Abstract
Lungs are directly exposed to the air, have enormous surface area and enable gas exchange in air-breathing animals. They are constantly “attacked” by microbes from both outside and inside and thus possess a unique, highly regulated local immune defense system which efficiently allows for microbial clearance while minimizing damaging inflammatory responses. As a prototypic host-adapted airborne pathogen, Mycobacterium tuberculosis traverses the lung and has several ‘interaction points (IPs)’ which it must overcome to cause infection. These interactions are critical, not only from a pathogenesis perspective but also in considering the effectiveness of therapies and vaccines in the lungs. Here we discuss emerging views on immunologic interactions occurring in the lungs for M. tuberculosis and their impact on infection and persistence.
Keywords: Mycobacterium tuberculosis, tuberculosis, respiratory track, airborne infection, mucosal immunity
Transmission and Establishment of Infection
Virtually all successful M. tuberculosis (M.tb) infections occur by airborne transmission of droplet nuclei (dried mucous droplets) containing a few viable bacteria produced by a sputum-positive individual with active tuberculosis (TB). As opposed to its rather well-characterized life inside host cells, we currently know very little about the characteristics of transmissible M.tb, how long M.tb remains in the air before entering the human body, and how M.tb interacts with and adapts to different lung environments during its pathway of infection.
M.tb starts its path to infection through a cough from an individual with active pulmonary TB. The average human cough bursts 1.5 liters of air out of the lungs containing about 3,000 droplets of saliva at speeds of up to 50 mph [1]. The majority of droplets containing M.tb are smaller than 100 microns [2]. Those greater than 5–10 microns are less effective in transmission, generally falling to the ground with gravity or potentially being deposited in the most upper airways with close contact. In contrast, those 0.5 to 3–5 microns remain in still or turbulent air for 2–40 hours before settling onto a surface [1], are transmitted over a greater distance, and are more effectively inhaled into the tracheobronchial tree and alveolar space [3]. Laryngeal TB is thought to be particularly infectious [4], due to both the stimulus for cough and the capacity to generate transmissible M.tb droplets.
M.tb must overcome several ‘Interactions Points (IPs)’ which typically serve as barriers to productive infection in the lung [5]. These evolutionarily developed IPs allow for multiple, highly effective host defense strategies, e.g., mucociliary clearance aimed at the physical removal of inhaled microbes; secretion of a variety of enzymes and pro- and anti-inflammatory mediators to fight infection [6]; and recognition of microbe-associated molecular patterns by pattern recognition receptors expressed on lung epithelial and myeloid cells. These strategies enable effective clearance of 99% of inhaled microbes in the nose and upper airways. If M.tb is able to effectively bypass these barriers and transit to the deep gas-exchange elements of the lung, this human-adapted pathogen has evolved multiple strategies to manipulate the host cell immune response during and after infection [7,8]. Here we discuss the pathway that M.tb follows to establish a successful infection, persist, and develop into TB disease from the point of view of the human respiratory track anatomy, physiology and immunology.
M.tb Transit Through the Respiratory System
M.tb can cause TB disease manifestations in all compartments of the respiratory tract (i.e. nose, sinuses, pharynx, larynx, trachea, bronchi, bronchioles, and lungs); although it is unclear if these disease manifestations occur as a result of primary infection or reactivation locally or elsewhere. In order for M.tb to infect a cell in the alveolar space, it must navigate lung anatomy, airway function and the laws of physics that govern flow dynamics, size, shape, velocity, and number of inhaled particles [3]. For example, M.tb-containing droplets must evade cough reflex arcs, airway geometry, humidity, mucocilary clearance, and mucosal bactericidal compounds, etc.
Depending on the droplet size, shape and speed, M.tb droplets can be deposited within the lung in four different ways [3]. Impaction occurs for larger particles where centrifugal force drives impaction of droplets against the walls of the upper bronchi [9]. Interception occurs for larger, elongated particles (e.g., M.tb clumps) that are deposited as soon as they contact the airway and are typically cleared by mucus and ciliary movement. These mucus-coated bacilli are typically expelled out and/or delivered into the stomach; the latter becomes another potential portal of entry. Sedimentation occurs for smaller particles that remain in the airways longer and, driven by the force of gravity, are deposited in the lower bronchi where air speed is slower [10]. Finally, suspension occurs for particles less than 0.5 microns where Brownian motion prevails and deposition in the lungs is very inefficient [3]. How M.tb droplet intrinsic (size, charge, viscosity, surface tension, density) and extrinsic (speed, concentration, clumping/agglomeration, humidity, temperature) properties determine successful transmission and infection is still uncertain. However it is believed that M.tb infection requires close contact with an individual with active TB.
First Major IP
The nasal cavity and sinuses represent the natural entrance points to the respiratory system for M.tb droplets (Figure 1, Key Figure) where several local physical and immune factors may halt a productive infection. Droplet nuclei that get past these environments encounter the first major IP: the trachea and main stem bronchi which bifurcate to enter each lung (Figure 1). Bronchial epithelial cells are ciliated, covered with mucous and undergo rhythmic movement. The mucosal lining of the tracheobronchial tree is comprised of pseudostratified ciliated columnar epithelium containing eight different cell types: basal, Kultschitzsky, intermediate, brush, serous, Goblet (mucous), ciliated and club (previously named Clara) cells [11]. Overall, we lack understanding of how these cells may contribute to M.tb pathogenesis. Basal cells are stem/progenitor cells of ciliated and mucosal cells, and are central to pulmonary host defense. Cigarette smoke alters the behavior and organization of these cells, affecting their stem cell capacity to regenerate the epithelium [12] and thereby likely contributes to M.tb infection susceptibility. Kultschitzsky, intermediate and brush cells have unknown functions in lung diseases, including TB. Serous and Goblet cells release watery and mucin-rich viscoelastic mucous, respectively, thus acting as lubricants and protectors of the respiratory system lining. This mucous is moved by beating ciliated cells contributing to mucociliary clearance, including potentially trapping M.tb droplets, allowing for their continual removal from the airways, thus limiting M.tb droplet access to the alveoli. Lastly, club cells have a dual functionality in the lung by acting as protectors (secreting mucus-like surfactant rich in glycosaminoglycans) and regenerators (acting as stem cells to restore damaged bronchial and alveolar epithelia) [13]. Airway inflammation can disrupt lung protection and regeneration by inducing swelling of mucous membranes lining the bronchi, increasing bronchial mucous production, and decreasing movement of the thick mucus by ciliated cells, thereby limiting microbial clearance. Respiratory disorders such as COPD [14], asthma [15], and silicosis [16], compounded by smoking [17] and corticosteroid use [15], are independent risk factors for M.tb infection because they cause airway inflammation driving airway obstruction, making airways smaller which increases air velocity and displaces air to unobstructed areas. Thus, airway inflammation can result in prolonging M.tb infection and deposition/movement of M.tb to healthy areas of the lung [3].
