Abstract
Many infants with complex congenital heart disease (CHD) do not develop the skills to feed orally and are discharged home on gastrostomy tube or nasogastric feeds. We aimed to identify risk factors for failure to achieve full oral feeding and evaluate the efficacy of oral motor intervention for increasing the rate of discharge on full oral feeds by performing a prospective study in the neonatal and cardiac intensive care units of a tertiary children’s hospital. 23 neonates born at ≥ 37 weeks gestation and diagnosed with single-ventricle physiology requiring a surgical shunt were prospectively enrolled and received oral motor intervention therapy. 40 historical controls were identified. Mean length of stay was 53.7 days for the control group and 40.9 days for the study group (p=0.668). 13/23 patients who received oral motor intervention therapy (56.5%) and 18/40 (45.0%) controls were on full oral feeds at discharge, a difference of 11.5% (95% CI −13.9% to 37.0%, p=0.378). Diagnosis of hypoplastic left heart syndrome, longer intubation and duration of withholding enteral feeds, and presence of gastroesophageal reflux disease were predictors of poor oral feeding on univariate analysis. Although we did not detect a statistically significant impact of oral motor intervention, we found clinically meaningful differences in hospital length of stay and feeding tube requirement. Further research should be undertaken to evaluate methods for improving oral feeding in these at-risk infants.
Keywords: oral motor intervention, oral feeding, gastrostomy tube feeding, nasogastric tube feeding, complex congenital heart disease
Purpose
Congenital heart disease (CHD) is the most common birth defect with a birth prevalence between 4 and 50 cases per 1000 live births in USA. The birth prevalence of severe heart disease which includes cyanotic lesions is 1.5 per 1000 live births (Van der Bom et al., 2011). Significant growth failure is well-recognized in infants undergoing congenital heart defect surgery due to multiple factors including increased metabolic demand, delayed enteral feeding secondary to prostaglandin infusion, risk of mesenteric hypoperfusion, and prolonged intubation (Golbus, Wojcik, Charpie, & Hirsch, 2011). While feeding algorithms have reduced the number of days to reach full enteral feeds (Braudis et al., 2009; Slicker et al., 2013), patients with cyanotic heart disease often continue to need nasogastric or gastrostomy tube feeds at discharge due to poor oral feeding skills (Jadcherla, Vijayapal, & Leuthner, 2009; Natarajan, Reddy Anne, & Aggarwal, 2010). Various studies have found that the proportion of neonates with CHD requiring tube feeding at discharge ranges from 29% to 45% (Einarson & Arthur, 2003; Kogon et al., 2007). The number of days to reach full oral feeds contributes significantly to prolonging ICU length of stay (Wheeler, Dent, Manning, & Nelson, 2008), and tube feeding is associated with less weight gain than exclusive oral feeding (Lambert et al., 2014; Medoff-Cooper et al., 2011). Gastrostomy tube placement bears anesthetic and infectious risks, and feeding by gastrostomy tube is associated with higher mortality prior to second stage cardiac surgery (Hebson et al., 2012) as well as neurodevelopmental delay at 6 and 12 months of age (Medoff-Cooper et al., 2015). Furthermore, it has been reported that parents have a feeling of inadequacy when their child is tube fed, and performing nasogastric or gastrostomy feeds can be a practical challenge (Craig, 2013; Rollins, 2006). Thus, there are several reasons to minimize the need for tube feeding.
The etiology of poor oral feeding in patients with cyanotic heart disease is unclear. Several previous studies have noted that patients with diagnosis of hypoplastic left heart syndrome (Davis et al., 2008; Golbus et al., 2011; Kelleher, Laussen, Teixeira-Pinto, & Duggan, 2006) and postoperative vocal cord paralysis (Jadcherla et al., 2009; Pham, Connelly, Wei, Sykes, & O’Brien, 2014) are more likely to require nasogastric or gastrostomy feeds at discharge. One study of 465 infants with single ventricle heart defects found that longer periods of intubation are associated with tube feeding requirement at discharge (Hill et al., 2014), though no other studies have been done to corroborate this finding. We sought to identify key variables that may predict which of these infants are at greater risk of requiring tube feeding at discharge, as recognition of risk factors for poor oral feeding may allow for early intervention in those infants who are most likely to have difficulty. Since oral feeding abilities in preterm infants have been enhanced by the application of oral motor intervention (Bingham, Ashikaga, & Abbasi, 2010; Fucile, Gisel, & Lau, 2005), we employed a similar intervention in infants born at gestational age 37 0/7 weeks and beyond who underwent complex cardiac surgery. We hypothesized that this novel approach would help improve their oral feeding skills and thereby reduce the need for nasogastric or gastrostomy feedings at discharge.
