Abstract
Day-night cycled light improves health outcomes in preterm infants, yet the best time to institute cycled light is unclear. The hypothesis of this study was that extremely preterm infants receiving early cycled light would have better health and developmental outcomes than infants receiving late cycled light. Infants born at ≤28 weeks gestation were randomly assigned to early cycled light (ECL) starting at 28 weeks postmenstrual age [PMA]) or late cycled light (LCL), starting at 36 weeks PMA. Daylight was 200–600 lux and night was 5–30 lux. Primary outcomes were weight over time and length of hospitalization. Secondary outcomes were hospital costs, sleep development, and neurodevelopment at 9, 18 and 24 months corrected age. Of 121 infants randomized, 118 were included in analysis. Weight gain in the two groups did not differ significantly but increased across time in both groups. In PMA weeks 36 to 44, the mean weight gain was 193.8 grams in the ECL group compared to 176.3 grams in the LCL group. Effect sizes for weight were Cohen d=0.26 and 0.36 for 36 and 44 weeks PMA. Infants in the ECL group went home an average of 5.5 days earlier than the LCL group, but this difference was not statistically significant. There were no group differences on neurodevelopmental outcomes. Although statistically non-significant, clinically important differences of improved weight gain and decreased hospital stay were observed with ECL. The small observed effect sizes on weight during hospitalization should be considered in future cycled light research with extremely preterm infants.
Keywords: cycled light, prematurity, extremely preterm, infant weight gain, child development, circadian rhythms, sleep
Preterm infants have poorer orientation, self-regulation, and reflexes and more excitability, hypotonia, and hypertonia at 40 weeks postmenstrual age (PMA) when compared to term infants (Pineda et al., 2013), but preterm infants’ growth and developmental outcomes vary with illness severity and degree of neurological insult (Boyle et al., 2012; Ge et al., 2013). Unexplained variations in outcomes exist even among healthy preterm infants (Schneider et al., 2014; Vohr, 2014). The variations in preterm infants’ outcomes have led to speculation that the neonatal intensive care unit (NICU) environment and light in particular may negatively affect the health and development of these infants, due to atypical sensory stimulation (Gressens, Rogido, Paindaveine, & Sola, 2002; Lickliter, 2000a) and the interruption of circadian rhythm development following preterm birth (Rivkees, 2003).
The sequential onset of fetal sensory system functioning, from somesthetic to vestibular, proprioceptive, olfactory, auditory, and visual (Gottlieb, 1971), and the relatively quiet intrauterine environment naturally limits sensory input, while the NICU environment provides atypical amounts of sensory stimulation following preterm delivery (Lickliter, 2000b). The fetus normally develops in a rich circadian environment, including maternal hormones and rest-activity cycles while remaining in near-darkness, but the NICU environment provides limited circadian cues and significant light exposure depending upon individual nursery practices. Factors with the potential to impact growth and circadian rhythm development in the NICU include light levels during the day and night (Watanabe et al., 2013), physical caregiving routines (Glotzbach, Edgar, Boeddiker, & Ariagno, 1994), and the timing and content of enteral feeding (Arslanoglu, Bertino, Nicocia, & Moro, 2012; Cubero et al., 2006; Glotzbach et al., 1994).
For extremely preterm infants (≤ 25 weeks) with prolonged intensive care stays, the unpredictable light environment of the inpatient setting has a greater potential for negative influences than the cycled light environment of the normal newborn. In a systematic review by the Cochrane Collaboration (Morag & Ohlsson, 2013), authors suggested that most preterm infant outcomes including growth, day-night activity, and length of hospital stay trended better with cycled light than either continuous near-darkness or bright light. However, few studies have been published, and all with small sample sizes (Boo, Chee, & Rohana, 2002; Brandon, Holditch-Davis, & Belyea, 2002; Guyer et al., 2012, 2015; Mirmiran, Baldwin, & Ariagno, 2003; Rivkees, Mayes, Jacobs, & Gross, 2004; Vasquez-Ruiz et al., 2014), and no clinical recommendation has been made.
The objective of this longitudinal randomized controlled trial was to evaluate infant health and developmental outcomes when provided early cycled light (ECL, beginning at 28 weeks PMA) versus late cycled light (LCL, beginning at 36 weeks PMA) in preterm infants born at ≤ 28 weeks gestation. We hypothesized that when compared to infants receiving LCL, infants receiving ECL would gain weight faster, be discharged from the hospital earlier with lower costs, and have better sleep and neurodevelopmental (mental, motor, visual acuity) outcomes. Retinopathy of prematurity (ROP) and hearing outcomes were measured to insure safety; we hypothesized there would be no differences between the ECL and LCL interventions, based on previous evidence that reduced light exposure has no effect on ROP severity (Jorge, Jorge, & El Dib, 2013) or sensory development (Brandon et al., 2002).
Methods
Design, Sample, and Setting
Infants born at ≤ 28 weeks gestation without a history of congenital anomalies associated with neurological or visual problems (e.g., Down Syndrome, congenital glaucoma) were enrolled between June 2003 and October 2006 with 2-year follow-up assessments completed by March 2009. All infants were recruited from the intensive care nursery at Duke University Hospital and received ongoing care in one of three step-down nurseries in the Duke Health System. All three step-down nurseries were staffed by the same neonatal medical provider group and the convalescent neonatal nursing care practices were standardized across all three settings. The study was approved by the Duke Medicine Institutional Review Board for Clinical Investigations. This trial is registered at clinicaltrials.gov, identifier NCT02146287.
Infants were randomly assigned to either the ECL or LCL, after obtaining informed consent from one parent. The assignment was based on stratified randomization with permuted blocks, using a computer-generated list obtained by the study statistician. The stratifying variable was gestational age, dichotomized as < 26 versus 26–28 weeks. Research staff enrolled and assigned infants to intervention groups. After hospital discharge, the infants were followed until 24 months CA in the NICU follow-up clinics.
Among the 121 infants randomized, 63 were assigned to ECL and 58 were assigned to LCL. A modified intent-to-treat approach was applied in that two ECL and one LCL infant who never received the interventions were excluded. The intent-to-treat sample, therefore, included 118 infants, with 61 in the ECL and 57 in the LCL group. A total of 83 infants contributed to outpatient data, but not all infants contributed to each outpatient outcome. See Figure 1 for a description of the participant flow.
Figure 1.
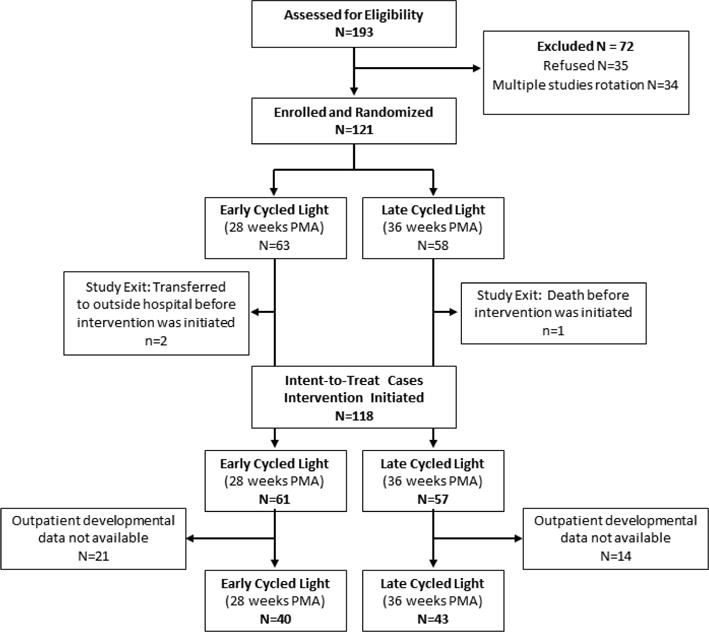
Patient Flow
Intervention and Intervention Fidelity
Before the intervention was initiated, ECL at 28 weeks and LCL at 36 weeks, infants in both groups received usual nursery care of continuous near-darkness. In the study nursery, all infants born at ≤ 28 weeks gestation were cared for in covered incubators unless light was required for caregiving procedures. Incubator and open bed covers were the same across the study settings and included a dark lining to maximize light-blocking capabilities.
If an infant was critically ill when scheduled for transition to cycled light, transition was delayed. Two study infants, both in the ECL group, were delayed for one week. Infants were also monitored for changes in apnea or bradycardia for 48 hours following transition. If they remained at baseline, the intervention continued. No infant had to be placed back into near-darkness after initiation of cycled light.
The intervention was implemented as part of normal caregiving by the nursing staff. Intervention instructions were posted at each infant’s bedside, and staff education occurred before and throughout the study. Near-darkness (5–30 lux) was provided using bed covers and dimmed individual lighting except for procedures. Daylight (200–600 lux) was provided with folded incubator covers to allow light in from four sides, removable light-blocking film, or with the bed cover off during daytime hours (0730–1830). Dimming of room lights as required to be within the intervention range was also used as necessary. The daylight lux range was above the 200 lux level known to stimulate the superchiasmatic nuclei in baboons (Rivkees, Hofman, & Fortman, 1997), yet within the recommended NICU lighting guidelines (White, 2007). The daylight range was also wide enough to be practical in most NICU light environments.
