) displayed significantly higher MABP after reperfusion compared to both shock groups.
B. Systemic Vasopressor Response
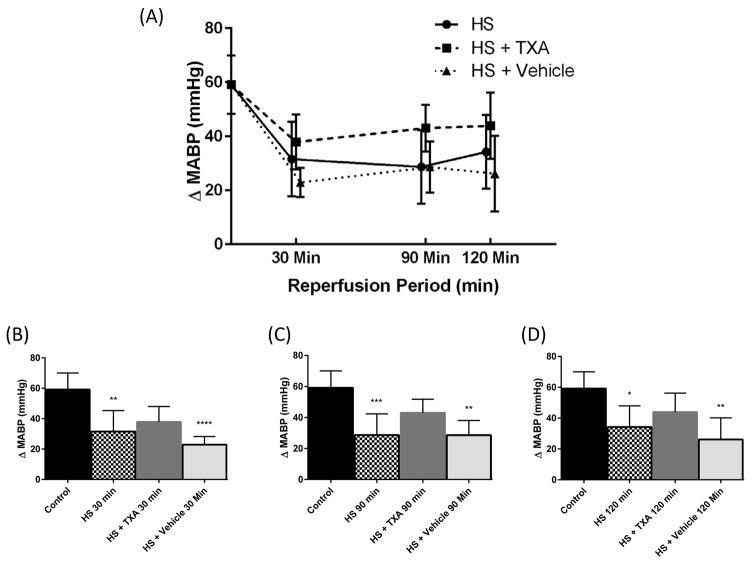
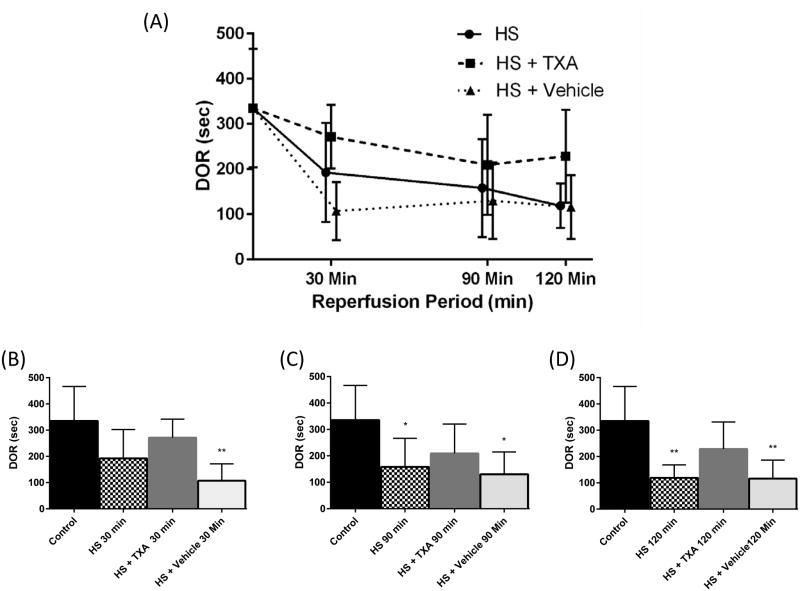
Vasopressor responsiveness to phenylephrine challenge in vivo during hemorrhagic shock was assessed by ΔMABP and DOR at baseline, and 30, 90 and 120 minutes after the start of reperfusion (Figure 2). There were no significant differences between groups for either ΔMABP or DOR at baseline. At 30 minutes after reperfusion there was a significant decrease in DOR for the HS + Vehicle group (p=0.001) and ΔMABP for both HS and HS + Vehicle groups compared to baseline (p=0.005, p<0.0001, respectively) (Figures 3 and 4)). This decrement in function in the untreated HS groups continued for the duration of the experiment in both HS and HS + Vehicle groups at 90 minutes in ΔMABP (p=0.001, p=0.003 respectively) and DOR (p=0.03, p=0.02 respectively), and at 120 minutes in ΔMABP (p=0.02, p=0.002 respectively), and DOR (p=0.002, p=0.006 respectively). At no time point during the study did ΔMABP or DOR in the HS + TXA group differ significantly from baseline. Changes in the ΔAUC from baseline (100%) were measured between the HS + Vehicle (26%, p=0.05) and HS groups (37%, p=0.064) vs. HS + TXA (71% of baseline response) at 90 minutes with significant differences between HS + Vehicle (23%, p=0.006) and HS (26%, p=0.002) vs. HS + TXA (71%) at 120 minutes.