Design and Methods
Study Population
The oral motor intervention was administered to a prospective cohort of consecutive patients ≥ 37 weeks gestational age with a diagnosis of hypoplastic left heart syndrome, transposition of great arteries, or any lesion which may require a systemic-pulmonary shunt placement in the neonatal period. Bedside laryngoscopy was offered to the patients in the study group as a screening tool to detect vocal cord palsy postoperatively. Patients were recruited to the study on admission to the neonatal intensive care unit prior to surgery at a single tertiary care children’s hospital for preoperative care during the study period (September 2012 to May 2015). Patients with significant central nervous system disease either clinically or by imaging (e.g. Grade III–IV intraventricular hemorrhage), orofacial anomalies interfering with feeding, and known chromosomal disorder were excluded. Infants over a month of age at the time of study entry were also excluded. The data from this group were then compared with a similar cohort of patients admitted to our neonatal intensive care unit or cardiothoracic intensive care unit from January 2010 to May 2012 who did not receive oral motor intervention therapy. Patients admitted after January 2010 were selected because a feeding algorithm (Braudis et al., 2009) was fully implemented in our cardiac and neonatal intensive care units by this time, leading to uniformity in practice. Prospective data collection for the study group was approved by a full UCLA Institutional Review Board in September 2012. Written informed consent was obtained from the parents for the administration of oral motor intervention and for postoperative bedside laryngoscopy before entry into the study. Parents were given the option to decline laryngoscopy while still participating in the study. Waiver of consent was obtained for retrospective chart review for the control group.
Intervention
Infants in the study group received oral motor intervention exercises based on the Beckman method (see Supplementary material), which has been shown to accelerate the transition to full oral feedings in preterm infants (Beckman, 2016; Fucile, Gisel & Lau, 2002; Younesian, Yadegari, & Soleimani, 2015). The exercises are simple, brief, and do not require the use of a device for oral stimulation. The intervention was initiated preoperatively in all patients in the study group by our occupational therapists after obtaining consent. All bedside neonatal and cardiac intensive care unit nurses received instruction on administration of the oral motor intervention exercises from an occupational therapist. Parents were also taught how to administer these exercises by an occupational therapist if interested. Occupational therapists, nurses, and parents were instructed to perform oral motor intervention 4 times per day with each session lasting 15 minutes. A laminated chart with pictures and description of exercises was placed at the bedside to serve as an aide memoire for administration of the intervention, and occupational therapists also continued to meet with nurses and parents to reinforce the protocol.
The oral motor intervention program consisted of a series of exercises stimulating gums, cheeks and lips by stretching and stroking (Coker-Bolt, Jarrard, Woodard, & Merrill, 2013; Fucile et al., 2005). Intubated patients received a modified set of oral intervention exercises. In the intervention group, the exercises were started as soon as they were enrolled in the study and continued unless the patient was deemed unstable by the primary medical team. Oral motor intervention was withheld for the first 24 hours after surgery as well as for any decompensation defined as start of a pressor or 15% increase from baseline dose, 15% decrease in pulse oximetry oxygen saturations, muscle relaxation, while receiving extracorporeal membrane oxygenation, or when the infant was deemed unstable by the primary team. Oral motor intervention was resumed once the patient was stabilized. Oral motor intervention was discontinued once the infant was taking all feeds by mouth for 24 hours.
Bedside laryngoscopy was performed prior to initiation of oral feeds by either the attending or trained resident head and neck surgeon using a flexible laryngoscope. This was done in extubated infants with consent for laryngoscopy at the time of study enrollment. The procedure was brief and did not require additional sedation.
Data collection
Data gathered for both study and control groups included date of birth, date of admission, diagnosis (including whether the diagnosis was made antenatally or postnatally), gestational age, birth weight, length and occipitofrontal circumference. Preoperative data including whether the patient was fed or intubated was collected. Feeding data collected included days to full oral feeds and presence of conditions affecting feeding such as gastroesophageal reflux disease and vocal cord palsy. Post-surgical complications including chylothorax, diaphragmatic palsy, and second surgery for any reason were noted. Method and volume of feeds and age at discharge were collected.