Cycled light was provided in an 11-hour-on, 11-hour-off pattern. Start times for each phase were allowed to vary between 6:30 and 7:30 AM and 6:30 and 7:30 PM based on change-of-shift nursing care needs. Light was provided with Philips Cool White fluorescent lamps and was measured as illuminance (lux), a measure of the reflective abilities of a surface. Lux levels were checked using an Extech light meter every shift by the bedside nurse to ensure they were in the appropriate range and environmental modifications were made as necessary. Research staff also assessed lux measures for every infant weekly.
Infants were monitored over their hospital stay and received the appropriate lux levels based upon their assigned light intervention 94% of the time (Near-darkness: M=5.9 lux, R=0–28 lux; Cycled light: M=351.4 lux, R=240–735 lux). There were no differences between groups in time when the lux levels were out of the appropriate range.
Outcome Measures
Primary outcomes were weight (in grams) over time and length of hospitalization. Secondary outcomes were hospital costs, sleep development, and neurodevelopment (mental, motor, and visual acuity).
Weight gain, length of hospitalization
Weight data was retrieved from the medical record weekly during hospitalization and at each developmental follow-up visit. Length of hospitalization (length of stay [LOS]) was calculated as the time from birth until day of discharge home.
Hospital costs
Hospital cost data were obtained from the hospital financial database and calculated as total hospital costs associated with the inpatient hospital stay.
Sleep development
Sleep was assessed during hospitalization with follow-up assessments after discharge until 24 months CA. Inpatient sleep data collection began at 30 weeks PMA if the infant was off of mechanical ventilation and approximately every 3 weeks thereafter until hospital discharge. Observations every 3 weeks were frequent enough to identify change without being overly burdensome. Data collection occurred with the infant positioned in bed during a 2-hour intra-feeding period between 9:00 and 12:00 in the morning and evening. Four sleep-wake states, active and quiet sleep, sleep-wake transition, and waking, were assessed using an instrumented sleep-wake state coding system, described elsewhere in detail (Brandon & Holditch-Davis, 2005). State was determined based on digitized waveforms of the infant’s body movements, respiration patterns, and eye movements acquired through a piezoelectric pad and EOG leads. Specific definitions are described in Table 1.
Table 1.
Sleep-Wake State Definitions
| Variable | Definitions |
|---|---|
| Quiet Sleep | Respiration is relatively regular (very regular or regular). No more than 20 seconds of continuous movement occurs in quiet sleep. |
| Active Sleep | Respirations are irregular. Sporadic motor movements may occur, but are not continuous. Rapid eye movements occur intermittently. |
| Sleep-Wake Transition | Behaviors of both wakefulness and sleep. Respirations are very irregular. Forty seconds of continuous movement in the middle of sleep is scored as sleep-wake transition. |
| Waking | Includes alert, non-alert waking and crying. Respirations are very irregular. Three minutes of continuous movement is scored as waking. |
| Very Regular Respiration | The smallest breath during a 10-second period is at least 80% of the height of the largest breath, and the narrowest peak-to-peak interval is at least 67% of the widest peak-to-peak interval. |
| Regular Respiration | Respiration during a 10-second period does not meet the criteria for very regular respiration but there is no more than one breath between 20–50% of the height of the largest breath, and the narrowest peak-to-peak interval is at least 50% of the widest peak-to-peak interval. |
| Irregular Respiration | Respiration is too irregular to qualify as regular respiration but does not meet the criteria for very irregular respiration. |
| Very Irregular Respiration | Scored when a 10-second period includes periodic respiration, continuous movement, and apneic pause defined as: (1) an absence of respiratory activity for an interval of more than 2 seconds between the end of expiration and the beginning of the next inspiration; (2) the presence of a detectable cardiac rhythm within the tracing of the respiratory pause; and (3) an obvious disruption of the ongoing respiratory pattern. |
Three raters coded the occurrences of sleep-wake states every 10 seconds during 2-hour observations and percent of the observation time in each of four states was calculated. Raters were trained until the reliability against the gold standard (last author) reached a kappa coefficient of 0.80 or greater. To minimize rater drift, raters were randomly paired and reliability checks were conducted on every 20th observation. Kappa coefficients were at the acceptable level (Bakeman & Gottman, 1997) for all four sleep-wake states (active sleep = 0.72, quiet sleep = 0.77, sleep-wake transition = 0.70, waking = 0.70).
Overall regularity of respiration in quiet sleep, i.e., quiet sleep regularity score, was further assessed because it is a strong predictor of neuro-maturation (Holditch-Davis & Edwards, 1998). The score was calculated by adding two times the percentage of very regular respiration in quiet sleep plus the percentage of regular respiration minus the percent of irregular respiration (Holditch-Davis, Scher, Schwartz, & Hudson-Barr, 2004). Possible scores on the quiet sleep regularity ranged from −100 (most immature) to 200 (most mature). Infants do not reach the full maturation on quiet sleep regularity until the post-neonatal period (Holditch-Davis & Edwards, 1998; Holditch-Davis, Edwards, & Helms, 1998).
Outpatient sleep development was assessed using parent-report sleep diaries for 7 consecutive days beginning at 4 months CA and continued every 5 months thereafter until 24 months CA (4, 9, 14, 19, 24 months CA). Parents recorded the time (divided into 30-minute epochs) their infant went to sleep and awakened from naps and nighttime sleep. The diary was reviewed with parents prior to discharge and over the phone the week before each measure was collected, to ensure their understanding of record completion. Reminder phone calls were made if diaries were not returned within 2 weeks, allowing parents 1 week to complete and 1 week to return the diaries. A copy of the sleep diary is included in Supplemental Digital Content 1.
Parents accurately reported sleep onset (r = .88) and sleep duration (r = .74) when evaluated against objective measures of sleep derived from activity monitoring (Sadeh, Dark, & Vohr, 1996). Day-to-day stability of the sleep diaries was tested with interclass correlations across 6 days within the 7 consecutive days of each data collection week. Interclass correlations were ≥ .90 for all variables.
Two coders calculated four sleep variables from the parent-reported sleep diaries: number of sleep bouts during the day (0700–1900) and night (1900–0700), length of longest sleep bout in 24 hours, and day-to-day variability of sleeping bedtime, i.e., first time the infant goes to bed after 1900. The discrepancies in the data calculated from two coders were resolved before the final analysis was conducted.
Neurodevelopmental outcomes
Neurodevelopmental outcomes included mental and motor development assessed by the Bayley Scales of Infant Development® (Bayley-II®) and visual acuity assessed with the Preferential Looking Test (Gwiazda, Wolfe, Brill, Mohindra, & Held, 1980). The Bayley-II® was administered at 9 and 18 months CA during infants’ routine Special Infant Care Clinic follow-up appointments, and the Preferential Looking test was administered during the 24-month Ophthalmology Clinic appointment. Evaluators were blinded to intervention assignment.
Descriptive and Covariate Variables
Data for the descriptive statistics and covariates were pulled from the medical record weekly during hospitalization and following each clinic visit after infants were discharged home. Length of intervention was calculated in days from the initiation of the intervention until hospital discharge.
Safety Measures
Ophthalmological exams were conducted by a pediatric ophthalmologist and graded according to the standard scoring of retinal development and retinopathy of prematurity (ROP) (“The International Classification of Retinopathy of Prematurity revisited,” 2005). Exams were repeated (inpatient and outpatient) until the retina was mature with no ROP disease or until the disease had regressed. Nursery audiologists obtained automated Auditory Brainstem Response (ABR) the week prior to discharge. All infants who failed the ABR were scheduled for repeat exams following discharge. Evaluators were blinded to intervention assignment.
Data Analysis
Data were analyzed using SAS version 9.3. Non-directional tests were conducted with the level of significance set at .05 for each test unless otherwise specified, with no adjustment for multiple outcomes due to the exploratory nature of this study.
Sample characteristics
Descriptive statistics were used to summarize demographic and clinical characteristics. Student t-test and Fisher’s Exact Test were used to examine differences in infant/mother characteristics of those assigned to the ECL versus to the LCL at enrollment and to identify covariates for the efficacy analyses. Sensitivity analyses were conducted to test for differences in characteristics of those included versus not included in the outpatient sample.
Weight gain
Weight gain during hospitalization was evaluated for PMA weeks 28 to 44 using a segmented random coefficients regression model (RRM), a type of hierarchical mixed-effects model for longitudinal data. These trajectory models compared intervention differences in rate and pattern of change in weight across time. The analysis of weight gain during hospitalization was segmented into two 8-week segments: PMA weeks 28 to 36, when only the ECL group received cycled light, and weeks 36 through 44, when both groups received cycled light.
For both segments, the initial model included fixed and random core components. Fixed effects were intervention, time, intervention-by-time, and birth weight as a covariate, while random effects were infant and infant-by-time. Each model was tested to determine if the change in weight over PMA weeks was non-linear. If so, the model was adjusted to incorporate time terms required to obtain the best-fitting model. In addition, the following covariates along with birth weight were evaluated in the initial model: (1) race: 0=non-Black, 1=Black; (2) gender: 0=male,1=female; (3) PMA week total calories, as a time-dependent covariate; (4) necrotizing enterocolitis (NEC): 0=none, 1=medical/surgical; (5) natural log + 1 of the number of ventilator days (because this variable was skewed); (6) length of intervention; and (7) gastroesophageal reflux disease (GERD): 0=no, 1=yes. Non-significant covariates were eliminated using backward elimination. The final model included the core components and covariates significant at the .05 level.