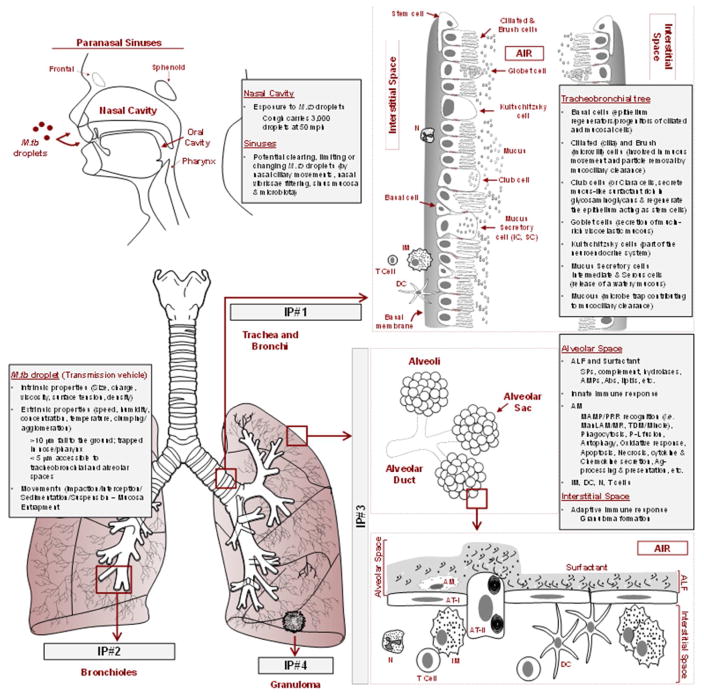
Figure 1. Key respiratory track interaction points for M. tuberculosis which must be overcome for establishment of infection.
M. tuberculosis infection requires transmission of an M. tuberculosis droplet. Intrinsic and extrinsic properties of the M. tuberculosis droplet will determine its success to reach the alveolar space. In this path to infection, M. tuberculosis droplets most transit the nose and sinuses, which contain mechanical and secretory mechanisms that limit entry of large M. tuberculosis droplets to the lower respiratory track. M. tuberculosis droplets of 3–5 microns encounter the tracheobronchial tree [Interaction Point (IP) #1] where they encounter several epithelial cell types that work together in facilitating mucociliary clearance. M. tuberculosis droplets next encounter divisions of bronchi into a myriad of bronchiolar types (IP#2) which terminate in alveolar ducts made up of alveolar sacs (IP#3). Here M. tuberculosis droplets will settle onto ALF which further disperses the droplets and reshapes M. tuberculosis bacilli as they encounter resident alveolar cells. The most abundant cells in the alveolar space are AT-I and AT-II cells. However, AMs are the main resident professional phagocyte which serves as the niche for M. tuberculosis survival within the host. The M. tuberculosis-infected AM will further trigger a series of complex and poorly understood innate immune events that result in generation of the adaptive cellular immune response with formation of granulomas (IP#4) that control infection but allow for bacterial persistence and serve as a barrier to therapy.
Although built for clearance, this first IP can also be exploited by M.tb as a portal of entry to the lymphoid tissue [18,19]. Indeed, bronchial Microfold (M) cells have been shown to translocate M.tb from the mucosa, enabling dissemination to the lymphatics [19,20]. Thus M cells, located in the nasal and bronchus-associated lymphoid tissue (NALT/BALT) [19,21], are positioned to participate in generating an early protective immune response against M.tb infection [19,20].
Bronchial epithelium has been described to harbor M.tb, where, on the one hand, induction of β-defensins may limit bacterial survival and on the other hand, residence in a non-acidified, late endosomal vacuole within bronchial epithelial cells may facilitate M.tb persistence [22,23]. Moreover, due to their anatomical localization and innate-like properties, mucosal associated invariant T (MAIT) cells are capable of detecting intracellular infection, and thus may also act as early sentinels in response to respiratory pathogens such as M.tb [24]. In fact, there is an inverse correlation between the extent of M.tb infection and the level of IFNγ secreted by MAIT cells [25].
Second and Third Major IPs
Following high velocity transit through the bronchi, M.tb droplets encounter the second major IP (Figure 1): Divisions of bronchi into bronchioles, terminal bronchioles, transitional bronchioles and respiratory bronchioles. Respiratory bronchioles divide into alveolar ducts, which compartmentalize into tiny globular compartments called alveoli which represent the majority of the lung surface area and are the third and most critical IP for M.tb (Figure 1). The adult lung is estimated to have 1,500 miles of airways, with 540 to 810 square feet (50 to 75 square meters) of total surface area, and ~4.8x108 alveoli [26], lined by a total of ~2.0x1010 cells [11]. The alveolar sac surface area is estimated to be 206.9 square millimeters [27] containing 8–12 alveolar macrophages (AMs), 40 alveolar epithelial cells type I (AT-I) and 67 type II (AT-II), with a total volume of 15 microliters of alveolar lining fluid (ALF) per alveolar sac [27]. Considering the barriers associated with interactions points 1 and 2, the likelihood of a single M.tb droplet reaching an alveolar sac is low.
ALF is composed of a surfactant lipid monolayer which reduces surface tension during inspiration and an aqueous hypophase. ALF surrounds innate immune cells such as resident ATs and AMs. Recruited cells to this space include monocytes, lymphocytes and neutrophils that participate in host defense against M.tb. Components of the human ALF hypophase layer [surfactant protein (SP) A and D, homeostatic hydrolases, mucosal antibody and the complement system, among others] are critical elements of the innate immune system during M.tb infection [8,28,29] and play important roles in M.tb-phagocyte encounters [28,29]. The importance of human ALF as a whole in determining the outcome of M.tb droplet deposition and subsequent M.tb infection is still uncertain. Recent studies indicate that M.tb exposed to human ALF is controlled more effectively by host phagocytes [30–32], potentially reducing tissue damage. This improved control is due to ALF-derived modifications of the M.tb cell wall during ALF exposure [30,32]. Human ALF contains a variety of homeostatic hydrolases secreted by resident alveolar host cells [6], including those producing surfactant [33], that resemble but are distinct from lysosomal enzymes [34]. Exposure of M.tb to ALF significantly reduces surface mannose-capped lipoarabinomannan (ManLAM) and trehalose dimycolate (TDM) [30]. Since ManLAM and TDM are critical M.tb virulence factors for survival in host cells [35], exposure to ALF is likely to impact how M.tb interacts with phagocytes, and ultimately affect the course of infection and persistence [30,31].