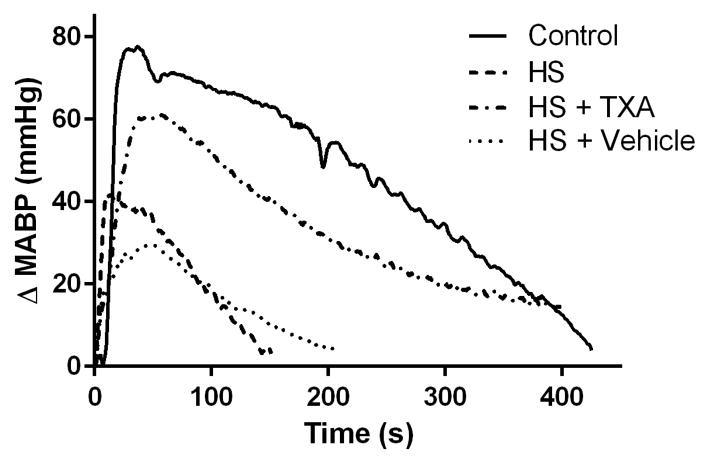
Figure 2.
Representative hemodynamic response to phenylephrine challenge (ΔMABP) at 120 minutes after start of reperfusion for a single animal in each group.
Figure 3.
(A) Average absolute change in MABP (ΔMABP) per group over time in response to phenylephrine challenge (Mean ± SD). (B) ΔMABP response to phenylephrine challenge 30 minutes after start of reperfusion, (C) ΔMABP response to phenylephrine challenge 90 minutes after start of reperfusion, (D) ΔMABP response to phenylephrine challenge 120 minutes after start of reperfusion. *p < 0.05 Control vs. HS, **p < 0.01 Control vs. HS, HS + Vehicle, ***p < 0.001 Control vs. HS, ****p < 0.0001 Control vs HS + Vehicle. Results plotted as Mean ± SD.
Figure 4.
(A) Average response duration to phenylephrine challenge (DOR) per group over time (Mean ± SD). (B) DOR to phenylephrine challenge 30 minutes after start of reperfusion, (C) DOR to phenylephrine challenge 90 minutes after start of reperfusion, (D) DOR to phenylephrine challenge 120 minutes after start of reperfusion. *p < 0.05 Control vs. HS, HS + Vehicle, **p < 0.01 Control vs. HS, HS + Vehicle. Results plotted as Mean ± SD.
C. α1 Adrenergic Receptor Levels
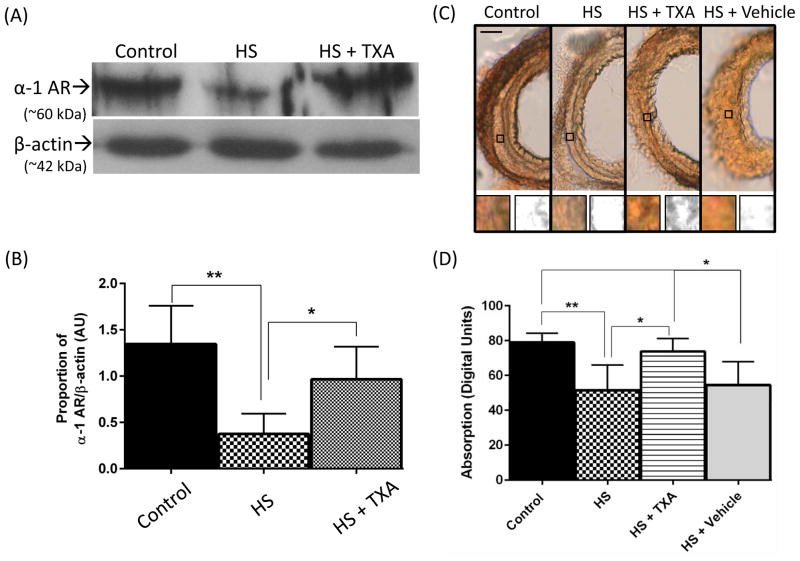
The α1 adrenergic receptor was examined by Western blot to determine whether there were changes in α1 receptor density in shock and whether these levels were preserved after treatment with enteral TXA. α1 receptor density was markedly decreased (p=0.001) by HS as measured by Western blot (Figure 5A) but maintained near Control levels by enteral treatment with TXA. Measurements of α1 receptor density were confirmed using IHC combined with digital image analysis (Figure 5C), where receptor levels as detected by immunolabeling were also significantly decreased in HS compared to Control (p=0.004). Enteral treatment with TXA resulted in light absorbance levels similar to those of the Control group.