Outpatient weight gain was evaluated based on the data from PMA week 28, CA month 9, and CA month 18. A RRM was used to compare intervention differences in rate and pattern of change in weight gain across these three time points. Birth weight and covariates noted above were included in the initial model. As for weight gain during hospitalization, non-significant covariates were eliminated from the final model. The 9- and 18-month assessments included weight obtained at months 9 and 18 ± 1 month.
Length of hospitalization and hospital cost
General linear models were then used to test for mean intervention differences in the LOS and total hospital costs, after co-varying for birth weight. Because the data for LOS and hospital cost were severely skewed, the data were transformed to rank values to normalize the data distributions for use in the analysis model. A natural log transformation was also considered but did not sufficiently address the non-normality issue. Spearman correlations were performed to examine the relationship between birth weight, days to full feeding, log + 1 of ventilator days, LOS, and cost.
Sleep development
Inpatient and outpatient sleep data were analyzed separately because we used two different means of data collection. For inpatient data, we first collapsed available sleep data to seven time intervals based on infant’s PMA: 30–31, 33–34, 35–36, 37–38, 39–40, 41–42, and 45–46 PMA weeks, to manage unbalanced sleep observations due to varying times of infant discharge or mechanical ventilation. Four of these time intervals (37–38, 41–42, and 45–46 weeks of PMA) were excluded from the analyses because more than 50% of infants had missing data.
A RRM was used to compare group differences in rate and pattern of change in infants’ sleep-wake states across time. A separate RRM was tested for each of five sleep outcomes (active sleep, quiet sleep, quiet sleep regularity score, sleep-wake transition, and waking) during daytime and nighttime. Each model was tested based on the initial model that included fixed effects of time, intervention group, and time-by-intervention along with the random effect of infants. Each model was also tested to determine if the change in sleep-wake patterns was non-linear, and, if so, the model was adjusted to incorporate time terms required to obtain the best fitting model.
Finally, the following infant characteristics were evaluated as covariates in the initial model because of their potential to influence sleep development (Holditch-Davis et al., 2004): (1) caffeine use: 1= yes, 0=no); (2) natural log + 1 of the number of ventilator days; (3) neurological risk: 0 =no risk (no periventricular leukomalacia [PVL], no intraventricular hemorrhage [IVH] or IVH grade 1–2), 1=risk (PVL or IVH grade 3–4); (4) gender: 0=male,1=female; and (5) race: 0=non-Black, 1=Black. Non-significant covariates were eliminated using backward elimination. The final model included the core components in the initial model and covariates that had a p value ≤ .10. A more lenient significance was set for the covariates in this exploratory, secondary outcome analysis.
Similar analysis steps were taken for the outpatient sleep data. We used a RRM to compare intervention differences in rate and change of infant sleep-wake patterns across times, separately for each of four sleep variables (number of sleep bouts during the day and night, length of longest sleep bouts, and variability of sleeping time from day to day). Each model was tested based on the initial model that included fixed effects of time, intervention, intervention–by-time and random effects of infant. Each model was also tested to determine if the change in sleep-wake patterns was non-linear, and, if so, the model was adjusted to incorporate time terms required to obtain the best fitting model. Finally, the following infant and mother characteristics were evaluated as covariates: (1) birth weight; (2) multiple birth: 0=single, 1=twin/triplet; (3) natural log + 1 of the number of ventilator days; neurological risk: 0=no risk, 1=risk; (4) gender: 0=male,1=female; (5) race: 0=non-Black, 1=Black; and (6) maternal marital status: 0=single, 1=married. Non-significant covariates (p >.1) were eliminated using backward elimination. As with the inpatient sleep data a more lenient significance was set for the covariates in this exploratory, secondary outcome analysis. The final model included the core components in the initial model and significant covariates.
Developmental outcomes
Intervention group differences were analyzed using Wilcoxon two-sample tests for the MDI and PDI, and Fisher’s Exact Test for visual acuity (Preferential Looking Test, both eyes normal or abnormal).
Safety outcomes
Safety measures were maximum stage of ROP, ABR hearing test (pass or fail). Fisher’s Exact Tests were used to test for intervention differences in proportions of the safety outcomes.
Power Analysis
Power analyses conducted prior to study enrollment indicated that a sample size of 100 (50/group) would be sufficient to achieve at least 80% power to detect significant between-intervention differences in the primary outcomes when applying hierarchical mixed-effects models for longitudinal data (inpatient weight gain) and general linear models for cross-sectional data (LOS), with the two-tailed significance set at .05. This estimate was based on the following assumptions: (1) 70% within-person correlation across time for the weight gain trajectory analysis; (2) medium effects for between-group differences in trajectory of change across the initial 36 weeks PMA (partial η2 =.70) and means at week 36 PMA (Cohen d=.55). With N=100, a medium intervention effect (Cohen d=.55) was also anticipated for LOS. In event of a smaller effect size (Cohen d=.25), a much larger sample would be required (N=568, 284/group). When viewed retrospectively, statistical power was less than 80% because the observed effects were smaller than anticipated.
Results
Infant Characteristics and Intervention Fidelity
In the inpatient sample, the ECL group had a significantly lower proportion of female infants than the LCL group. The sensitivity analysis did not otherwise reveal any significant differences in the characteristics of infant/mother dyads who were included in the inpatient sample only and those who were included in both samples. Table 2 summarizes infant and maternal characteristics for the ECL and LCL groups in the inpatient and outpatient samples. There were also no group differences in the attrition rates over time (Figure 1).
Table 2.
Infant and Maternal Characteristics: Inpatient and Outpatient Samples
| Variable | Inpatient Sample, N = 118 | Outpatient Sample, n = 83 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| ECL, n = 61 (28 week PMA) |
LCL, n = 57 (36 week PMA) |
pa | ECL, n = 40 (28 week PMA) |
LCL, n = 43 (36 week PMA) |
pb | |||||
|
| ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Gestational age at birth | 26.3 | 1.4 | 26.3 | 1.5 | .94 | 26.0 | 1.3 | 26.2 | 1.5 | .28 |
| Birthweight | 874.1 | 219.7 | 872.7 | 232.7 | .97 | 845.8 | 219.9 | 848.3 | 228.9 | .34 |
| Apgar score at 1 min | 4.0 | 2.2 | 3.7 | 2.4 | .38 | 4.1 | 2.2 | 3.7 | 2.2 | .35 |
| Apgar score at 5 min | 6.3 | 1.9 | 6.7 | 1.7 | .20 | 6.2 | 1.9 | 6.5 | 1.8 | .47 |
| # days phototherapy | 6.1 | 3.5 | 5.3 | 2.7 | .16 | 6.3 | 3.4 | 5.1 | 2.5 | .09 |
| SNAP on day of life 1 | 14.4 | 10.7 | 14.5 | 10.7 | .95 | 14.7 | 10.6 | 14.2 | 11.4 | .60 |
| SNAP on day of life 14 | 2.7 | 6.9 | 2.6 | 6.6 | .92 | 3.0 | 7.3 | 3.0 | 7.2 | .91 |
| Maternal age | 26.6 | 5.3 | 27.6 | 6.6 | .34 | 27.0 | 5.8 | 27.4 | 6.9 | .56 |
| Maternal education | 12.5 | 2.5 | 13.6 | 3.0 | .06 | 12.6 | 2.4 | 12.8 | 2.5 | .79 |
|
| ||||||||||
| n | % | n | % | n | % | n | % | |||
|
| ||||||||||
| Gender | .04 | .45 | ||||||||
| Female | 22 | (36.1) | 31 | (54.4) | 15 | (37.5) | 21 | (48.8) | ||
| Male | 39 | (63.9) | 26 | (45.6) | 25 | (62.5) | 22 | (51.2) | ||
| Race | .63 | .67 | ||||||||
| African-American | 40 | (65.6) | 40 | (70.2) | 26 | (65.0) | 33 | (76.7) | ||
| Caucasian | 15 | (24.6) | 14 | (24.6) | 10 | (25.0) | 7 | (16.3) | ||
| Hispanic-Latino | 6 | (9.8) | 3 | (5.3) | 4 | (10.0) | 3 | (7.0) | ||
| Multiple Birth | .64 | .56 | ||||||||
| Singleton | 53 | (86.9) | 46 | (80.7) | 37 | (92.5) | 36 | (83.7) | ||
| Twins | 6 | (9.8) | 7 | (12.3) | 4 | (9.3) | 4 | (9.3) | ||
| Triplets | 2 | (3.3) | 4 | (7.0) | 1 | (2.5) | 3 | (7.0) | ||
| Neurological risk | 9 | (15.5) | 9 | (16.7) | .86 | 5 | (12.5) | 8 | (18.6) | .55 |
| Sepsis | .79 | .71 | ||||||||
| None | 35 | (57.4) | 36 | (63.2) | 22 | (55.0) | 27 | (62.8) | ||
| Yes | 14 | (23.0) | 12 | (21.1) | 9 | (22.5) | 8 | (18.6) | ||
| Multiple | 12 | (19.7) | 9 | (15.8) | 9 | (22.5) | 8 | (18.6) | ||
| Patent ductus arteriosus | .50 | .55 | ||||||||
| None/No treatment | 26 | (42.6) | 18 | (32.1) | 14 | (35.0) | 14 | (32.6) | ||
| Medical | 26 | (42.6) | 28 | (50.0) | 17 | (42.5) | 21 | (48.8) | ||
| Surgical | 9 | (14.8) | 10 | (17.9) | 9 | (22.5) | 8 | (18.6) | ||
| Necrotizing enterocolitis | .06 | .15 | ||||||||
| None | 41 | (70.7) | 41 | (74.6) | 27 | (67.5) | 30 | (69.8) | ||
| Medical | 14 | (24.1) | 6 | (10.9) | 11 | (27.5) | 6 | (14.0) | ||
| Surgical | 3 | (5.2) | 8 | (14.6) | 2 | (5.0) | 7 | (16.3) | ||
| Maternal marital status | .24 | .30 | ||||||||
| Married | 19 | (32.8) | 25 | (44.6) | 12 | (30.8) | 18 | (42.9) | ||
| Not Married | 38 | (65.5) | 31 | (55.4) | 26 | (66.7) | 24 | (57.1) | ||
| Unknown | 1 | (1.7) | 0 | (0.0) | 1 | (2.6) | 0 | (0.0) | ||
Notes. ECL=Early cycled light; LCL=late cycled light; PMA=postmenstrual age. Neurological risk=Grade III or IV Intraventricular hemorrhage and/or Periventricular leukomalacia.