Statistical Methods
Patient demographics were compared between the historical controls and the oral motor intervention group using t-tests for continuous variables and chi-square or Fisher’s Exact test for categorical variables (as appropriate). Postoperative measures were compared between groups using t-tests or Wilcoxon tests. To assess which pre-operative factors were associated with feeding status discharge (full oral feeds vs. not), univariate and multivariate logistic regression models were constructed. The terms of univariate models included diagnosis status, intubation time, days with oral feeds withheld, and presence of gastroesophageal reflux disease. Multivariate models were constructed with the same outcome and each of these variables and treatment + the treatment by pre-operative factor interaction. Statistical analyses were performed in SPSS V22 (IBM Corp., Armonk, NY) and p-values <0.05 were considered statistically significant.
Results
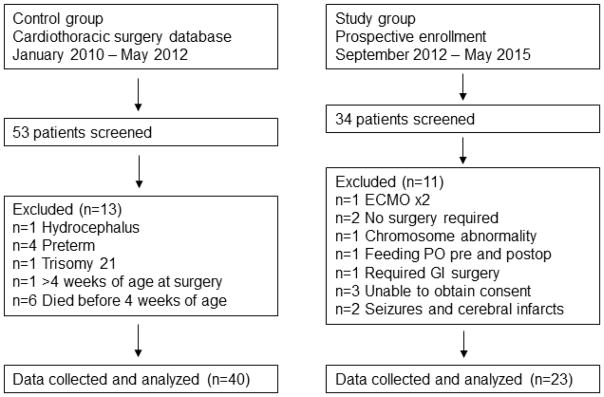
Thirty-four neonates with hypoplastic left heart syndrome, transposition of the great arteries, or other shunt-dependent cardiac lesion were screened for the intervention. Twenty-three patients met entry criteria and were enrolled into the intervention group. Forty infants met criteria for inclusion in the control group (Figure 1). As shown in Table 1, there were no significant differences between the two groups for gestational age, birth weight, length, and occipitofrontal circumference. The two groups were not significantly different in the timing of diagnosis, type of cardiac defect, mean age at surgery, or type of surgery performed.
Figure 1.
Identification of patients.
Number of patients screened, reasons for exclusions, and final number of included patients.
Table 1.
Demographicsa
| Characteristics | Intervention (n=23) | Control (n=40) | p-value |
|---|---|---|---|
|
| |||
| Gestational age (mean, weeks) | 39.1 (1.3) | 39.0 (0.8) | 0.626 |
|
| |||
| Birth weight (mean, kg) | 3.3 (0.5) | 3.2 (0.5) | 0.532 |
|
| |||
| Birth length (mean, cm) | 50.0 (2.5) | 49.7 (2.5) | 0.645 |
|
| |||
| OFC (mean, cm) | 33.7 (1.2) | 33.8 (1.8) | 0.694 |
|
| |||
| Diagnosis | 0.397 | ||
| HLHS | 5 (22%)b | 15 (38%)b | |
| TGA | 10 (44%)b | 3 (8%)b | |
| Tetralogy of Fallot requiring shunt | 1 (4%)b | 16 (40%)b | |
| Other | 7 (30%)b | 6 (15%)b | |
|
| |||
| Antenatal diagnosis | 15 (65%)b | 20 (50%)b | 0.242 |
|
| |||
| Age at surgery (mean, days) | 7.0 (3.2) | 6.7 (4.3) | 0.767 |
|
| |||
| Surgery | 0.441 | ||
| Norwood and Sano | 2 (9%)b | 8 (20%)b | |
| Blalock-Taussig shunt ± Norwood | 14 (61%)b | 17 (43%)b | |
| Arterial switch operation | 6 (26%)b | 14 (35%)b | |
| Other | 1 (4%)b | 1 (3%)b | |
Unless otherwise denoted, mean (SD) is reported.
n (%) is reported.
OFC=occipitofrontal circumference; HLHS=hypoplastic left heart syndrome; TGA=transposition of the great arteries
In the intervention group, 8 out of 23 patients had parental participation in performing oral motor intervention. No adverse effects related to oral motor intervention were reported, including unplanned extubation, gagging, or vomiting.
Postoperatively, there was no statistically significant difference in the length of intubation, duration of withholding enteral feeds, and days to full enteral or oral feeds (Table 2). In the control group, 18 out of 40 patients (45%) were discharged from the hospital on full oral feeds, and 13 out of 23 patients (57%) in the intervention group were discharged on full oral feeds, an 11.5% difference (95% CI −13.9% to 37.0%). This difference was not statistically significant (p=0.378). Mean length of stay was reduced in the study group (40.9 vs 53.7 days), though this also was not a significant difference (p=0.668).