SP-A/-D also impact the establishment of M.tb infection [8,28,29]. SP-A enhances M.tb phagocytosis and apoptotic cell clearance by macrophages, and regulates inflammation, the oxidative burst, and expression and activity of the macrophage mannose receptor (MR, CD206) [8,28,29]. The MR is a C-type lectin highly expressed on AMs, which among others, is important for the successful establishment of M.tb infection [36]. SP-D agglutinates M.tb with two consequences: a decrease in M.tb phagocytosis by macrophages [37], and a better control of those that get phagocytosed via increased phagosome-lysosome fusion [38]. Other innate immune components of ALF are complement proteins and their cleavage products [39]. Complement levels and function are highly regulated in the lung to promote opsonization of pathogens while limiting potential harmful inflammation [39]. Indeed, the role of complement C3 in opsonizing M.tb within ALF and the contribution of complement receptors in M.tb phagocytosis has been described [40,41]. Other major ALF constituents (antibodies [42], transferrin [43], defensins [44], lysozyme [45], albumin [45], α-1-antitrypsin [45], and secretory leucoprotease inhibitor, among others [29]), and low molecular antioxidants (ascorbate, urate, glutathione [46]) are detectable at different levels in human ALF among healthy individuals and patients with lung inflammatory diseases, including TB [47,48]. Their functions in limiting or promoting the establishment of M.tb infection are uncertain.
Surfactant lipids are also major determinants of alveolar function and inflammation. Dipalmitoylphosphatidylcholine comprises almost 50% of total surfactant and is its major surface-active component [49]. The other surfactant lipids are charged phospholipids, cholesterol, acylglycerols and free fatty acids [50]. Surfactant lipids modulate the release of oxidative and inflammatory mediators from inflammatory cells [51] and alterations in their composition lead to lung surfactant dysfunction [52] including a decrease in SP-A function [53] with consequences in M.tb infection [28,29]. Surfactant lipids are also involved in regulating the production of the neutrophil chemoattractant CXCL8 (or IL-8) by ATs [54]. The accumulation of lipids in AMs has been used clinically as an indicator of lung inflammation and obstruction [55]; however how these lipids modulate inflammatory responses mediated by AMs and other alveolar resident cells during M.tb infection is poorly understood. A lipid-laden macrophage index score has been proposed for chronic pulmonary diseases [56]. Pulmonary alveolar proteinosis which is associated with abnormal surfactant recycling and metabolism fosters infection by intracellular pathogens, including mycobacteria [57]. Surfactant uptake and metabolism also occurs during the normal recycling process in healthy AMs which contain lipid inclusions and these lipid-containing AMs are particularly susceptible to M.tb infection [58].
M.tb interacts with ATs and AMs in the alveolar space. AMs, the first professional phagocytes to encounter M.tb [8], are uniquely situated within ALF [59] in the interface between air and lung tissue, and constitute the first phagocyte defense against inhaled bacteria [60]. They possess high phagocytic and clearance properties but with a uniquely regulated inflammatory response [61] with reduced microbicidal capacity [62]. Efficient microbial phagocytosis followed by slow intracellular killing may be sufficient for AMs to control infection with many routinely encountered extracellular pathogens; however, host-adapted intracellular pathogens like M.tb may take advantage of AM’s properties to reside and multiply within them [63]. Recent metabolic information on immune cells has now been integrally linked to their plasticity in inflammatory phenotypes with implications for AMs and M.tb control [64].
M.tb invasion of ATs can also play a role in the establishment of infection [65]. During primary infection, contact of M.tb with ATs is at low frequency. However, ATs may become key players when larger numbers of bacteria are released from dying infected phagocytes or from disrupted granulomas [66,67]. Interactions with ATs could also allow time for M.tb to alter its metabolism with subsequent implications in bacterial recognition by phagocytes. Thus ATs may represent a more transient intracellular niche for M.tb to adapt and survive. In support of this, M.tb has the capacity to infect and replicate within the permissive intracellular environment of AT-II cells [67]. ATs (along with macrophages and dendritic cells) could also serve as a portal of entrance to the interstitium, crossing through the epithelial basement membrane, where they encounter interstitial phagocytic cells. Clearly, interactions between M.tb and ATs are dynamic and further examination is important as these interactions could affect the outcome of infection and progression to TB [65].
M.tb encounters monocytes and neutrophils in the alveolar space as inflammation progresses. Circulating and progenitor/fetal monocytes as well as interstitial macrophages enter the lung to create and maintain AM and dendritic cell populations, and also appear during lung inflammation [68,69]. Alveolar deposition of M.tb drives an influx of monocytes along with characteristic recruitment of neutrophils [8,70]; the latter may play an important role in controlling M.tb infection [31,70] through both oxidative and non-oxidative processes [71,72].
Fourth Major IP
The fourth major IP (Figure 1) is that occurring between M.tb and granuloma in the lung. Granulomas allow for bacterial persistence during latent TB infection (LTBI) and provide a niche where bacteria are more recalcitrant to anti-TB treatment. They consist of a mixture of well differentiated innate host cells such as foamy macrophages and epithelioid cells, large multinucleated giant cells and occasionally, natural killer cells, dendritic cells and neutrophils. These cells are surrounded by T and B cells, the latter can be found in adjacent B cell follicles [73]. Granulomas are heterogeneous, dynamic structures that change in size during M.tb infection [74,75]. The nature, physiology and immunological complexity of M.tb granuloma are discussed in detail elsewhere [76–79]. It is a challenge to develop appropriate models for understanding the dynamics of granulomas. Nevertheless, in vitro human-like granuloma models indicate that granuloma formation and response to infection depend on the priming status of host cells and immune status of the host, and that M.tb develops a unique transcriptional profile in response to how the immune response within the granuloma is orchestrated to control infection [74,80]. The evolution of M.tb-containing granulomas is mainly studied using animal models. Although the mouse model is the most commonly used, mice do not generate structural granulomas as found in humans. Recently, new mouse models (e.g., inbred C3HeB/FeJ mouse [81] and genetically altered mice [82–84]) have been shown to generate more mature granulomas (necrotizing/caseating) upon M.tb infection. Zebrafish, Guinea pigs, rabbits and non-human primates also generate necrotic granulomas [85], the latter have been particularly useful in defining granuloma characteristics [86].