Two-tailed test results: t-tests for continuous and chi-square/Fisher’s Exact tests for categorical variables.
Two-tailed test results: Wilcoxon Two-Sample Tests for continuous and chi-square/Fisher’s Exact tests for categorical variables.
Weight Gain
Weight gain during hospitalization
The final RRM analysis for weight gain from 28 to 36 weeks PMA (N=118) included the fixed effects of time, time2, intervention-by-time, intervention-by-time2, birth weight, total calories, and total calories-by-time interaction. Random effects were infant, infant-by-time, and infant-by-time2. The effects of intervention, intervention-by-time, and intervention-by-time2 were not statistically significant. Cross-sectional contrasts did not reveal any intervention differences at any PMA weeks (Figure 2A). There was a significant increase in weight across time in both groups (time2: F1,402=64.24, p<.001). Weight gain over time was associated with birth weight (F1,123=572.03, p<.001), with less weight gain observed in lower birth weight infants. A significant total calories-by-time interaction (F1,655=6.63, p<.010) indicated that greater weight gain was associated with increased caloric intake over time.
Figure 2.
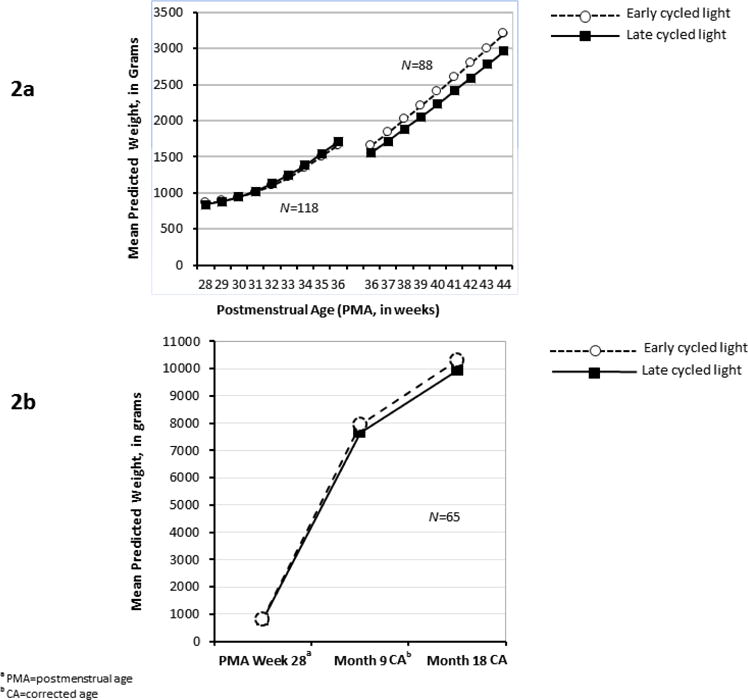
Weight Gain Over Time
aPMA=postmenstrual age
The final RRM for weight gain from 36 to 44 weeks PMA (N=88) included the fixed effects of time, intervention, intervention-by-time, birth weight, gender, and natural log + 1 of the number of ventilator days as well as total calories per week and its interaction with time. Random effects were infant and infant-by-time. The effects of intervention and the intervention-by-time were not statistically significant. Cross-sectional contrasts did not reveal any significant intervention difference at any PMA week. The trajectory analysis indicated that there was a significant increase in weight in both groups (time: F1,207=322.49, p<.001). Lower birth weight (F1,82.2=32.74, p<.001), male gender (F1,78=7.00, p<.010), and more days on the ventilator (natural log + 1, F1,81.2=8.07, p<.010) each predicted lower weight. As before, a significant total calories-by-time interaction was observed (F1,382=11.04, p<.001), with weight over time increasing as total caloric intake over time increased. The mean adjusted weights at each PMA week are available in Supplemental Digital Content 2. The intervention effect sizes were Cohen d = .26 and .36 for 36 and 44 weeks PMA, respectively.
Figure 2a displays the results for the two segments of the analysis, PMA 28–36 weeks (N=118, segment 1) and PMA 36–44 weeks (N=88, segment 2). Although the latter segment had a smaller sample, sensitivity analyses did not reveal any significant differences in infant/maternal characteristics between the 88 included and the 30 excluded (all p>.05). Additionally, infant/maternal characteristics did not differ significantly between those included and excluded among infants in the (a) ECL group (segment 1: N=61; segment 2: N=44) and (b) LCL group (segment 1: N=57; segment 2: N=44). Although the mean predicted weight at PMA 36 weeks for the LCL infants was greater at the end of segment 1 (M=1707.2, SD=488.8, N=57) than at the beginning of segment 2 (M=1555.7, SD=386.6, N=44), an independent t-test indicated that the predicted means were not statistically different (p=.085).
Outpatient weight gain
This subsample included 65 infants with weight data at month 9 and/or month 18. The final RRM included the fixed effects of intervention, time, intervention-by-time, and birth weight along with the random effects of infants and infant-by-time interactions. There was a significant increase in weight over time in both intervention groups (F2,3.34=728.85, p<.001). However, the intervention (F1,16.7=0.69, p=.418) or intervention-by-time (F2,3.33=0.31, p=.750) effects were not statistically significant. Birth weight significantly influenced weight gain across time (F1,36.6=14.75, p<.001), with smaller birth weight infants presenting with lower weights gains over time than larger birth weight infants. Contrasts at each time did not reveal any significant intervention differences in mean weight at PMA week 28, month 9, and month 18 (Figure 2b). Intervention effect sizes were 0.54 at month 9 and 0.59 at month 18 CA. (The mean adjusted weights at PMA week 28, month 9 CA, and month 18 CA are provided in the Supplemental Digital Content 2.)
Length of hospitalization and hospital cost
The rank values of LOS and hospital costs were analyzed due to severe skewness of the data. The GLM results, co-varying for birth weight, indicated that the mean LOS in the ECL and LCL groups did not differ (F1,115=0.30, p=.586, Cohen d=.11). Infants in the ECL group went home on average 5.5 days earlier than the LCL group. The unadjusted mean LOS was 91.4 days in the ECL group (SD=45.0) and 96.9 days (SD=48.2) in the LCL group.
In terms of total cost, the ECL and LCL groups also did not differ (F1,114=0.58, p=.4460, Cohen d=.15), although the unadjusted mean cost was less in the ECL group (M=$127650.70, SD=75744.00) than in the LCL group (M=$140501.80, SD=93260.00). For both outcomes, smaller birth weight was significantly associated with increased LOS (F1,115=40.3, p<.001) and total cost (F1,114=47.9, p<.001).
Spearman correlations showed lower birth weight was significantly associated with greater number of ventilator days (rs= -.60, p<.001) and increased days until full feeding (rs= -.50, p<.001). Increased LOS and cost were also related to greater number of ventilator days (LOS rs=0.68, cost rs=.75, both p<.001) and days until full feeding (LOS rs=.70, cost rs=.71, both p<.001). Because birth weight was inversely correlated with ventilator days (rs= -.60, p<.001) and days to full feeding (rs= -.49, p<.001), only birth weight was included as a covariate in the above analyses to avoid unstable models due to multicollinearity. GERD and Score for Neonatal Acute Physiology (SNAP) of day 1 of life were not related to birth weight (both r ≤ -.18, p>.05) and were evaluated as potential covariates but were omitted from the final model due to their statistical non-significance (p>.05).