Table 2.
Postoperative results
| Characteristics | Intervention (n=23) | Control (n=40) | p-value |
|---|---|---|---|
| Number of patients NPO pre-op (n, %) | 16 (70%) | 28 (70%) | 0.971 |
| Mean days intubated pre-op (range, SD) | 2.3 (0–7, 2.29) | 3.53 (0–13, 3.45) | 0.275 |
| Mean days intubated post-op (range, SD) | 7.4 (2–43, 8.8) | 12.4 (1–98, 16.5) | 0.060 |
| Mean days NPO post-op (range, SD) | 8.7 (2–45, 9.7) | 11.6 (1–37, 9.5) | 0.090 |
| Number of patients taking full oral feeding at discharge (n, %) | 13 (57%) | 18 (45%) | 0.378 |
| Mean days to full enteral feeds (range, SD) | 4.7 (1–21, 6.5) | 7.1 (1–60, 11.0) | 0.139 |
| Mean days to full oral feeds (range, SD)a | 3.9 (1–14, 3.7) | 5.2 (1–15, 4.9) | 0.840 |
| GT placement and/or Nissen fundoplication performed (n, %) | 8 (35%) | 12 (30%) | 0.695 |
| Number of patients with GERD (n, %) | 6 (26%) | 10 (25%) | 0.924 |
| Mean length of hospital stay in days (range, SD) | 40.9 (14–117, 28.9) | 53.7 (11–450, 71.1) | 0.668 |
| Number of patients transferred out before full oral feeds were achieved (n, %) | 2 (9%) | 9 (23%) | 0.193 |
NPO=nil per os, indicating that patients were not taking any feeds by mouth; SD=standard deviation; GT=gastrostomy tube; GERD=gastroesophageal reflux disease
Denotes the number of days following achievement of full enteral feeds until achievement of full oral feeds.
Longer total duration of intubation and number of days withholding enteral feeds postoperatively were both associated with failure to attain full oral feeds by discharge (p=0.004 and p=0.001, respectively). On multivariate analysis, neither the treatment group, nor the interaction effect of total duration of intubation and number of days without enteral feeds with treatment group were significant (p=0.464 and p=0.797, respectively). This means that although longer total duration of intubation and number of days withholding enteral feeds postoperatively were associated with failure to attain full oral feeds, we did not find evidence that this effect was mitigated in the treatment group.
This study identified that 25% of the control group and 26% of the study group had gastroesophageal reflux disease diagnosed either clinically or by routine imaging prior to placement of a surgical gastrostomy tube (p=0.924). On univariate analysis, presence of gastroesophageal reflux disease was associated with higher likelihood of requiring tube feeding at discharge (p=0.003). Oral motor intervention did not have a differential effect on discharge feeding route for patients with or without gastroesophageal reflux disease (p=0.992).
Examination of baseline characteristics demonstrated that diagnosis of hypoplastic left heart syndrome was associated with higher rates of discharge with tube feedings when compared to patients with other cardiac diagnoses (p=0.012). No interaction effect was found between diagnosis (hypoplastic left heart syndrome versus other diagnosis) and effect of oral motor intervention on rates of full oral feeding at discharge (p=0.479).
Four out of 40 patients in the control group (10%) were found to have vocal cord palsy; a total of 5 patients were evaluated by flexible laryngoscopy, and this was done only when they developed signs and symptoms concerning for vocal cord palsy. In the study group, 6 out of 23 patients (26%) were diagnosed with vocal cord palsy. Three of these 6 patients were screened after extubation but prior to the development of symptoms.
Conclusions
This study identified diagnosis of hypoplastic left heart syndrome, duration of intubation and withholding of oral feeds, and presence of gastroesophageal reflux disease as predictors of failure to achieve full feeding at discharge for infants who underwent surgery for complex CHD. The mechanisms of these associations are not well understood. In the case of hypoplastic left heart syndrome, there may be occult neurodevelopmental differences related to the physiology of the specific cardiac defect, or these infants may be more clinically unstable and unable to feed orally as early and as often. Likewise, withholding oral feeds may directly prevent appropriate development of oral feeding ability, or there may be an underlying pathology that both predisposes infants to clinical instability and impairs oromotor skill development. Gastroesophageal reflux disease has been associated with feeding refusal and need for nasogastric or gastrostomy feedings in infants without CHD (Mathisen, Worrall, Masel, Wall, & Shepherd, 1999; Rudolph & Link, 2002), though it is not directly tied to abnormal development of oromotor skills. Our study did not ascertain whether the link between gastroesophageal reflux disease and tube feeding requirement was related to feeding refusal behavior, oromotor dysfunction, or both; this may be the subject of future investigation. Nevertheless, gastroesophageal reflux disease has been observed at greater frequency in infants with CHD than the general population (Steltzer, Rudd, & Pick, 2015; Weesner & Rosenthal, 1983), so identification and treatment of gastroesophageal reflux disease may be beneficial for promoting oral feeding in this subpopulation.