When M.tb infection progresses to active disease, granulomas grow in size and fail to contain M.tb leading to the various TB disease manifestations. Individuals with pulmonary TB develop large acellular necrotic granulomas resulting in cavities. The human T cell response is thought to be involved in cavity formation [87]. Cavities are rich in neutrophils and macrophages and contain caseous (milky, thick substance) necrotic material [77]. Individuals with advanced cavitary pulmonary TB can discharge numerous M.tb bacilli to the airways, facilitating efficient expectoration of M.tb-containing droplets via coughing and thereby initiating the transmission cycle.
Concluding Remarks
Lungs are a unique organ in directly interfacing with the environment and possessing an enormous surface area to enable its critical primary function of breathing and gas exchange. In performing its function, it must limit the inhalation of unwanted particulates, and those that do enter the lung must be cleared efficiently while limiting lung damage, in part by dampening the pro-inflammatory immune response. Given its primary function, inhaled microbes represent a particular challenge, especially host-adapted pathogens like M.tb that have evolved to enter and survive following their entry in the lung where they can get an upper hand early in their pathogenesis. On the other hand, lungs also have uniquely effective clearance capabilities, including rapid recruitment of lymphocytes to the airways and the existence of tissue-resident memory T cells within the lung [88]. Herein we describe several IPs for M.tb as it transits the lung and point out how little we know about these interactions.
Moving forward, it is critical that we have accurate models available to study M. tuberculosis-lung interactions. For humans, we must resort to the availability of bronchoalveolar lavage material which contains primarily AMs and ALF or biopsy material that provides a typically late and static view. Bronchoscopy-based research is costly and there is difficulty with recruitment of donors on a regular basis, both facts are important limitations of this procedure. More importantly, bronchoscopy-based research cannot directly address the relationship between immune events of the airways and those than cannot be assessed via bronchoscopy (i.e., antigen presentation within local lymph nodes, trafficking of immune cells to granulomas, etc.). Hence insights could be found by using animal models to assess how these processes may be reflected in immune findings that are amenable to bronchoscopy-based evaluation. Animal models will also continue to be very important to study M.tb transmission dynamics, latency and disease development. Such models are essential since it is well-known that effective treatments and vaccine strategies are particularly problematic in the lung. However, these models must take into account lung anatomy, physiology and immunology which all dictate the outcome of airborne infection. Finally, there is also the need for development of more tractable in vitro human models and refined in silico models, both of which are being described [30,89,90].
Maintenance of LTBI in the lung requires particular balance and coordination of innate and adaptive immune responses. Any disturbance, e.g., pollutants, cigarette smoke, lung inflammatory disorders, change in the microbiota, or co-infections (HIV, helminth) etc. can drive different types of lung inflammation resulting in a propensity for active TB disease and enhanced transmission potential. For example, HIV co-infection can alter how M.tb interacts with lung mucosal function [91] and AMs [92], and subsequently granuloma formation by modulating TNF and possibly cell trafficking, leading to an impaired immune response [93]. TB patients co-infected with helminths have more severe radiological pulmonary disease, with a higher number of involved lung zones at the end of TB treatment [94]. Since concomitant helminth infection in newly diagnosed TB patients drives a permissive T helper 2 response, this favors persistent M.tb infection and a more extended clinical course of the disease [94].
Advanced knowledge of the pulmonary elements described in this review is also necessary from the standpoint of effectively engineering inhaled immunotherapies and/or inhaled vaccines [95]. Impacting this is the realization that lungs are constantly exposed to high levels of cigarette and environmental smoke as well as other pollutants in many countries where TB is endemic. This fact will require us to understand in detail the influence of these pollutants on the lung environment, and how this impacts M.tb infection, progression to disease and response to inhaled therapies and vaccines. Smoking and other particulate exposures have major effects on AM function and immune responses [96].
Other external factors such as age, nutritional status and host genetics likely also impact the four interactions points described. Several human population studies have emphasized the importance of genetics in host susceptibility to TB (reviewed in [97] and [98]). Rare genetic disorders such as cystic fibrosis which impacts several of the IPs described has been associated with different mycobacterial infections [99], although the relationship to TB is unclear [100]. Assessing the contributions and functional consequences of each IP in the setting of M.tb infection and subsequent progression to disease remains a major challenge. Many questions related to the roles of lung anatomy, physiology and gas exchange in M.tb transmission and successful establishment of the infection remain unanswered (see Outstanding Questions).
Outstanding questions Box.
What is the metabolic status of transmitted M. tuberculosis?
What is the metabolic status of the lung that renders it favorable to accommodate M. tuberculosis?
How many M. tuberculosis droplets cause an infection?
What is the nature of the host cells that interact with M. tuberculosis during its transit in the lung via its various interaction points?
Does inhalation of airborne M. tuberculosis ever truly lead to an aborted infection?
What is the extent of cell types that harbor M. tuberculosis in the lung?
How exactly does M. tuberculosis leave the alveolus to traffic to the lymph nodes? Is it via trafficking of host cells (Trojan horse) through the Pores of Kohn?
What is the relationship between M. tuberculosis infection in the lung and granuloma formation?
How often are lung granulomas resolved or maintained to drive latency or active TB?
What triggers cavity formation in the lung?
What level of M. tuberculosis dissemination outside of the lung through the lymphatics is necessary to develop a sustained adaptive immune response manifested by a positive TB skin test or an interferon-gamma (IFN-γ) release assay?
Trends Box.
Mycobacterium tuberculosis is an airborne, human to human transmissible bacterial pathogen that causes tuberculosis (TB).
Airborne infection requires that M. tuberculosis bacilli on dried mucous droplets of the right size effectively enter the lung alveolar spaces following transit through an elaborate and complex respiratory system designed to eliminate inhaled particulates.
Within the respiratory system, there are numerous interaction points for airborne M. tuberculosis droplets that include soluble and cellular elements involved in innate immunity.
A more complete understanding of how lung anatomy, physiology and gas exchange participate in M. tuberculosis transmission and successful establishment of infection is required to advance our approaches to effective treatments and vaccines targeted for the lung
Acknowledgments
Support of our research from the National Institute of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) AI093570 (to JBT); NIH/NIAID AI059639 and AI116039, and the Bill and Melinda Gates Foundation (to LSS); and from the NIH/National Institute of Aging (NIA) AG051428 (to JBT and LSS).
Glossary
- Alveolar epithelial cell (AT)
cellular constituent of the alveolar epithelium.
- Alveolar epithelial cell type I (AT-I)
cellular constituent of the alveolar epithelium with structural function.
- Alveolar epithelial cell type II (AT-II)
cellular constituent of the alveolar epithelium that secretes alveolar lining fluid.
- Alveolar lining fluid (ALF)
secreted by alveolar epithelial cells type II into the alveolar space. It is comprised of surfactant lipids and proteins, and an aqueous hypophase containing an array of soluble enzymes and immunomodulators.