Sleep Development
Sleep data was available for a subsample of 106 infants (ECL: n=53, LCL: n=53) for inpatient sleep outcomes and for 53 infants (ECL: n=26, LCL: n=27) for outpatient sleep outcomes. Comparisons of infant and maternal characteristics in these subgroups are presented in Supplemental Digital Content 3. Briefly, in the inpatient sample (n=106), there were no differences on any of the infants’ and mothers’ characteristics between the groups. In the outpatient sample (n=53), infants in the ECL had significantly longer days on ventilation treatment than those in the LCL, so we controlled number of ventilator days as a covariate when it was significant for the analyses in the outpatient sample. Sensitivity analyses were also conducted to test for differences in the characteristics of those included versus not included in the outpatient sample. The outpatient sample was significantly different from the inpatient-only sample on GA at birth (Z=0.025, p=.027) and maternal marital status (Fisher’s exact test p=.024) with younger GA and proportionally more single mothers in the outpatient sample.
Sleep development during hospitalization
The final model for active sleep during the day included the fixed effects of time and intervention along with the random effect of infants. Active sleep decreased significantly over time in both intervention groups (F3,58=15.16, p<.001), but the intervention effect was not significant (F1,88.3=.010, p=.931) (Figure 3a).
Figure 3.
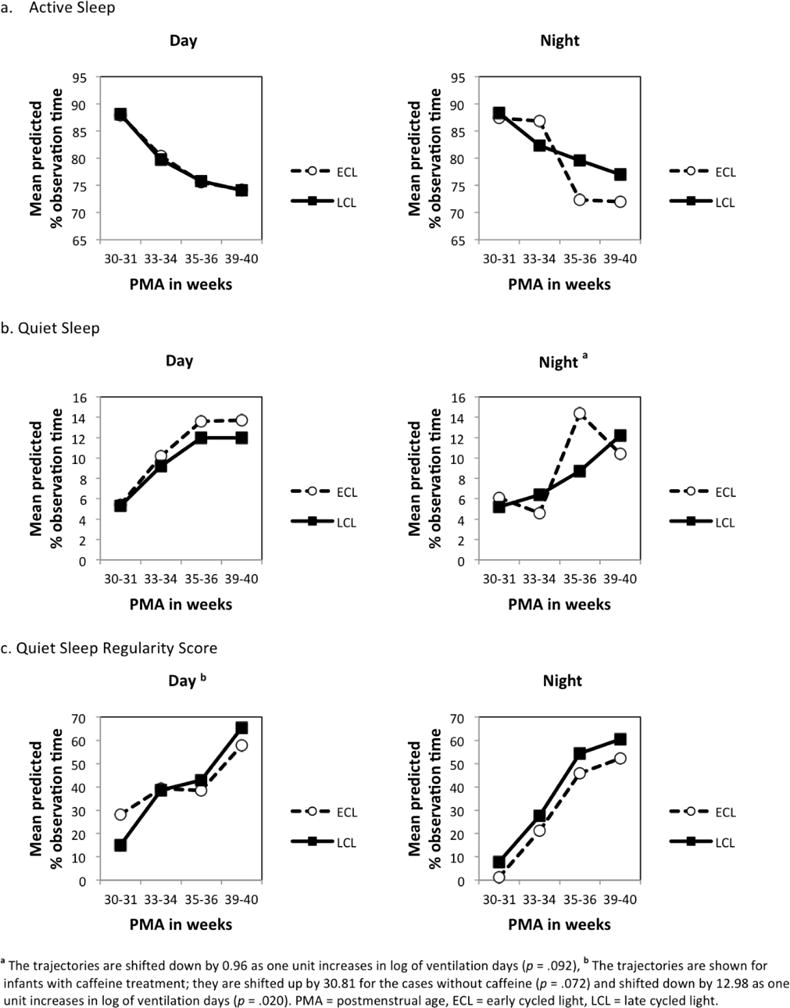
Changes in Sleep Variables During Hospitalization by Intervention Group
aThe trajectories are shifted down by 0.96 as one unit increases in log of ventilation days (p = .092),b The trajectories are shown for infants with caffeine treatment; they are shifted up by 30.81 for the cases without caffeine (p = .072) and shifted down by 12.98 as one unit increases in log of ventilation days (p = .020). PMA = postmenstrual age, ECL = early cycled light, LCL = late cycled light.
The final model for active sleep during the night included the fixed effects of time, intervention, and time-by-intervention, along with the random effect of infants. Active sleep also decreased significantly during the night (F3,68.3=14.84, p<.001) and there was a significant interaction effect between time and intervention (F3,68.3=2.92, p=.040), suggesting the rate of decrease in active sleep was accelerated at 35–36 weeks of PMA for infants receiving the ECL compared to those in the LCL group (Figure 3a).
The final model for quiet sleep during the day included the fixed effects of time and intervention along with the random effect of infants. Quiet sleep significantly increased over time during the day in both groups (F3,53.4=5.51, p=.002), however the intervention effect was not significant (F1,69.7=0.62, p=.435) (Figure 3b). The final model for quiet sleep during the night included the fixed effects of time, intervention, time-by-intervention, and natural log +1 of the number of ventilator days along with the random effect of infants. Quiet sleep during the night also increased significantly over time (F3,59.7=8.45, p<.001) and the rate of increase in quiet sleep was larger after 35–36 weeks PMA for infants receiving the ECL compared to those in the LCL group, but this difference was not significant (time-by-intervention interaction, F3,60.7=2.50, p=.068) (Figure 3b). Infants with longer ventilation treatment averaged less time in quiet sleep during the night, but this difference was not significant (F1, 67.1=2.91, p=.092).
The final model for quiet sleep regularity during the day included the fixed effects of time, intervention, caffeine use, and natural log +1 of the number of ventilator days along with the random effect of infants. Quiet sleep regularity increased significantly over time during the day in both intervention groups (F3,64.6=3.00, p=.037), but the intervention effect was not significant (F1,79.2=0.03, p= 863). Also, caffeine treatment (F1, 95.3=3.31, p=.072) and number of ventilator days (F1,80.4=5.68, p=.020) also influenced quiet sleep regularity during the day, suggesting infants with longer ventilation treatment and on caffeine treatment had a lower score on quiet sleep regularity (Figure 3c). Quiet sleep regularity during the night included the fixed effects of time and intervention along with the random effect of infants. Quiet sleep during the night increased significantly over time (F3,43.2=3.82, p=.016), but the intervention effect was not significant (F1,75.2=0.36, p=.551) (Figure 3c).
The final model for waking both during the day and at night included the fixed effects of time and intervention along with the random effect of infants. During the day, there was no significant change in waking over time in either group (F3,67.6=1.25, p=.298); however, the intervention effect was significant, with increased waking for infants in the LCL group (F1,83=5.84, p=.018). During the night, waking significantly increased over time (F3,69.6=6.36, p=.001), but the intervention effect was not significant (F1,73.3=0.52, p=.474) (Figure 4a).
Figure 4.
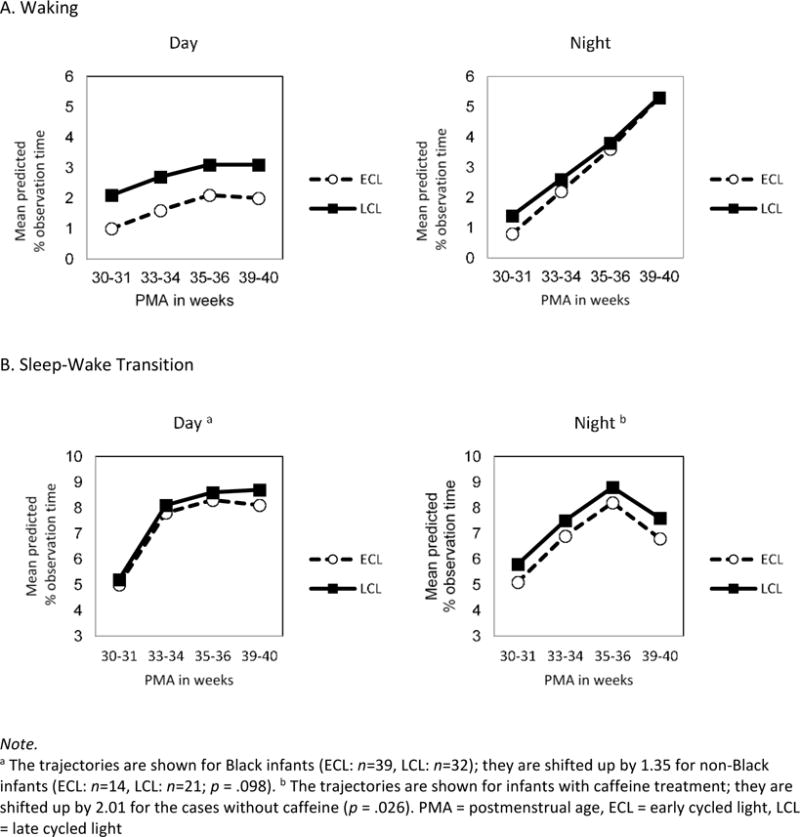
Changes in Sleep-related Variables During Hospitalization by Intervention Group
Note.
aThe trajectories are shown for Black infants (ECL: n=39, LCL: n=32); they are shifted up by 1.35 for non-Black infants (ECL: n=14, LCL: n=21; p = .098).b The trajectories are shown for infants with caffeine treatment; they are shifted up by 2.01 for the cases without caffeine (p = .026). PMA = postmenstrual age, ECL = early cycled light, LCL = late cycled light
The final model for sleep-wake transition during the day included the fixed effects of time, intervention, and infant race along with the random effect of infants. During the day, sleep-wake transition increased significantly over time (F3,72.4=10.31, p<.001), but the intervention effect was not significant (F1,104=0.29, p=.589). Black infants demonstrated less time in sleep-wake transition during the day than non-Black infants, but this difference was not significant (F1,113=2.79, p=.098) (Figure 4b). The final model for sleep-wake transition during the night included the fixed effects of time, intervention, and caffeine use along with the random effect of infants. During the night there was no significant effects of time (F3,90.1=1.61, p=.192) or intervention (F1,89.8=1.09, p=.299). Infants who received caffeine treatment spent significantly less time in sleep-wake transition than those without caffeine (F1,190=5.02, p=.026) (Figure 4b).