The dysphagia seen in infants with CHD is similar to that of premature infants, particularly lack of suck-swallow-breathe coordination (Pereira Kda et al., 2015). Though there is little data available on the use of oral motor intervention in infants born at or later than 37 weeks gestation, efficacy of oral motor intervention has been demonstrated in preterm infants. For example, in preterm infants receiving gavage feeds, oral motor intervention has been shown to increase non-nutritive sucking pressure and activity (Barlow, Finan, Lee, & Chu, 2008; Poore & Barlow, 2009). This resulted in decreased time to achieve full oral feeds (Lessen, 2011), higher milk transfer rates, and greater volume taken during oral feeds (Zhang et al., 2014). Data for reduction in length of stay has been mixed (Bache, Pizon, Jacobs, Vaillant, & Lecomte, 2014; Zhang et al., 2014), though a recent metaanalysis including these studies concluded that length of stay was reduced by a mean of 3.64 days (95% CI, −5.57 to −1.71) (Tian et al., 2015). Our study was one of the first to employ oral motor intervention exercises in infants with complex CHD born at or beyond 37 weeks gestation. Our results showed that the group that received oral motor intervention had a mean reduction in length of stay of 12.8 days which was clinically meaningful, though not statistically significant. Coker-Bolt and colleagues recently employed an oral intervention protocol similar to that used in our study in infants with univentricular physiology and demonstrated a significant decrease in length of stay in their intervention group by 6.7 days (p=0.04) (Coker-Bolt et al., 2013).
In our study, bedside nurses and parents in addition to occupational therapists were able to administer the intervention. Although occupational therapists provided verbal and written instructions for the exercises, we did not assess fidelity testing, and this limitation may be addressed in future studies. Few parents chose to perform the exercises; our study did not survey parents regarding the reason for this, but we speculate that they may have been intimidated by the detailed exercises and could have benefitted from additional encouragement from nurses and occupational therapists. No adverse events, such as unintended extubation or gagging, were reported from the study group. Although the study group did have a higher percentage of patients discharged on full oral feedings, this difference (11.5%) was not statistically significant. However, given our observed sample sizes, the minimally detectable effect size was approximately 30%, so this study may be underpowered to detect a true difference. The use of historical controls is also a limitation, as management strategies may have differed in parameters that were not measured. Additionally, some patients in the control group may have also received feeding therapy from an occupational therapist when clinically appropriate; though typical occupational therapy treatment regimens do not include the rigorous Beckman protocol that was used in the oral motor intervention group, this could potentially have decreased the magnitude of the difference between the groups. Two patients in the study group and 9 patients in the control group were also transferred to another hospital prior to discharge, so it is unknown whether they were able to take full oral feeds by discharge. We hope that further studies will be conducted to assess the efficacy of oral motor intervention in this population.
We also evaluated for the presence of vocal cord palsy postoperatively, as this is a known complication of cardiac surgery due to mechanisms including injury to the recurrent laryngeal nerve and trauma secondary to intubation, and this in turn is associated with feeding difficulties, poor growth, and need for GT placement (Einarson & Arthur, 2003; Hill et al., 2014; Pham et al., 2014; Sachdeva et al., 2007). The reported incidence of vocal cord palsy in children undergoing cardiac surgery varies from 1.7% to 8% and is associated with lower gestational age, lower birth weight, and lower surgical weight (Carpes et al., 2011; Dewan, Cephus, Owczarzak, & Ocampo, 2012; Strychowsky, Rukholm, Gupta, & Reid, 2014). In our study, bedside flexible laryngoscopy was performed safely with no additional sedation required, and there were no adverse effects from the procedure. Although our study was underpowered to detect effects of vocal cord palsy on route of feeding at discharge, we did note that 3 patients without symptoms of vocal cord palsy in the study group who underwent screening flexible laryngoscopy were found to have vocal cord palsy. This suggests that screening is likely to show a higher incidence of vocal cord palsy. Given that bedside flexible laryngoscopy is safe and can be performed by otolaryngologists as well as neonatologists (Kohelet, Arbel, & Shinwell, 2011), our results are consistent with a recent meta-analysis that recommends the routine use of postoperative laryngoscopy for vocal cord assessment in children undergoing cardiac surgery (Strychowsky et al., 2014).