- Alveolar macrophage (AM)
resident professional phagocyte of the alveolar space.
- Bronchus-associated lymphoid tissues (BALT)
loosely organized clusters of lymph tissue beneath the bronchus epithelium in the lungs.
- Chronic obstructive pulmonary disease (COPD)
a chronic inflammatory lung disease that causes obstructed airflow from the lungs.
- Complement component 3 (C3)
innate immune system protein that plays a central role in the complement system and contributes to innate immunity.
- Interleukin (IL)
an immunomodulator (cytokine) secreted by host cells in response to a stimulus.
- Interleukin 8 [IL8 or chemokine (C-X-C motif) ligand 9, CXCL8]
a chemoattractant produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells and endothelial cells.
- Interaction points (IPs)
refers to points of contacts (or barriers) that Mycobacterium tuberculosis needs to overcome as traverses the lung during its pathway to infection.
- Latent TB infection (LTBI)
refers to an individual infected with M. tuberculosis, but without the signs and symptoms of active tuberculosis. Active pulmonary tuberculosis is contagious while latent tuberculosis infection is not.
- Mannose-capped lipoarabinomannan (ManLAM)
an important component of the M. tuberculosis cell wall involved in macrophage recognition and bacterial intracellular survival.
- Mannose receptor (MR; CD206)
a host cell homeostatic receptor that recognizes mannosylated components on the cell wall surface of M. tuberculosis and enables entry and survival in macrophages.
- Microfold cell (M cell)
found in bronchus-associated lymphoid tissue.
- Mucosal associated invariant T (MAIT) cells
evolutionarily conserved T cells restricted by the non-classical MHC-1b molecule, MR1.
- Mycobacterium tuberculosis (M.tb)
a bacterium that causes tuberculosis.
- Nasal-associated lymphoid tissue (NALT)
loosely organize clusters of lymph tissue beneath the nasal epithelium in the nasal cavity.
- Surfactant protein-A or –D (SP-A/-D)
soluble C-type lectins in ALF with innate immune function.
- T cell
white blood cell of key importance to the adaptive immune system.
- Trehalose dimycolate (TDM)
cell wall component of M. tuberculosis that interacts with the host cell receptor macrophage inducible Ca2+ dependent lectin.
- Tuberculosis (TB)
an infectious disease caused by a bacterium within the M. tuberculosis complex (where M. tuberculosis infection is the leading cause of TB). Active TB generally affects the lungs; however it can also affect other body parts. Classical clinical manifestations of active TB are chronic cough with blood-containing sputum, fever, weight loss and night sweats.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kulkarni P, Baron P, Willeke K. In: Aerosol Measurement: Principles, Techniques, and Applications. Kulkarni P, Baron P, Willeke K, editors. New Jersey: Wiley; 2001. pp. 15–30. [Google Scholar]
- 2.Heyder J, Gebhart J, Stahlhofen W, Stuck B. Biological variability of particle deposition in the human respiratory tract during controlled and spontaneous mouth-breathing. Ann Occup Hyg. 1982;26:137–147. [PubMed] [Google Scholar]
- 3.Fernandez TA, Casan CP. Deposition of inhaled particles in the lungs. Arch Bronconeumol. 2012;48:240–246. doi: 10.1016/j.arbres.2012.02.003. S0300-2896(12)00064-6 [pii]; [DOI] [PubMed] [Google Scholar]
- 4.Espinoza CG, Montano P, Saba SR. Laryngeal tuberculosis. Laryngoscope. 1981;91:110–113. doi: 10.1288/00005537-198101000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Arnett E, Krishnan N, Roberston BD, Schlesinger L. Host pathogen biology for airborne Mycobacterium tuberculosis. In: Hickey A, Misra A, Fourie P, editors. Drug delivery systems for tuberculosis preveniton and treatment. Chichester, UK: John Wiley & Sons, Ltd; 2016. pp. 11–47. [Google Scholar]
- 6.Nicod LP. Lung defenses: An overview. Eur Resp Rev. 2005;14:45–50. [Google Scholar]
- 7.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Guirado E, Schlesinger LS, Kaplan G. Macrophages in tuberculosis: Friend or foe. Semin Immunopathol. 2013;35:563–583. doi: 10.1007/s00281-013-0388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heyder J. Alveolar deposition of inhaled particles in humans. Am Ind Hyg Assoc J. 1982;43:864–866. doi: 10.1080/15298668291410710. [DOI] [PubMed] [Google Scholar]
- 10.Heyder J. Particle transport onto human airway surfaces. Eur J Respir Dis Suppl. 1982;119:29–50. [PubMed] [Google Scholar]
- 11.Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 12.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. dmm.006031 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkinson JJ, Adair-Kirk TL, Kelley DG, Demello D, Senior RM. Clara cell adhesion and migration to extracellular matrix. Respir Res. 2008;9:1. doi: 10.1186/1465-9921-9-1. 1465-9921-9-1 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Gao B, Xu W, Chen L, Xiong S. The defect in autophagy induction by clinical isolates of Mycobacterium tuberculosis is correlated with poor tuberculosis outcomes. PLoS ONE. 2016;11:e0147810. doi:10.1371/journal.pone.0147810;PONE-D-15-40011 [pii] [Google Scholar]
- 15.Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55:19–26. doi: 10.1002/art.21705. [DOI] [PubMed] [Google Scholar]
- 16.Barboza CE, Winter DH, Seiscento M, Santos UP, Terra FM. Tuberculosis and silicosis: Epidemiology, diagnosis and chemoprophylaxis. J Bras Pneumol. 2008;34:959–966. doi: 10.1590/s1806-37132008001100012. S1806-37132008001100012 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Davies PD, Yew WW, Ganguly D, Davidow AL, Reichman LB, Dheda K, Rook GA. Smoking and tuberculosis: The epidemiological association and immunopathogenesis. Trans R Soc Trop Med Hyg. 2006;100:291–298. doi: 10.1016/j.trstmh.2005.06.034. S0035-9203(05)00321-4 [pii]; [DOI] [PubMed] [Google Scholar]
- 18.Behr MA, Waters WR. Is tuberculosis a lymphatic disease with a pulmonary portal? Lancet Infect Dis. 2014;14:250–255. doi: 10.1016/S1473-3099(13)70253-6. S1473-3099(13)70253-6 [pii]; [DOI] [PubMed] [Google Scholar]