Outpatient sleep development
The final model for number of sleep bouts during the night included the fixed effects of time, time2, intervention, time-by-intervention, and neurological risk along with the random effect of infants. The number of sleep bouts during the night decreased significantly over time in both intervention groups (time: F1,126=37.7, p<.001; time2: F1,121=14.4, p<.001). After 14 months CA, infants in the ECL group had fewer night sleep bouts than the LCL group, but this difference was non-significant (F1,135=2.97, p=.087) (Figure 5A). Also, infants with neurological risk had fewer night sleep bouts (F1,33.2=5.22, p=.029) than infants without neurological risk.
Figure 5.
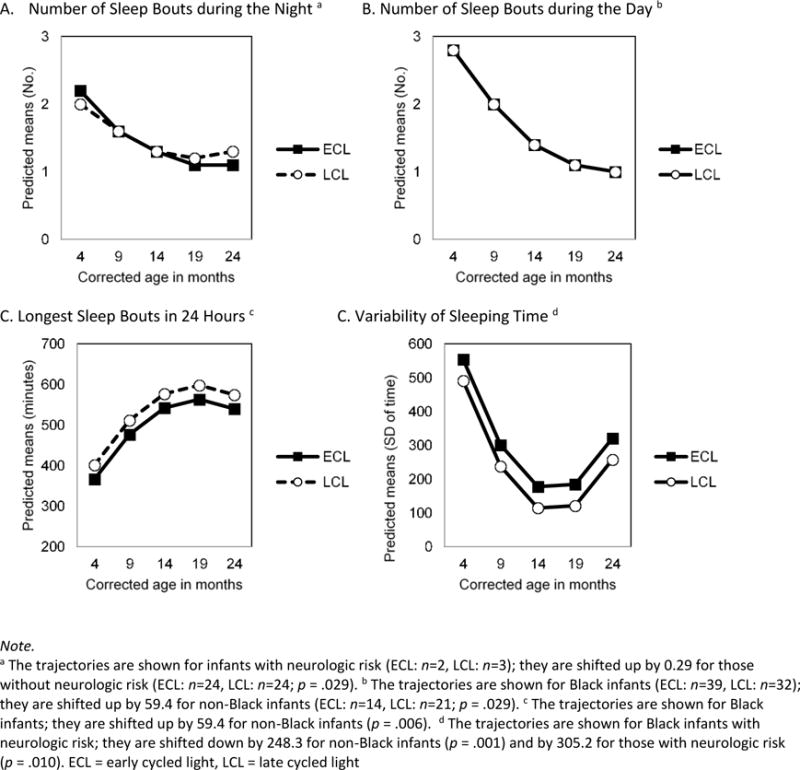
Sleep Development After Discharge up to 2 Years by the Intervention Group
Note.
aThe trajectories are shown for infants with neurologic risk (ECL: n-2, LCL: n=3); they are shifted up by 0.29 for those without neurologic risk (ECL: n=24, LCL: n=24; p = .029). bThe trajectories are shown for Black infants (ECL: n-39, LCL: n=32); they are shifted up by 59.4 for non-Black infants (ECL: n=14, LCL: n=21; p = .029).cThe trajectories are shown for Black infants; they are shifted up by 59.4 for non-Black infants (p = .006). dThe trajectories are shown for Black infants with neurologic risk; they are shifted down by 248.3 for non-Black infants (p = .001) and by 305.2 for those with neurologic risk (p = .010). ECL = early cycled light, LCL = late cycled light
The final model for number of sleep bouts during the day included the fixed effects of time, time2, intervention, and race along with the random effect of infants. Number of sleep bouts during the day also decreased significantly over time in both intervention groups (time: F1,121=74.5, p<.001; time2: F1,115=20.9, p<.001), however the intervention effect was not significant (F1,44.8=0.26, p=.615) (Figure 5B). Non-Black infants had significantly more day sleep bouts (F1,43.5=5.11, p=.029) than Black infants.
The final model for length of longest sleep bouts in 24 hours included the fixed effects of time, time2, intervention, and infant race along with the random effect of infants. Longest sleep bouts increased significantly over time in both groups (time: F1,124=44.87, p<.001; time2: F1,119=21.2, p<.001), and infants’ longest sleep bouts were significantly longer in the LCL group than in the ECL group (F1,44.1=4.43, p=.041) (Figure 5C). Non-Black infants’ longest sleep bouts were significantly longer (F1, 42.6=8.24, p=.006) than that of Black infants.
The final model for variability of bedtime from day to day included the fixed effects of time, time2, intervention, infant race, and neurological risk along with the random effect of infants. Day-to-day variability of bedtime decreased significantly over time in both groups (time: F1,120=27.6, p<.001; time2: F1,114=19.53, p<.001), but the intervention effect was not significant (F1,45.6=2.26, p=.140) (Figure 5D). Significant predictors for variability of bedtime were infant race (F1,45=11.70, p=.001) and neurological risk (F1,39.1=7.25, p=.010). That is, Black infants or infants with neurological risk had more day-to-day variability in sleeping time than non-Black or those without neurologic risk.
Neurodevelopmental Outcomes
MDI data was available for 46 infants at 9 months CA and 42 infants at 18 months CA. PDI data was available for 45 infants at 9 months CA and 40 infants at 18 months CA. Covariates were not evaluated due to the small sample sizes. The intervention groups did not differ in MDI or PDI scores at 9 or 18 months. Mean MDI scores at 9 and 18 months in the ECL group were 93.9 (SD=45.0) and 79.4 (SD=19.2) respectively and, were 95.8 (SD=11.0) and 79.3 (SD=16.7) respectively in the LCL group. The intervention groups also did not differ in visual acuity scores at 24 months of age. Infants in the ECL group had normal acuity 84.4% of the time and infants in the LCL group had normal acuity 75.0% of the time.
Safety Outcomes
The intervention groups did not differ significantly on the maximum stage of ROP or hearing test abnormalities (Table 3).
Table 3.
Safety Outcomes
| ECL, n=61 | LCL, n=57 | ||||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | pa | |
| Retinopathy of Prematurity, Maximum Stage | 54 | 54 | .54 | ||
| 0 = Immature Retina | 10 | (18.5) | 9 | (16.7) | |
| 1 = ROP Stage 1 | 10 | (18.5) | 14 | (25.9) | |
| 2 = ROP Stage 2 | 20 | (37.0) | 23 | (42.6) | |
| 3 = ROP Stage 3 | 13 | (24.1) | 8 | (14.8) | |
| 4 = ROP Stage 4 | 1 | (1.8) | 0 | (0.0) | |
|
| |||||
| Brainstem Auditory Evoked Response Hearing Test | 52 | 53 | 1.00 | ||
| Pass | 49 | (94.2) | 50 | (94.3) | |
| Fail | 3 | (5.8) | 3 | (5.7) | |
Note. ECL = Early-cycled light; LCL = Late-cycled light.
Two-tailed Fisher’s exact test results.
Discussion
This was the first known study of the impact of the timing of a cycled light environment in a sample composed exclusively of extremely preterm (<28 weeks) infants. While we found no statistically significant differences in our primary outcomes of weight gain over time and length of hospitalization, clinically important differences were observed. The mean rate of weight gain over the 8 weeks from PMA week 36 to 44 was 193.8 grams per week in the ECL group, compared to 176.3 grams per week in the LCL group. As expected, weight gain over time was larger in infants with larger birth weight and caloric intake. Despite weight increasing from PMA week 36 to 44, our intervention effect sizes were smaller than expected (Morag & Ohlsson, 2013), and therefore we were underpowered to evaluate group differences in weight. Our smaller effect sizes are likely attributable to the more immature sample (≤ 28 weeks at birth) than included in previous studies (Boo et al., 2002; Brandon et al., 2002; Mirmiran et al., 2003). While moderate effect sizes were observed in the outpatient weight analysis, attrition limited those findings.