Figure 5.
(A) α1D renal artery band density by Western blot with representative α1D renal artery band (α-1 AR). The average of the control band densities was used as a reference. β-actin is shown as a loading control for the same animals. (B) Histogram of the average relative band intensities per group expressed as ratios of α1R/β-actin (n=6 animals/group). *p < 0.05, HS vs. HS + TXA; **p < 0.01, Control vs. HS. Results plotted as Mean ± SD. (C) Representative femoral artery sections labeled for α1D. Insets indicate sample smooth muscle regions of interest (squared boxes) both before and after 8-bit conversion. (D) Histogram of labeling density values in digital units. Scale bar is = 100 microns. **p < 0.01 Control vs. HS, *p<0.05 HS + TXA vs all other groups. Results plotted as Mean ± SD.
D. Human Carotid SMC α1 Adrenergic Receptor Levels
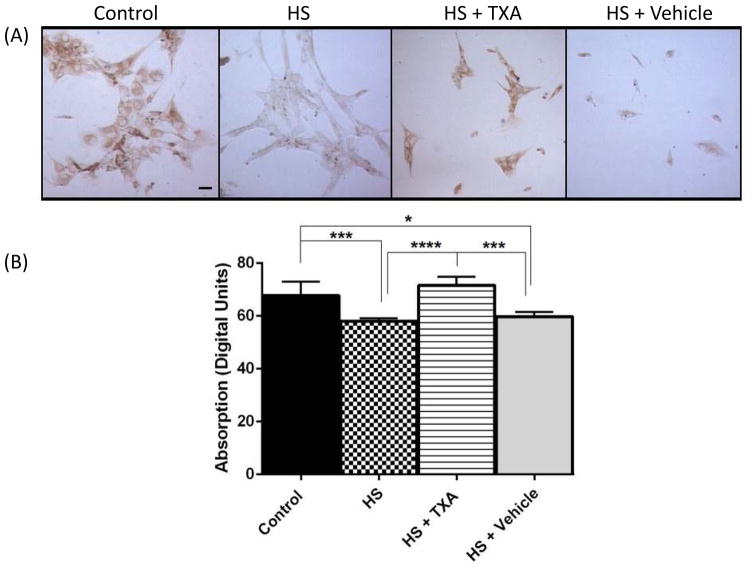
SMCs were examined for α1 using ICC (Figure 6). α1 receptor density was significantly decreased in HS and HS + Vehicle compared to Control groups (p=0.001, p=0.018, respectively), while incubation with plasma from the HS + TXA group resulted in levels similar to Control. α1 receptor levels from the HS + Vehicle and HS groups did not differ significantly from each other.
Figure 6.
(A) Human carotid SMCs labeled for α1D adrenergic receptor following 3 hour incubation with rat plasma, normalized with respect to added plasma volume. Histograms display labeling density values in digital units. Scale bar is = 10 microns. *p<0.05 Control vs. HS + Vehicle, ***p < 0.001 Control vs. HS, ***p<0.001 HS + TXA vs. HS + Vehicle, ****p<0.0001 HS + TXA vs. HS. Results plotted as Mean ± SD.