Practice Implications
We advocate for identifying infants with complex CHD at greater risk for poor oral feeding such as those with vocal cord palsy and gastroesophageal reflux disease so that intervention can be timely, and routine screening for these factors may be warranted. To aid these at-risk infants, we demonstrated in this study that oral motor intervention can be safely performed in a hospital setting and may potentially decrease length of stay and improve rates of full oral feeds, though additional data is required to confirm our results. Although our data did not show that oral motor intervention was more beneficial for such high-risk infants than those without these risk factors, further exploration into such therapies should continue. The optimal frequency and duration of oral motor intervention should also be evaluated. In this study, parents were also able to participate in performing oral motor intervention, and future studies may evaluate potential long-term value of ongoing oral motor intervention at home following hospital discharge. Our study adds to a growing body of literature suggesting that oral motor intervention may have a positive effect on hospital length of stay and rates of oral feeding, and it remains to be seen whether this will translate into financial, developmental, and quality-of-life benefits.
Supplementary Material
Highlights.
Infants with complex congenital heart disease are at risk for poor oral feeding.
Specific factors associated with poor oral feeding are identified.
Oral motor intervention may decrease hospital length of stay.
Oral motor intervention may decrease feeding tube requirements.
Screening bedside laryngoscopy may identify vocal cord dysfunction.
Acknowledgments
We would like to thank Debra Beckman for allowing us to include a description of her exercises as well as Courtney Jarrard and Sandra Fucile for providing assistance with developing the instructional materials for the oral stimulation exercises.
Financial Support
Statistical analyses for this research were supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124.
Footnotes
Conflicts of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bache M, Pizon E, Jacobs J, Vaillant M, Lecomte A. Effects of pre-feeding oral stimulation on oral feeding in preterm infants: A randomized clinical trial. Early Human Development. 2014;90(3):125–129. doi: 10.1016/j.earlhumdev.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Finan DS, Lee J, Chu S. Synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. Journal of Perinatology. 2009;28:541–548. doi: 10.1038/jp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman DA. Beckman oral motor assessment and intervention. Beckman & Associates Inc; 620 Wymore Rd, Suite 230, Maitland Fl 32751–4253: 1986. p. 90.p. 91.p. 95.p. 96.p. 97.p. 98.p. 102.p. 104. Rev.2016. [Google Scholar]
- Bingham PM, Ashikaga T, Abbasi S. Prospective study of non-nutritive sucking and feeding skills in premature infants. Archives of Disease in Childhood–Fetal and Neonatal Edition. 2010;95(3):F194–F200. doi: 10.1136/adc.2009.164186. [DOI] [PubMed] [Google Scholar]
- Braudis NJ, Curley MA, Beaupre K, Thomas KC, Hardiman G, Laussen P, … Thiagarajan RR. Enteral feeding algorithm for infants with hypoplastic left heart syndrome poststage I palliation. Pediatric Critical Care Medicine. 2009;10(4):460–466. doi: 10.1097/PCC.0b013e318198b167. [DOI] [PubMed] [Google Scholar]
- Carpes LF, Kozak FK, Leblanc JG, Campbell AI, Human DG, Fandino M, … Fiori H. Assessment of vocal fold mobility before and after cardiothoracic surgery in children. Archives of Otolaryngology–Head and Neck Surgery. 2011;137(6):571–575. doi: 10.1001/archoto.2011.84. [DOI] [PubMed] [Google Scholar]
- Coker-Bolt P, Jarrard C, Woodard F, Merrill P. The effects of oral motor stimulation on feeding behaviors of infants born with univentricle anatomy. Journal of Pediatric Nursing. 2013;28(1):64–71. doi: 10.1016/j.pedn.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Craig GM. Psychosocial aspects of feeding children with neurodisability. European Journal of Clinical Nutrition. 2013;67:S17–S20. doi: 10.1038/ejcn.2013.226. [DOI] [PubMed] [Google Scholar]