Infants receiving ECL also went home 5.5 days earlier when compared to LCL. Because adequate weight gain and steady growth trajectories are one requirement for discharge to home, shorter LOS in the ECL may be have been affected by their faster weight gain. As in previous research, LOS was influenced by time required to reach full feedings (Brandon et al., 2002). In addition, LOS was influenced by the number of ventilator days, a likely proxy for severity of illness in this extremely preterm population. Hospital costs were examined as a secondary measure to quantify any intervention cost savings. As expected, lower birthweight and longer hospitalization significantly increased total hospital costs. The total hospital cost was less in the ECL than in LCL infants but the difference was not significant.
Infant sleep development during hospitalization and following hospital discharge revealed few intervention effects, possibly because all infants had the benefit of cycled light exposure to promote circadian rhythms before hospital discharge. There were only two significant intervention effects in inpatient sleep development. Infants in the ECL group had accelerated active sleep development after 35–36 weeks PMA when compared to the LCL group, while infants in the LCL group had more waking over time during the daytime. The sleep states are similar to sleep patterns described other studies (Guyer et al., 2015; Holditch-Davis et al., 2004; Llaguno et al., 2015). In addition, the change in sleep-wake states was seen in both day and nighttime observations. During both day and nighttime, active sleep decreased over time, while quiet sleep and quiet sleep regularity increased over time. The only day-night difference was an increase in sleep-wake transitions during the day with no change during the night.
In both intervention groups, parents reported developmentally appropriate changes in sleep patterns following hospital discharge. The number of day and night sleep bouts decreased over time, while the longest sleep bout each 24 hours increased in length over time, indicating a consolidation of sleep. In addition, the variability in the time an infant typically went to bed for the night also decreased over time, indicating an increasing predictability of nighttime routines. There was only one significant intervention effect; infants’ longest sleep bout in the LCL group was longer than infants in the ECL group.
Secondary outcomes of neurodevelopmental status were explored at 9, 18 and 24 months CA. Mental and motor development was in the normal range at 9 months CA and in the delayed range at 18 months CA for both groups, consistent with prior patterns of early outcomes in extremely preterm infants (Janssen et al., 2011; Vohr et al., 2012). Consistent with other research (O’Connor, Spencer, & Birch, 2007), most infants (75%) had normal visual acuity.
The sensitivity analysis revealed no differences in the characteristics of infants and mothers included in the inpatient sample only versus both samples. There were no significant intervention differences in attrition rates over time. However, the overall attrition rate resulted in inadequate power to determine group differences at the later points. Because developmental follow-up was standard care for this population, attrition may be linked to health system factors in the US or parents’ lack of concern over their infant’s developmental status (Guillén, DeMauro, Ma, & et al., 2012).
ROP and hearing outcomes were deemed important safety outcomes from prior research. Several teams have explored the relationship between light and the development of ROP in preterm infants (Brandon et al., 2002; Kennedy et al., 2001; Phelps & Watts, 2001). As expected, there were no group differences in severity of ROP. In addition, there were no group differences in hearing development. Two critically ill infants in the ECL group had their implementation of cycled light delayed by one week. Changes in apnea and bradycardia from their baseline did not change for any infant after beginning cycled light.
This study was not designed specifically to test the benefits of cycled light over near darkness but rather to determine the most appropriate time to institute cycled light. It is possible the timing of the intervention should be located somewhere between the two start points tested here, 28 and 36 weeks PMA. In addition, while the practice patterns were consistent across the study settings, unmeasured differences in the three stepdown units may have affected study findings. The attrition of participants limited the power to be certain of the post-discharge outcomes. Furthermore, subtle differences in developmental outcomes may not be discernable by the Bayley II or until the infant is older, such as a sensory processing disorder.
Extremely preterm infants were the population of interest for our study because they are most vulnerable to loss of fetal programming of circadian rhythms, due to shorter gestations and long hospitalizations. They are also exposed to the negative sensory stimuli of the NICU environment. Melatonin readily crosses the maternal placenta (Okatani et al., 1998; Schenker et al., 1998), with levels that are typically 10–12 times higher at night (Stehle et al., 2011). Therefore, melatonin may play a significant role in fetal development of circadian rhythms (Reiter, Tan, Korkmaz, & Rosales-Corral, 2014). Following delivery, human milk and associated melatonin levels may also be important for infant development (Arslanoglu et al., 2012; Cubero et al., 2006). Human milk consumption during hospitalization is associated with greater weight gain, shorter LOS and fewer hospital costs (Bhatia et al., 2013). Few nurseries attempt to provide human milk to infants at the time of day it was pumped, but newer nursery designs including single care-by-family rooms may make provision of human milk at the time of day it is pumped more feasible. In this study, human milk consumption across the hospitalization was not different between the early and late groups. Given the interdependency of infant health and developmental outcomes, future environmental researchers should explore the impact of cycled light and other environmental events such as feeding methods (type and frequency), parental interaction, and provider stimulation, on the development of circadian rhythms.
Conclusions
While most NICUs in the US modify the light environment in some fashion, a national standard of care for environmental light remains absent. Day-night cycling of light is essential to the development of circadian rhythms and maintenance of health (Brooks & Canal, 2013), but in current practice, preterm infants often must adjust to a continuously bright environment when they are sickest, to allow for close observation, and then transition back to a dim or cycled light environment when they are no longer critically ill. In this study, the initiation of low-intensity cycled light at 28 weeks gestation was timed to reduce potential negative effects of very early light exposure on visual or auditory development (Brandon et al., 2002) and to match the developmental point when the retinohypothalamic pathway is functional and able to facilitate development of circadian rhythms (Hao & Rivkees, 1999).
The smaller than expected intervention effect sizes and the attrition following hospital discharge left our study underpowered to detect significant differences. Additional studies with larger samples are still required to explicate the benefits of and best time to institute cycled light. Future researchers should take into consideration the small effect sizes found in our study when considering sample size.
However, a clinically important trend towards improved weight gain and decreased LOS was observed in the group receiving early cycled light. Consistent with previous work, we found no safety concerns with the use of day-night cycling of light. Some light exposure in the NICU environment is inevitable, but early day and night cycling of light may promote preterm infants’ circadian rhythms (Guyer et al., 2015) and low-intensity cycled light may be the best stimulus available to minimize the disruption of the maternal circadian rhythm environment. Given the safety of low-intensity cycled light and the inevitable transition to cycled light in the home environment, NICUs should ensure transition to cycled light prior to discharge.
Supplementary Material
Acknowledgments
Supported by R01NR008044, from the National Institute of Nursing Research, National Institutes of Health (NIH).The authors wish to thank Angel Barnes, Donna Ryan, Kathryn Gustafson, Ricki Goldstein, and Sharon Freedman for their extensive support of subject recruitment and data collection.
Footnotes
Financial Disclosure: The authors have no financial relationships to disclose.
Conflict of Interest: The authors do not have any conflict of interests.
Clinical Trial Registration: clinicaltrials.gov identifier NCT02146287
Contributor Information
Debra H. Brandon, School of Nursing, Department of Pediatrics, School of Medicine, Duke University, DUMC 3322, 307 Trent Dr. Durham, NC 27710, debra.brandon@duke.edu.
Susan G. Silva, Duke University School of Nursing, Durham, NC.
Jinhee Park, Boston College William F. Connell School of Nursing, Chestnut Hill, MA.
William Malcolm, Department of Pediatrics, School of Medicine, Duke University, Durham, NC.
Heba Kamhawy, Duke University School of Nursing, Durham, NC.
Diane Holditch-Davis, Marcus Hobbs Distinguished Professor Emerita of Nursing, Duke University School of Nursing, Durham, NC.