DISCUSSION
Fulminate decompensated hemorrhagic shock (Class IV),19 characterized by limited responsiveness to fluid bolus and vasopressor therapy, carries a poor prognosis, in part because no effective interventions exist. Lack of responsiveness to vasopressors and failure to maintain vascular tone, commonly described in septic shock, are not well documented for hemorrhagic shock, and although several hypotheses have been proposed, there currently exists no consensus as to mechanism.20 To study this problem, we examined the role of the α1 adrenergic receptor in experimental hemorrhagic shock. The α1 receptor is the archetypical adrenergic receptor most responsible for maintaining systemic vascular tone (vasoconstriction) and we hypothesized that failure of this receptor, either through reduced density or function, results in decreased vascular tone and subsequent hemodynamic collapse in hemorrhagic shock.8 Furthermore, we hypothesized that enteral treatment with tranexamic acid (TXA), a mild trypsin inhibitor,21 would lead to improved hemodynamics in hemorrhagic shock, in part by maintaining α1 receptor activity by prevention of proteolytically-mediated processes. The mechanisms of action by which enteral TXA is effective in experimental hemorrhagic shock appears unrelated to TXA’s well-known clinical role as an antifibrinolytic. Although IV TXA has some efficacy in hemorrhagic shock,16 this has not been consistently reported.22 Other protease inhibitors with differing effects on the coagulation system have also been found to be effective when given enterally to treat experimental shock,23, 24 and the limited patient information on the subject appears to support the hypothesis that the protease inhibitors must be given enterally to display marked clinical improvement.25
This study confirms previous reports that enteral inhibition with TXA leads to improved hemodynamics in experimental hemorrhagic shock.15 Using phenylephrine as a probe for α1 adrenergic receptor responsiveness, we report here that systemic sensitivity to this receptor decreases in experimental hemorrhagic shock with concomitant decrements in systemic pressure maintenance. The decrease in hemodynamic response is manifest as measured by attenuated changes in mean systemic blood pressure (ΔMABP) and in the duration of response (DOR) to the pressor agent phenylephrine, as well as by changes in the total hemodynamic effectiveness of phenylephrine (ΔAUC). This reduction in hemodynamic responsiveness begins early in the reperfusion period and continues unabated through the end of the experiment. Western blot analysis and IHC confirm decreased α1 adrenergic receptor density in hemorrhagic shock; the decrement in density appears to be a mechanism by which α1 receptor-mediated hyporesponsiveness is mediated. This result is consistent with reports from other investigators on adrenergic function in shock, which appears to be predominantly related to changes in receptor density rather than function.26
The mechanisms by which vascular α1 adrenergic receptor density is modulated in shock are unknown, but results from our study demonstrate that circulating factors in the blood may be operant. These circulating mediators may include digestive pancreatic proteases15 and products of proteolysis that either directly,27 or indirectly through vasoactive degradation products14 affect receptor function, and thus vascular responsiveness. Supporting this hypothesis are our current findings that enteral TXA restores receptor levels to baseline concentrations with commensurate improvements in hemodynamics in experimental hemorrhagic shock by protecting the ischemic small bowel from proteolytic degradation;11, 12, 15 this protection is not seen with intravenous protease inhibitor treatment.28
There are several limitations to this study. Among these is the lack of a detailed described mechanism by which enteral TXA inhibition in the bowel restores α1 receptor function systemically. Previous studies have demonstrated increased survival after experimental shock with enteral protease inhibition,15 but not with intravenous protease inhibition.28, 29 The reasons for this are two-fold: first, the high concentrations of proteolytic enzymes in the bowel (millimolar range) can only be overcome by high concentrations of inhibitor in the bowel lumen, which is not feasible in the systemic circulation. Second, and more importantly, by limiting proteolytic degradation of the bowel mucosa and resulting permeability changes,13, 17, 18, 30 enteral protease inhibition prevents the systemic egress of non-protease gut mediators that might otherwise affect α1 receptor function and result in subsequent hypotension. Further studies are necessary to identify the mediators and mechanisms responsible for this phenomenon. Likewise, although changes in α1 receptor responsiveness appear to be secondary to changes in density as shown by Western blot and IHC analysis, the possibility of abnormal α1 receptor binding or malfunction in hemorrhagic shock cannot be excluded. Finally, it is acknowledged that other receptors, both adrenergic (e.g., β1, β2) and otherwise (e.g., vasopressin), are also operant and may be affected in hemorrhagic shock; further studies are necessary to clarify the roles of these receptors in this condition and the possible effect of enteral TXA in modulating them.
In conclusion, this study proposes a possible mechanism for vasopressor resistance-induced refractory shock by demonstrating that hemorrhagic shock decreases α1 adrenergic receptor responsiveness and density. Decrements in α1 adrenergic receptor activity and concentrations are mitigated by enteral treatment with TXA, with resultant improved hemodynamics and vascular responsiveness. Restoration of α1 receptor density and functionality may be a key mechanism by which enteral TXA improves systemic blood pressure and survival in experimental hemorrhagic shock.