- Davis D, Davis S, Cotman K, Worley S, Londrico D, Kenny D, Harrison AM. Feeding difficulties and growth delay in children with hypoplastic left heart syndrome versus d-transposition of the great arteries. Pediatric Cardiology. 2008;29(2):328–33. doi: 10.1007/s00246-007-9027-9. [DOI] [PubMed] [Google Scholar]
- Dewan K, Cephus C, Owczarzak V, Ocampo E. Incidence and implication of vocal fold paresis following neonatal cardiac surgery. The Laryngoscope. 2012;122(12):2781–2785. doi: 10.1002/lary.23575. [DOI] [PubMed] [Google Scholar]
- Einarson KD, Arthur HM. Predictors of oral feeding difficulty in cardiac surgical infants. Pediatric Nursing. 2003;29(4):315–319. [PubMed] [Google Scholar]
- Fucile S, Gisel E, Lau C. Oral stimulation accelerates the transition from tube to oral feeding in preterm infants. The Journal of Pediatrics. 2002;141(2):230–236. doi: 10.1067/mpd.2002.125731. [DOI] [PubMed] [Google Scholar]
- Fucile S, Gisel EG, Lau C. Effect of an oral stimulation program on sucking skill maturation of preterm infants. Developmental Medicine and Child Neurology. 2005;47(3):158–162. doi: 10.1017/s0012162205000290. [DOI] [PubMed] [Google Scholar]
- Golbus JR, Wojcik BM, Charpie JR, Hirsch JC. Feeding complications in hypoplastic left heart syndrome after the norwood procedure: A systematic review of the literature. Pediatric Cardiology. 2011;32(4):539–552. doi: 10.1007/s00246-011-9907-x. [DOI] [PubMed] [Google Scholar]
- Hebson CL, Oster ME, Kirshbom PM, Clabby ML, Wulkan ML, Simsic JM. Association of feeding modality with interstage mortality after single-ventricle palliation. The Journal of Thoracic and Cardiovascular Surgery. 2012;144(1):173–177. doi: 10.1016/j.jtcvs.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Hill GD, Hehir DA, Bartz PJ, Rudd NA, Frommelt MA, Slicker J, … Ghanayem NS. Effect of feeding modality on interstage growth following stage I palliation: A report from the national pediatric cardiology quality improvement collaborative. The Journal of Thoracic and Cardiovascular Surgery. 2014;148(4):1534–1539. doi: 10.1016/j.jtcvs.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadcherla SR, Vijayapal AS, Leuthner S. Feeding abilities in neonates with congenital heart disease: a retrospective study. Journal of Perinatology. 2009;29(2):112–118. doi: 10.1038/jp.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006;22(3):237–244. doi: 10.1016/j.nut.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kohelet D, Arbel E, Shinwell ES. Flexible fiberoptic bronchoscopy--a bedside technique for neonatologists. The Journal of Maternal-Fetal and Neonatal Medicine. 2011;24(3):531–535. doi: 10.3109/14767058.2010.501123. [DOI] [PubMed] [Google Scholar]
- Kogon BE, Ramaswamy V, Todd K, Plattner C, Kirshbom PM, Kanter KR, Simsic J. Feeding difficulty in newborns following congenital heart surgery. Congenital Heart Disease. 2007;2(5):332–337. doi: 10.1111/j.1747-0803.2007.00121.x. [DOI] [PubMed] [Google Scholar]
- Lambert LM, Pike NA, Medoff-Cooper B, Zak V, Pemberton VL, Young-Borkowski L, … Williams RV. Variation in feeding practices following the Norwood procedure. The Journal of Pediatrics. 2014;164(2):237–242. doi: 10.1016/j.jpeds.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessen BS. Effect of the premature infant oral motor intervention on feeding progression and length of stay in preterm infants. Advances in Neonatal Care. 2011;11(2):129–139. doi: 10.1097/ANC.0b013e3182115a2a. [DOI] [PubMed] [Google Scholar]
- Mathisen B, Worrall L, Masel J, Wall C, Shepherd RW. Feeding problems in infants with gastro-oesophageal reflux disease: a controlled study. Journal of Paediatrics and Child Health. 1999;35(2):163–169. doi: 10.1046/j.1440-1754.1999.t01-1-00334.x. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B, Irving SY, Marino BS, García-España JF, Ravishankar C, Bird GL, Stallings VA. Weight change in infants with a functionally univentricular heart: From surgical intervention to hospital discharge. Cardiology in the Young. 2011;21(2):136–144. doi: 10.1017/S104795111000154X. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B, Irving SY, Hanlon AL, Golfenshtein N, Radcliffe J, Stallings VA, … Ravishankar C. The association among feeding mode, growth, and developmental outcomes in infants with complex congenital heart disease at 6 and 12 months of age. The Journal of Pediatrics. 2015;169:154–159. doi: 10.1016/j.jpeds.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan G, Reddy Anne S, Aggarwal S. Enteral feeding of neonates with congenital heart disease. Neonatology. 2010;98(4):330–336. doi: 10.1159/000285706. [DOI] [PubMed] [Google Scholar]