References
- Arslanoglu S, Bertino E, Nicocia M, Moro GE. WAPM Working Group on Nutrition: Potential chronobiotic role of human milk in sleep regulation. Journal of Perinatal Medicine. 2012;40:1–8. doi: 10.1515/JPM.2011.134. [DOI] [PubMed] [Google Scholar]
- Bakeman R, Gottman JM. Observing interaction: An introduction to sequential analysis. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- Boo NY, Chee SC, Rohana J. Randomized controlled study of the effects of different durations of light exposure on weight gain by preterm infants in a neonatal intensive care unit. Acta Paediatrica. 2002;91:674–679. doi: 10.1111/j.1651-2227.2002.tb03301.x/. [DOI] [PubMed] [Google Scholar]
- Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, Quigley MA. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: Population based cohort study. BMJ. 2012;344:e896. doi: 10.1136/bmj.e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon DH, Holditch-Davis D. Validation of an instrumented sleep-wake assessment against a biobehavioral assessment. Newborn and Infant Nursing Reviews. 2005;5(3):109–115. doi: 10.1053/j.nainr.2005.04.002. [DOI] [Google Scholar]
- Brandon DH, Holditch-Davis D, Belyea M. Preterm infants born at less than 31 weeks’ gestation have improved growth in cycled light compared with continuous near darkness.[see comment] Journal of Pediatrics. 2002;140:192–199. doi: 10.1067/mpd.2002.121932. [DOI] [PubMed] [Google Scholar]
- Brooks E, Canal MM. Development of circadian rhythms: Role of postnatal light environment. Neuroscience and Biobehavioral Reviews. 2013;37:551–560. doi: 10.1016/j.neubiorev.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Cubero J, Narciso D, Aparicio S, Garau C, Valero V, Rivero M, Barriga C. Improved circadian sleep-wake cycle in infants fed a day/night dissociated formula milk. Neuro Endocrinology Letters. 2006;27:373–380. [PubMed] [Google Scholar]
- Ge WJ, Mirea L, Yang J, Bassil KL, Lee SK, Shah PS. Prediction of neonatal outcomes in extremely preterm neonates. Pediatrics. 2013;132:e876–885. doi: 10.1542/peds.2013-0702. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Edgar DM, Boeddiker M, Ariagno RL. Biological rhythmicity in normal infants during the first 3 months of life. Pediatrics. 1994;94:482–488. [PubMed] [Google Scholar]
- Gottlieb G. Ontogenesis of sensory function in birds and mamuals. In: Tobach E, Aronson L, Shaw E, editors. The Biopsychology of development. New York: Academic Press; 1971. pp. 67–128. [Google Scholar]
- Gressens P, Rogido M, Paindaveine B, Sola A. The impact of neonatal intensive care practices on the developing brain. Journal of Pediatrics. 2002;140:646–653. doi: 10.1067/mpd.2002.123214. [DOI] [PubMed] [Google Scholar]
- Guillén Ú, DeMauro S, Ma L, et al. Relationship between attrition and neurodevelopmental impairment rates in extremely preterm infants at 18 to 24 months: A systematic review. Archives of Pediatrics and Adolescent Medicine. 2012;166:178–184. doi: 10.1001/archpediatrics.2011.616. [DOI] [PubMed] [Google Scholar]
- Guyer C, Huber R, Fontijn J, Bucher HU, Nicolai H, Werner H, Jenni OG. Cycled light exposure reduces fussing and crying in very preterm infants. Pediatrics. 2012;130:e145–151. doi: 10.1542/peds.2011-2671. [DOI] [PubMed] [Google Scholar]
- Guyer C, Huber R, Fontijn J, Bucher HU, Nicolai H, Werner H, Jenni OG. Very preterm infants show earlier emergence of 24-hour sleep-wake rhythms compared to term infants. Early Human Development. 2015;91:37–42. doi: 10.1016/j.earlhumdev.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Wolfe JM, Brill S, Mohindra I, Held R. Quick assessment of preferential looking acuity in infants. American Journal of Optometry and Physiological Optics. 1980;57:420–427. doi: 10.1097/00006324-198007000-00003. [DOI] [PubMed] [Google Scholar]
- Hao H, Rivkees SA. The biological clock of very premature primate infants is responsive to light. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2426–2429. doi: 10.1073/pnas.96.5.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holditch-Davis D, Edwards LJ. Modeling development of sleep-wake behaviors. II. Results of two cohorts of preterms. Physiology and Behavior. 1998;63:319–328. doi: 10.1016/s0031-9384(97)00396-x. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Edwards LJ, Helms RW. Modeling development of sleep-wake behaviors: I. Using the mixed general linear model. Physiology and Behavior. 1998;63:311–318. doi: 10.1016/s0031-9384(97)00459-9. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Scher M, Schwartz T, Hudson-Barr D. Sleeping and waking state development in preterm infants. Early Human Development. 2004;80:43–64. doi: 10.1016/j.earlhumdev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- Janssen AJ, Akkermans RP, Steiner K, de Haes OA, Oostendorp RA, Kollee LA, Nijhuis-van der Sanden MW. Unstable longitudinal motor performance in preterm infants from 6 to 24 months on the Bayley Scales of Infant Development–Second edition. Research in Developmental Disabilities. 2011;32:1902–1909. doi: 10.1016/j.ridd.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Jorge EC, Jorge EN, El Dib RP. Early light reduction for preventing retinopathy of prematurity in very low birth weight infants. Cochrane Database of Systematic Reviews. 2013;8:Cd000122. doi: 10.1002/14651858.CD000122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KA, Fielder AR, Hardy RJ, Tung B, Gordon DC, Reynolds JD, Group LRC Reduced lighting does not improve medical outcomes in very low birth weight infants. Journal of Pediatrics. 2001;139:527–531. doi: 10.1067/mpd.2001.117579. [DOI] [PubMed] [Google Scholar]
- Lickliter R. Atypical perinatal sensory stimulation and early perceptual development: Insights from developmental psychobiology. Journal of Perinatology. 2000a;20(8 Pt 2):S45–54. doi: 10.1038/sj.jp.7200450. [DOI] [PubMed] [Google Scholar]
- Lickliter R. The role of sensory stimulation in perinatal development: Insights from comparative research for care of the high-risk infant. Journal of Developmental and Behavioral Pediatrics. 2000b;21(6):437–447. [PubMed] [Google Scholar]
- Llaguno NS, Pedreira Mda L, Avelar AF, Avena MJ, Tsunemi MH, Pinheiro EM. Polysomnography assessment of sleep and wakefulness in premature newborns. Revista Brasileira de Enfermagem. 2015;68:1109–1115. doi: 10.1590/0034-7167.2015680616i. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Baldwin RB, Ariagno RL. Circadian and sleep development in preterm infants occurs independently from the influences of environmental lighting. Pediatric Research. 2003;53:933–938. doi: 10.1203/01.PDR.0000061541.94620.12. [DOI] [PubMed] [Google Scholar]
- Morag I, Ohlsson A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database of Systematic Review. 2013;8:Cd006982. doi: 10.1002/14651858.CD006982.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AR, Spencer R, Birch EE. Predicting long-term visual outcome in children with birth weight under 1001g. Journal of AAPOS. 2007;11:541–545. doi: 10.1016/j.jaapos.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Okamoto K, Hayashi K, Wakatsuki A, Tamura S, Sagara Y. Maternal-fetal transfer of melatonin in pregnant women near term. Journal of Pineal Research. 1998;25(3):129–134. doi: 10.1111/j.1600-079x.1998.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Phelps DL, Watts JL. Early light reduction for preventing retinopathy of prematurity in very low birth weight infants. Cochrane Database of Systematic Reviews. 2001;1:CD000122. doi: 10.1002/14651858.CD000122. [update of Cochrane Database Syst Rev. 2000;(2):CD000122; PMID: 10796144] [DOI] [PubMed] [Google Scholar]
- Pineda RG, Tjoeng TH, Vavasseur C, Kidokoro H, Neil JJ, Inder T. Patterns of altered neurobehavior in preterm infants within the neonatal intensive care unit. Journal of Pediatrics. 2013;162:470–476.e471. doi: 10.1016/j.jpeds.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Human Reproduction Update. 2014;20:293–307. doi: 10.1093/humupd/dmt054. [DOI] [PubMed] [Google Scholar]
- Rivkees SA. Developing circadian rhythmicity in infants. Pediatrics. 2003;112(2):373–381. doi: 10.1542/peds.112.2.373. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Hofman PL, Fortman J. Newborn primate infants are entrained by low intensity lighting. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:292–297. doi: 10.1073/pnas.94.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkees SA, Mayes L, Jacobs H, Gross I. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics. 2004;113:833–839. doi: 10.1542/peds.113.4.833. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Dark I, Vohr BR. Newborns’ sleep-wake patterns: The role of maternal, delivery and infant factors. Early Human Development. 1996;44:113–126. doi: 10.1016/0378-3782(95)01698-8. [DOI] [PubMed] [Google Scholar]
- Schenker S, Yang Y, Perez A, Acuff RV, Papas AM, Henderson G, Lee MP. Antioxidant transport by the human placenta. Clinical Nutrition. 1998;17(4):159–167. doi: 10.1016/s0261-5614(98)80052-6. [DOI] [PubMed] [Google Scholar]
- Schneider LA, Burns NR, Giles LC, Higgins RD, Nettelbeck TJ, Ridding MC, Pitcher JB. Cognitive abilities in preterm and term-born adolescents. Journal of Pediatrics. 2014;165:170–177. doi: 10.1016/j.jpeds.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Stehle JH, Saade A, Rawashdeh O, Ackermann K, Jilg A, Sebesteny T, Maronde E. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. Journal of Pineal Research. 2011;51:17–43. doi: 10.1111/j.1600-079X.2011.00856.x. [DOI] [PubMed] [Google Scholar]
- Vasquez-Ruiz S, Maya-Barrios JA, Torres-Narvaez P, Vega-Martinez BR, Rojas-Granados A, Escobar C, Angeles-Castellanos M. A light/dark cycle in the NICU accelerates body weight gain and shortens time to discharge in preterm infants. Early Human Development. 2014;90:535–540. doi: 10.1016/j.earlhumdev.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Vohr BR. Neurodevelopmental outcomes of extremely preterm infants. Clinics in Perinatology. 2014;41:241–255. doi: 10.1016/j.clp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, Fuller J. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. Journal of Pediatrics. 2012;161:222–228.e223. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Akiyama S, Hanita T, Li H, Nakagawa M, Kaneshi Y, Ohta H. Designing artificial environments for preterm infants based on circadian studies on pregnant uterus. Frontiers in Endocrinology. 2013;4:113. doi: 10.3389/fendo.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. (Report of the Seventh Conference on NICU Design).Recommendations for newborn ICU design. 2007 Retrieved from from www.nd.edu/~nicudes/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