Acknowledgments
Sources of Funding
NIH grant GM 85072
Career Development Award (CDA2) 1IK2BX001277-01A1 from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, GM 85072
“CelSys Shock” Marie Curie International Outgoing Fellowship (PIOF-GA-2012-328796) of the European Union in support of the corresponding author
“ShockOmics” Project of the European Union (Grant#602706).
Supported by NIH grant GM 85072, Career Development Award (CDA2) 1IK2BX001277-01A1 from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, GM 85072, the “CelSys Shock” Marie Curie International Outgoing Fellowship (PIOF-GA-2012-328796) of the European Union in support of the corresponding author, and the “ShockOmics” Project of the European Union (Grant#602706).
Footnotes
Conflict of interest
Geert W. Schmid-Schönbein owns shares in InflammaGen, a company by Leading Bioscience Inc., San Diego, CA, U.S.A..
Meetings where partial data from this manuscript were presented
38th Annual Conference on Shock in Denver (2015).
LEVEL OF EVIDENCE: N/A
STUDY TYPE: therapeutic
AUTHOR CONTRIBUTION STATEMENT
MS, FA, JBL, AT, MC, and JL participated in data acquisition, analysis and experimental design; FA, GWS and EBK participated in experimental design, data analysis, data interpretation and drafting of the manuscript.
References
- 1.Bassi E, Park M, Azevedo LC. Therapeutic strategies for high-dose vasopressor-dependent shock. Crit Care Res Pract. 2013;2013:654708. doi: 10.1155/2013/654708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL. Comparison of dopamine and norepinephrine in the treatment of shock. The New England journal of medicine. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 3.Levy B, Collin S, Sennoun N, Ducrocq N, Kimmoun A, Asfar P, Perez P, Meziani F. Vascular hyporesponsiveness to vasopressors in septic shock: From bench to bedside. Intensive Care Med. 2010;36:2019–2029. doi: 10.1007/s00134-010-2045-8. [DOI] [PubMed] [Google Scholar]
- 4.Landry DW, Levin HR, Gallant EM, Ashton RC, Jr, Seo S, D’Alessandro D, Oz MC, Oliver JA. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122–1125. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 5.Thiemermann C, Szabo C, Mitchell JA, Vane JR. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:267–271. doi: 10.1073/pnas.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander JH, Reynolds HR, Stebbins AL, Dzavik V, Harrington RA, Van de Werf F, Hochman JS. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: The triumph randomized controlled trial. Jama. 2007;297:1657–1666. doi: 10.1001/jama.297.15.joc70035. [DOI] [PubMed] [Google Scholar]
- 7.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546c88: Effect on survival in patients with septic shock. Critical care medicine. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 8.Michelotti GA, Price DT, Schwinn DA. Alpha 1-adrenergic receptor regulation: Basic science and clinical implications. Pharmacol Ther. 2000;88:281–309. doi: 10.1016/s0163-7258(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 9.Thiele RH, Nemergut EC, Lynch C., 3rd The clinical implications of isolated alpha(1) adrenergic stimulation. Anesth Analg. 2011;113:297–304. doi: 10.1213/ANE.0b013e3182120ca5. [DOI] [PubMed] [Google Scholar]
- 10.DeLano FA, Schmid-Schonbein GW. Proteinase activity and receptor cleavage: Mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension. 2008;52:415–423. doi: 10.1161/HYPERTENSIONAHA.107.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altshuler AE, Kistler EB, Schmid-Schonbein GW. Autodigestion: Proteolytic degradation and multiple organ failure in shock. Shock. 2016;45:483–489. doi: 10.1097/SHK.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kistler EB, Alsaigh T, Chang M, Schmid-Schonbein GW. Impaired small-bowel barrier integrity in the presence of lumenal pancreatic digestive enzymes leads to circulatory shock. Shock. 2012;38:262–267. doi: 10.1097/SHK.0b013e31825b1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang M, Alsaigh T, Kistler EB, Schmid-Schonbein GW. Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PLoS One. 2012;7:e40087. doi: 10.1371/journal.pone.0040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsaigh T, Chang M, Richter M, Mazor R, Kistler EB. In vivo analysis of intestinal permeability following hemorrhagic shock. World J Crit Care Med. 2015;4:287–295. doi: 10.5492/wjccm.v4.i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLano FA, Hoyt DB, Schmid-Schonbein GW. Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock. Sci Transl Med. 2013;5:169ra111. doi: 10.1126/scitranslmed.3005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejia-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yutthakasemsunt S. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (crash-2): A randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 17.Peng Z, Ban K, LeBlanc A, Kozar RA. Intraluminal tranexamic acid inhibits intestinal sheddases and mitigates gut and lung injury and inflammation in a rodent model of hemorrhagic shock. J Trauma Acute Care Surg. 2016;81:358–365. doi: 10.1097/TA.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diebel ME, Diebel LN, Manke CW, Liberati DM, Whittaker JR. Early tranexamic acid administration: A protective effect on gut barrier function following ischemia/reperfusion injury. J Trauma Acute Care Surg. 2015;79:1015–1022. doi: 10.1097/TA.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 19.Choi SB, Choi JY, Park JS, Kim DW. Atls hypovolemic shock classification by prediction of blood loss in rats using regression models. Shock. 2016 doi: 10.1097/SHK.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 20.Duan C, Yang G, Li T, Liu L. Advances in vascular hyporeactivity after shock: The mechanisms and managements. Shock. 2015;44:524–534. doi: 10.1097/SHK.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 21.Altshuler AE, Lamadrid I, Li D, Ma SR, Kurre L, Schmid-Schonbein GW, Penn AH. Transmural intestinal wall permeability in severe ischemia after enteral protease inhibition. PLoS One. 2014;9:e96655. doi: 10.1371/journal.pone.0096655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sondeen JL, Hanson MA, Prince MD, de Guzman R, Polykratis IA, Aden JK, 3rd, Cap AP, Dubick MA. Double-blinded, placebo-controlled study of early tranexamic acid treatment in swine uncontrolled hemorrhage model. J Trauma Acute Care Surg. 2016;80:81–88. doi: 10.1097/TA.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 23.Doucet JJ, Hoyt DB, Coimbra R, Schmid-Schonbein GW, Junger WG, Paul LW, Loomis WH, Hugli TE. Inhibition of enteral enzymes by enteroclysis with nafamostat mesilate reduces neutrophil activation and transfusion requirements after hemorrhagic shock. J Trauma. 2004;56:501–510. doi: 10.1097/01.ta.0000114536.98447.f7. discussion 510–501. [DOI] [PubMed] [Google Scholar]
- 24.Fitzal F, DeLano FA, Young C, Rosario HS, Schmid-Schonbein GW. Pancreatic protease inhibition during shock attenuates cell activation and peripheral inflammation. J Vasc Res. 2002;39:320–329. doi: 10.1159/000065544. [DOI] [PubMed] [Google Scholar]
- 25.Lee YT, Wei J, Chuang YC, Chang CY, Chen IC, Weng CF, Schmid-Schonbein GW. Successful treatment with continuous enteral protease inhibitor in a patient with severe septic shock. Transplant Proc. 2012;44:817–819. doi: 10.1016/j.transproceed.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Mizumachi K, Yahagi M, Kawabata H, Tezuka S, Honda T, Okada K. Decreased beta-adrenergic receptor density in rat myocardium during hemorrhagic shock. J Anesth. 1991;5:404–411. doi: 10.1007/s0054010050404. [DOI] [PubMed] [Google Scholar]
- 27.DeLano FA, Schmid-Schonbein GW. Pancreatic digestive enzyme blockade in the small intestine prevents insulin resistance in hemorrhagic shock. Shock. 2014;41:55–61. doi: 10.1097/SHK.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kistler EB, Lefer AM, Hugli TE, Schmid-Schonbein GW. Plasma activation during splanchnic arterial occlusion shock. Shock. 2000;14:30–34. doi: 10.1097/00024382-200014010-00006. [DOI] [PubMed] [Google Scholar]
- 29.Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- 30.Chang M, Kistler EB, Schmid-Schonbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enzymes into the intestinal wall. Shock. 2012;37:297–305. doi: 10.1097/SHK.0b013e318240b59b. [DOI] [PMC free article] [PubMed] [Google Scholar]