- 19.Nair VR, Franco LH, Zacharia VM, Khan HS, Stamm CE, You W, Marciano DK, Yagita H, Levine B, Shiloh MU. Microfold Cells Actively Translocate Mycobacterium tuberculosis to Initiate Infection. Cell Rep. 2016;16:1253–1258. doi: 10.1016/j.celrep.2016.06.080. S2211-1247(16)30849-X [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teitelbaum R, Schubert W, Gunther L, Kress Y, Macaluso F, Pollard JW, McMurray DN, Bloom BR. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity. 1999;10:641–650. doi: 10.1016/s1074-7613(00)80063-1. [DOI] [PubMed] [Google Scholar]
- 21.Pankow W, von WP. M cell in the immune system of the lung. Respiration. 1988;54:209–219. doi: 10.1159/000195527. [DOI] [PubMed] [Google Scholar]
- 22.Rivas-Santiago B, Contreras JC, Sada E, Hernandez-Pando R. The potential role of lung epithelial cells and beta-defensins in experimental latent tuberculosis. Scand J Immunol. 2008;67:448–452. doi: 10.1111/j.1365-3083.2008.02088.x. SJI2088 [pii]; [DOI] [PubMed] [Google Scholar]
- 23.Harriff MJ, Cansler ME, Toren KG, Canfield ET, Kwak S, Gold MC, Lewinsohn DM. Human lung epithelial cells contain Mycobacterium tuberculosis in a late endosomal vacuole and are efficiently recognized by CD8(+) T cells. PLoS ONE. 2014;9:e97515. doi: 10.1371/journal.pone.0097515. doi:10.1371/journal.pone.0097515;PONE-D-13-45565 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold MC, Napier RJ, Lewinsohn DM. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev. 2015;264:154–166. doi: 10.1111/imr.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang J, Chen X, An H, Yang B, Zhang F, Cheng X. Enhanced immune response of MAIT cells in tuberculous pleural effusions depends on cytokine signaling. Sci Rep. 2016;6:32320. doi: 10.1038/srep32320. srep32320 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, Richter J, Gundersen HJ. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120–124. doi: 10.1164/rccm.200308-1107OC. doi:10.1164/rccm.200308-1107OC;200308-1107OC [pii] [DOI] [PubMed] [Google Scholar]
- 27.Stone KC, Mercer RR, Freeman BA, Chang LY, Crapo JD. Distribution of lung cell numbers and volumes between alveolar and nonalveolar tissue. Am Rev Respir Dis. 1992;146:454–456. doi: 10.1164/ajrccm/146.2.454. [DOI] [PubMed] [Google Scholar]
- 28.Torrelles JB, Azad AK, Henning LN, Carlson TK, Schlesinger LS. Role of C-type lectins in mycobacterial infections. Curr Drug Targets. 2008;9:102–112. doi: 10.2174/138945008783502467. [DOI] [PubMed] [Google Scholar]
- 29.Carlson TK, Brooks M, Meyer D, Henning L, et al. Pulmonary innnate immunity: Soluble and cellular host defenses of the lung. In: Marsh C, Tridandapani S, Piper M, editors. Regulation of Innate Immune Function. Kerala: Transworld Research Network; 2010. pp. 165–211. [Google Scholar]
- 30.Arcos J, Sasindran SJ, Fujiwara N, Turner J, Schlesinger LS, Torrelles JB. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J Immunol. 2011;187:372–381. doi: 10.4049/jimmunol.1100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arcos J, Diangelo L, Scordo J, Sasindran J, Moliva J, Turner J, Torrelles J. Lung mucosa lining fluid modifies Mycobacterium tuberculosis to reprogram human neutrophil killing mechanisms. J Infect Dis. 2015;212:948–958. doi: 10.1093/infdis/jiv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arcos J, Sasindran SJ, Moliva JI, Scordo JM, Sidiki S, Guo H, Venigalla P, Kelley HV, Lin G, Diangelo L, Silwani SN, Zhang J, Turner J, Torrelles JB. Mycobacterium tuberculosis cell wall released fragments by the action of the human lung mucosa modulate macrophages to control infection in an IL-10-dependent manner. Mucosal Immunol. 2017:mi2016115. doi: 10.1038/mi.2016.115. [pii]; doi:10.1038/mi.2016.115; [ePUb Ahead of Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11(Suppl):S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 34.Hook GE, Gilmore LB. Hydrolases of pulmonary lysosomes and lamellar bodies. J Biol Chem. 1982;257:9211–9220. [PubMed] [Google Scholar]
- 35.Torrelles JB. Broadening our view about the role of Mycobacterium tuberculosis cell envelope components during infection: A battle for survival. In: Cardona PJ, editor. Understanding Tuberculosis - Analyzing the Origin of Mycobacterium tuberculosis Pathogenicity. Rijeka, Croatia: Intech; 2012. pp. 77–122. [Google Scholar]
- 36.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, Desjardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS. Surfactant protein D binds to Mycobacterium tuberculosis bacili and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol. 1999;163:312–321. [PubMed] [Google Scholar]
- 38.Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB, Voelker DR, Crouch EC, Schlesinger LS. Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun. 2006;74:7005–7009. doi: 10.1128/IAI.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolger MS, Ross DS, Jiang H, Frank MM, Ghio AJ, Schwartz DA, Wright JR. Complement levels and activity in the normal and LPS-injured lung. Am J Physiol Lung Cell Mol Physiol. 2007;292:L748–L759. doi: 10.1152/ajplung.00127.2006. 00127.2006 [pii]; [DOI] [PubMed] [Google Scholar]
- 40.Schlesinger LS. Mycobacterium tuberculosis and the complement system. Trends Microbiol. 1998;6:47–49. doi: 10.1016/S0966-842X(97)01203-1. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson JS, Weis JJ, Martin JL, Schlesinger LS. Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun. 2004;72:2564–2573. doi: 10.1128/IAI.72.5.2564-2573.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandler MJ, Zeiss CR, Leach CL, Hatoum NS, Levitz D, Garvin PJ, Patterson R. Levels and specificity of antibody in bronchoalveolar lavage (BAL) and serum in an animal model of trimellitic anhydride-induced lung injury. J Allergy Clin Immunol. 1987;80:223–229. doi: 10.1016/0091-6749(87)90133-3. doi:0091-6749(87)90133-3 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Shigemura M, Nasuhara Y, Konno S, Hattori T, Shimizu C, Matsuno K, Nishimura M. Levels of transferrin in bronchoalveolar lavage fluid in sarcoidosis. Lung. 2010;188:151–157. doi: 10.1007/s00408-009-9218-7. [DOI] [PubMed] [Google Scholar]
- 44.Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, Conway BA, Greenberg EP, Valore EV, Welsh MJ, Ganz T, Tack BF, McCray PB., Jr Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci U S A. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bicer E, Forbes B, Somers G, Blomberg A, Behndig A, Mudway I. Characterizing the composition of human respiratory tract lining fluids in health and disease. Am J Respir Crit Care Med. 2012:A4661. [Google Scholar]