- da Pereira KR, Firpo C, Gasparin M, Teixeira AR, Dornelles S, Bacaltchuk T, Levy DS. Evaluation of swallowing in infants with congenital heart defect. International Archives of Otorhinolaryngology. 2015;19(1):55–60. doi: 10.1055/s-0034-1384687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham V, Connelly D, Wei JL, Sykes KJ, O’Brien J. Vocal cord paralysis and dysphagia after aortic arch reconstruction and Norwood procedure. Otolaryngology–Head and Neck Surgery. 2014;150(5):827–833. doi: 10.1177/0194599814522413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore MA, Barlow SM. Suck predicts neuromotor integrity and developmental outcomes. Perspectives on Speech Science and Orofacial Disorders. 2009;19(1):44–51. [Google Scholar]
- Rollins H. The psychosocial impact on parents of tube feeding their child. Paediatric Nursing. 2006;18(4):19–22. [PubMed] [Google Scholar]
- Rudolph CD, Link DT. Feeding disorders in infants and children. Pediatric Clinics of North America. 2002;49(1):97–112. doi: 10.1016/s0031-3955(03)00110-x. [DOI] [PubMed] [Google Scholar]
- Sachdeva R, Hussain E, Moss MM, Schmitz ML, Ray RM, Imamura M, Jaquiss RD. Vocal cord dysfunction and feeding difficulties after pediatric cardiovascular surgery. The Journal of Pediatrics. 2007;151(3):312–315. e2. doi: 10.1016/j.jpeds.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Slicker J, Hehir DA, Horsley M, Monczka J, Stern KW, Roman B, … Anderson JB. Nutrition algorithms for infants with hypoplastic left heart syndrome; birth through the first interstage period. Congenital Heart Disease. 2013;8(2):89–102. doi: 10.1111/j.1747-0803.2012.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steltzer M, Rudd N, Pick B. Nutrition care for newborns with congenital heart disease. Clinics in Perinatology. 2005;32(4):1017–1030. doi: 10.1016/j.clp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Strychowsky JE, Rukholm G, Gupta MK, Reid D. Unilateral vocal fold paralysis after congenital cardiothoracic surgery: A meta-analysis. Pediatrics. 2014;133(6):e1708–1723. doi: 10.1542/peds.2013-3939. [DOI] [PubMed] [Google Scholar]
- Tian X, Yi LJ, Zhang L, Zhou JG, Ma L, Ou YX, … Song GM. oral motor intervention improved the oral feeding in preterm infants: Evidence based on a meta-analysis with trial sequential analysis. Medicine (Baltimore) 2015;94(31):e1310. doi: 10.1097/MD.0000000000001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ. The changing epidemiology of congenital heart disease. Nature Reviews Cardiology. 2011;8(1):50–60. doi: 10.1038/nrcardio.2010.166. [DOI] [PubMed] [Google Scholar]
- Weesner KM, Rosenthal A. Gastroesophageal reflux in association with congenital heart disease. Clinical Pediatrics. 1983;22(6):424–426. doi: 10.1177/000992288302200606. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Dent CL, Manning PB, Nelson DP. Factors prolonging length of stay in the cardiac intensive care unit following the arterial switch operation. Cardiology in the Young. 2008;18(1):41–50. doi: 10.1017/S1047951107001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younesian S, Yadegari F, Soleimani F. Impact of oral sensory motor stimulation on feeding performance, length of hospital stay, and weight gain of preterm infants in NICU. Iranian Red Crescent Medical Journal. 2015;17(7):e13515. doi: 10.5812/ircmj.17(5)2015.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lyu T, Hu X, Shi P, Cao Y, Latour JM. Effect of nonnutritive sucking and oral stimulation on feeding performance in preterm infants: A randomized controlled trial. Pediatric Critical Care Medicine. 2014;15(7):608–614. doi: 10.1097/PCC.0000000000000182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.