- 46.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 47.Madebo T, Lindtjorn B, Aukrust P, Berge RK. Circulating antioxidants and lipid peroxidation products in untreated tuberculosis patients in Ethiopia. Am J Clin Nutr. 2003;78:117–122. doi: 10.1093/ajcn/78.1.117. [DOI] [PubMed] [Google Scholar]
- 48.Reddy YN, Murthy SV, Krishra DR, Prabhakar MC. Role of free radicals and antioxidants in Tuberculosis patients. Indian J Tuberc. 2003;51:213–218. [Google Scholar]
- 49.van Golde LM. Synthesis of surfactant lipids in the adult lung. Annu Rev Physiol. 1985;47:765–774. doi: 10.1146/annurev.ph.47.030185.004001. [DOI] [PubMed] [Google Scholar]
- 50.Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta. 1998;1408:90–108. doi: 10.1016/s0925-4439(98)00061-1. S0925-4439(98)00061-1 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 52.Reid KB, Clark H, Palaniyar N. Surfactant and lung inflammation. Thorax. 2005;60:620–622. doi: 10.1136/thx.2004.036699. 60/8/620 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu SH, Possmayer F. Effect of pulmonary surfactant protein A and neutral lipid on accretion and organization of dipalmitoylphosphatidylcholine in surface films. J Lipid Res. 1996;37:1278–1288. [PubMed] [Google Scholar]
- 54.Abate W, Alghaithy AA, Parton J, Jones KP, Jackson SK. Surfactant lipids regulate LPS-induced interleukin-8 production in A549 lung epithelial cells by inhibiting translocation of TLR4 into lipid raft domains. J Lipid Res. 2010;51:334–344. doi: 10.1194/jlr.M000513. jlr.M000513 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazachkov MY, Muhlebach MS, Livasy CA, Noah TL. Lipid-laden macrophage index and inflammation in bronchoalveolar lavage fluids in children. Eur Respir J. 2001;18:790–795. doi: 10.1183/09031936.01.00047301. [DOI] [PubMed] [Google Scholar]
- 56.Gibeon D, Zhu J, Sogbesan A, Banya W, Rossios C, Saito J, Rocha JP, Hull JH, Menzies-Gow AN, Bhavsar PK, Chung KF. Lipid-laden bronchoalveolar macrophages in asthma and chronic cough. Respir Med. 2014;108:71–77. doi: 10.1016/j.rmed.2013.10.005. S0954-6111(13)00415-0 [pii]; [DOI] [PubMed] [Google Scholar]
- 57.Dragomir A, Ciontu M, Martius M, Munteanu I, Stoica R, Ulmeanu R, Serbescu A, Mihaltan F. Superinfection with Mycobacterium tubercuosis in a patient with pulmonary alveolar proteinosis. Maedica J Clin Med. 2008;3:59–63. [Google Scholar]
- 58.Dodd CE, Pyle CJ, Glowinski R, Rajaram MV, Schlesinger LS. CD36-mediated uptake of surfactant lipids by human macrophages promotes intracellular growth of Mycobacterium tuberculosis. J Immunol. 2016;197:4727–4735. doi: 10.4049/jimmunol.1600856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jonsson S, Musher DM, Goree A, Lawrence EC. Human alveolar lining material and antibacterial defenses. Am Rev Respir Dis. 1986;133:136–140. doi: 10.1164/arrd.1986.133.1.136. [DOI] [PubMed] [Google Scholar]
- 60.Lohmann-Matthes ML, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J. 1994;7:1678–1689. [PubMed] [Google Scholar]
- 61.Hussell T, Bell TJ. Alveolar macrophages: Plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. nri3600 [pii]; [DOI] [PubMed] [Google Scholar]
- 62.Fels A, Cohn ZA. The alveolar macrophage. J Appl Physiol. 1986;60:353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- 63.Day J, Friedman A, Schlesinger LS. Modeling the immune rheostat of macrophages in the lung in response to infection. Proc Natl Acad Sci U S A. 2009;106:11246–11251. doi: 10.1073/pnas.0904846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gleeson LE, Sheedy FJ, Palsson-McDermott EM, Triglia D, O’Leary SM, O’Sullivan MP, O’Neill LA, Keane J. Cutting Edge: Mycobacterium tuberculosis induces aerobic glycolysis in human alveolar macrophages that is required for control of intracellular bacillary replication. J Immunol. 2016;196:2444–2449. doi: 10.4049/jimmunol.1501612. jimmunol.1501612 [pii]; [DOI] [PubMed] [Google Scholar]
- 65.Scordo JM, Knoell DL, Torrelles JB. Alveolar epithelial cells in Mycobacterium tuberculosis infection: Active players or innocent bystanders? J Innate Immun. 2016;8:3–14. doi: 10.1159/000439275. 000439275 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDonough KA, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–4811. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dobos KM, Spotts EA, Quinn FD, King CH. Necrosis of lung epithelial cells during infection with Mycobacterium tuberculosis is preceded by cell permeation. Infect Immun. 2000;68:6300–6310. doi: 10.1128/iai.68.11.6300-6310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: Implications for understanding lung disease in humans. J Immunol. 2014;192:2821–2829. doi: 10.4049/jimmunol.1302269. jimmunol.1302269 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guilliams M, De KI, Henri S, Post S, Vanhoutte L, De PS, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. jem.20131199 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: Friend or foe? Trends Immunol. 2012;33:14–25. doi: 10.1016/j.it.2011.10.003. S1471-4906(11)00182-7 [pii]; [DOI] [PubMed] [Google Scholar]
- 71.Brown AE, Holzer TJ, Andersen BR. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987;156:985–989. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- 72.Jones GS, Amirault HJ, Andersen BR. Killing of Mycobacterium tuberculosis by neutrophils: A nonoxidative process. J Infect Dis. 1990;162:700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- 73.Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA, Fountain JJ, Martino C, Pearl JE, Tighe M, Lin YY, Slight S, Kolls JK, Reinhart TA, Randall TD, Cooper AM. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol. 2011;187:5402–5407. doi: 10.4049/jimmunol.1101377. jimmunol.1101377 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orme IM, Basaraba RJ. The formation of the granuloma in tuberculosis infection. Semin Immunol. 2014;26:601–609. doi: 10.1016/j.smim.2014.09.009. S1044-5323(14)00091-8 [pii]; [DOI] [PubMed] [Google Scholar]
- 75.Lin PL, Maiello P, Gideon HP, Coleman MT, Cadena AM, Rodgers MA, Gregg R, O’Malley M, Tomko J, Fillmore D, Frye LJ, Rutledge T, DiFazio RM, Janssen C, Klein E, Andersen PL, Fortune SM, Flynn JL. PET CT identifies reactivation risk in Cynomolgus macaques with latent M. tuberculosis. PLoS Pathog. 2016;12:e1005739. doi: 10.1371/journal.ppat.1005739;PPATHOGENS-D-16-00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sasindran J, Torrelles JB. Mycobacterium tuberculosis infection and inflammation: What is beneficial for the host and for the bacterium? Front Microbiol. 2011;2:1–16. doi: 10.3389/fmicb.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guirado E, Schlesinger LS. Modeling the Mycobacterium tuberculosis Granuloma - the Critical Battlefield in Host Immunity and Disease. Front Immunol. 2013;4:98. doi: 10.3389/fimmu.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ehlers S, Schaible UE. The granuloma in tuberculosis: Dynamics of a host-pathogen collusion. Front Immunol. 2012;3:411. doi: 10.3389/fimmu.2012.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marakalala MJ, Raju RM, Sharma K, Zhang YJ, Eugenin EA, Prideaux B, Daudelin IB, Chen PY, Booty MG, Kim JH, Eum SY, Via LE, Behar SM, Barry CE, III, Mann M, Dartois V, Rubin EJ. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat Med. 2016;22:531–538. doi: 10.1038/nm.4073. nm.4073 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guirado E, Mbawuike U, Keiser TL, Arcos J, Azad AK, Wang SH, Schlesinger LS. Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model. MBio. 2015;6:e02537-14. doi: 10.1128/mBio.02537-14. mBio.02537-14 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kramnik I, Demant P, Bloom BB. Susceptibility to tuberculosis as a complex genetic trait: Analysis using recombinant congenic strains of mice. Novartis Found Symp. 1998;217:120–131. doi: 10.1002/0470846526.ch9. [DOI] [PubMed] [Google Scholar]
- 82.Heitmann L, Abad DM, Schreiber T, Erdmann H, Behrends J, McKenzie AN, Brombacher F, Ehlers S, Holscher C. The IL-13/IL-4Ralpha axis is involved in tuberculosis-associated pathology. J Pathol. 2014;234:338–350. doi: 10.1002/path.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cyktor JC, Carruthers B, Kominsky RA, Beamer GL, Stromberg P, Turner J. IL-10 inhibits mature fibrotic granuloma formation during Mycobacterium tuberculosis infection. J Immunol. 2013;190:2778–2790. doi: 10.4049/jimmunol.1202722. jimmunol.1202722 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR, Kramnik I. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434:767–772. doi: 10.1038/nature03419. nature03419 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams A, Orme IM. Animal models of tuberculosis: An overview. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.TBTB2-0004-2015. [DOI] [PubMed] [Google Scholar]
- 86.Flynn JL, Gideon HP, Mattila JT, Lin PL. Immunology studies in non-human primate models of tuberculosis. Immunol Rev. 2015;264:60–73. doi: 10.1111/imr.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. doi: 10.1038/nri3259. nri3259 [pii]; [DOI] [PubMed] [Google Scholar]
- 88.Perdomo C, Zedler U, Kuhl AA, Lozza L, Saikali P, Sander LE, Vogelzang A, Kaufmann SH, Kupz A. Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. MBio. 2016;7 doi: 10.1128/mBio.01686-16. mBio.01686-16 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Linderman JJ, Kirschner DE. In silico models of M. tuberculosis infection provide a route to new therapies. Drug Discov Today Dis Models. 2015;15:37–41. doi: 10.1016/j.ddmod.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doryab A, Amoabediny G, Salehi-Najafabadi A. Advances in pulmonary therapy and drug development: Lung tissue engineering to lung-on-a-chip. Biotechnol Adv. 2016;34:588–596. doi: 10.1016/j.biotechadv.2016.02.006. S0734-9750(16)30013-1 [pii]; [DOI] [PubMed] [Google Scholar]
- 91.Jambo KC, French N, Zijlstra E, Gordon SB. AIDS patients have increased surfactant protein D but normal mannose binding lectin levels in lung fluid. Respir Res. 2007;8:42. doi: 10.1186/1465-9921-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jambo KC, Banda DH, Kankwatira AM, Sukumar N, Allain TJ, Heyderman RS, Russell DG, Mwandumba HC. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol. 2014;7:1116–1126. doi: 10.1038/mi.2013.127. mi2013127 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diedrich CR, O’Hern J, Wilkinson RJ. HIV-1 and the Mycobacterium tuberculosis granuloma: A systematic review and meta-analysis. Tuberculosis (Edinb) 2016;98:62–76. doi: 10.1016/j.tube.2016.02.010. S1472-9792(15)30263-8 [pii]; [DOI] [PubMed] [Google Scholar]
- 94.Workineh M, Mathewos B, Tekeste Z. Effect of helminths on immunity, clinical response and vaccination agaisnt tuberculosis: A review. Ad J Biol Sci Res. 2013;1:13–21. [Google Scholar]
- 95.O’Connor G, Gleeson LE, Fagan-Murphy A, Cryan SA, O’Sullivan MP, Keane J. Sharpening nature’s tools for efficient tuberculosis control: A review of the potential role and development of host-directed therapies and strategies for targeted respiratory delivery. Adv Drug Deliv Rev. 2016;102:33–54. doi: 10.1016/j.addr.2016.04.024. S0169-409X(16)30134-X [pii]; [DOI] [PubMed] [Google Scholar]
- 96.Crotty Alexander LE, Shin S, Hwang JH. Inflammatory diseases of the lung induced by conventional cigarette smoke: A review. Chest. 2015;148:1307–1322. doi: 10.1378/chest.15-0409. S0012-3692(15)50243-2 [pii]; [DOI] [PubMed] [Google Scholar]
- 97.Khalilullah SA, Harapan H, Hasan NA, Winardi W, Ichsan I, Mulyadi M. Host genome polymorphisms and tuberculosis infection: What we have to say? Egypt J Chest Dis Tuberc. 2014;63:173–185. doi: 10.1016/j.ejcdt.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun. 2012;80:3343–3359. doi: 10.1128/IAI.00443-12. IAI.00443-12 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martiniano SL, Nick JA, Daley CL. Nontuberculous mycobacterial infections in cystic fibrosis. Clin Chest Med. 2016;37:83–96. doi: 10.1016/j.ccm.2015.11.001. S0272-5231(15)00140-9 [pii]; [DOI] [PubMed] [Google Scholar]
- 100.Poolman EM, Galvani AP. Evaluating candidate agents of selective pressure for cystic fibrosis. J R Soc Interface. 2007;4:91–98. doi: 10.1098/rsif.2006.0154. YG8973T965872665 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]