Abstract
Rationale
Methamphetamine is one of the most largely consumed ilicit drugs, and its use is associated with abuse liability and several adverse health effects, such as sleep impairment. Importantly, sleep quality can influence addiction treatment outcomes. Evidence suggests that tolerance can develop to the sleep-disrupting effects of stimulant drugs.
Objective
The aim of the present study was to investigate the development of tolerance to the actigraphy-based sleep-disrupting and stimulant effects of methamphetamine self-administration in rhesus monkeys.
Methods
Methamphetamine (0.03 mg/kg/inf, i.v.) self-administration was carried out following 3 different protocols: 14 consecutive days of self-administration; 5 days per week for 3 weeks, with a 2-day interval between 5-day blocks of self-administration; and 3 days per week for 3 weeks, with a 4-day interval between 3-day blocks of self-administration. Daytime activity and activity-based sleep measures were evaluated with Actiwatch monitors a week before (baseline parameters) and throughout each protocol.
Results
Methamphetamine self-administration markedly disrupted sleep-like measures and increased daytime activity. Tolerance developed to those effects with repeated methamphetamine intake exceeding 5 consecutive days. Inclusion of washout periods (2 or 4 days) between blocks of methamphetamine self-administration attenuated the development of tolerance, with longer breaks from methamphetamine intake being more effective in maintaining the sleep-disrupting and stimulant effects of methamphetamine.
Conclusions
Tolerance can develop to the stimulant and sleep-disrupting effects of methamphetamine self-administration. Interruption of drug intake extends the effects of methamphetamine on sleep-like measures and daytime activity.
Keywords: methamphetamine, sleep, self-administration, activity, actigraphy, rhesus monkeys
Introduction
According to the United Nations Office on Drugs and Crime (UNODC), the use of amphetamine-type stimulants has become widespread in the past decades, with the global number of users having reached nearly 35.7 million people in 2014 (UNODC 2016). Among these drugs, methamphetamine has accounted for the largest increase in the use and availability of amphetamine-type stimulants in the past 5 years (UNODC 2016). Amphetamine-type stimulants are largely consumed for recreational purposes, mainly due to the ease of access to these drugs. As alertness-enhancing and stimulant drugs, amphetamines are the active ingredient in several products currently approved for medical use (Mitler et al. 1993; Morgenthaler et al. 2007; Barateau et al. 2016; Briars and Todd 2016). Amphetamines are also illicitly consumed in order to promote wakefulness due to professional demands, such as in shift workers (Bonnefond et al. 2004) and truck drivers (Girotto et al. 2014).
The broad pharmacological actions of amphetamines translate into the generation of adverse effects and abuse liability. Currently, the abuse of amphetamine-type stimulants is a global public health problem (UNODC 2016), and their use is associated with numerous adverse physical, behavioral, and mental health outcomes (Herbeck et al. 2015; Rommel et al. 2015). Disturbed sleep is one of the main adverse effects of the use of amphetamine-type stimulants (Cruickshank and Dyer 2009). Sleep impairment has been associated with the use of amphetamines in the clinics (Huang et al. 2011; Santisteban et al. 2014; Mitler et al. 1993; Barateau et al. 2016), as well as in the context of amphetamine and methamphetamine ongoing abuse (Kirkpatrick et al. 2012a; Mahoney et al. 2014).
Evidence suggests that tolerance can develop to the sleep-disrupting effects of stimulant drugs. Studies conducted in children with attention deficit hyperactivity disorder (ADHD) indicate that while treatment with stimulants continued to exert therapeutic effects for up to 5 years, tolerance developed to some adverse effects, including subjective daytime sleepiness and fatigue (Huang and Tsai 2011). In addition, tolerance has been reported to the sleep-disrupting effects of methamphetamine following repeated (up to 3 consecutive days) administration of low oral doses in healthy humans, a phenomenon that was accompanied by the development of tolerance to methamphetamine’s positive subjective effects (Comer et al. 2001; Kirkpatrick et al. 2012a). However, it remains unclear wether tolerance can develop to the sleep-disrupting effects of psychostimulants in the context of drug abuse.
Sleep quality directly influences addiction treatment outcomes, with individuals with history of sleep problems showing a higher risk of relapse and for the development of drug abuse (Ford and Kamerow 1989; Brower and Perron 2010; Wong et al. 2010; Hasler et al. 2012). Sleep patterns should always be considered in treatment strategies, emphasizing the importance of investigating the factors influencing the time course of stimulant-induced sleep impairment. Thus, the aim of the present study was to investigate the development of tolerance to the stimulant and sleep-disrupting effects of methamphetamine self-administration (SA) in rhesus monkeys and the influence of the drug regimen on this phenomenon.
Material and Methods
Subjects
Three adult male (ROf8, RLk4 and RJl8) and 2 adult female (RVm8 and RZs9) rhesus monkeys (Macaca mulatta) weighing 9–15 kg, served as subjects for the studies. Animals were fitted with collars (Primate Products) prior to the initiation of the studies. Each subject was individually housed in stainless steel home cages and fed Purina monkey chow (Ralston Purina, St. Louis, MO), supplemented with fruit and vegetables daily. Water was continuously available in the colony. Environmental enrichment was provided on a regular basis. The colony was maintained at an ambient temperature of 22±2°C at 45–50% humidity, and the lights were set to a 12-h light/dark cycle (lights on at 7h; lights off at 19h). All subjects had a long history of exposure to methamphetamine, having consistently self-administered methamphetamine 5–7 days a week for the past 4 years, except for weeks when SA was discontinued between the end of a protocol and the beginning of a new experiment (Andersen et al. 2013; Berro et al. 2016). All protocols and animal care and handling strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985) and the recommendations of the American Association for Accreditation of Laboratory Animal Care, and were approved by the Institutional Animal Care and Use Committee of Emory University.
Drugs
(+/−) Methamphetamine hydrochloride was provided by the National Institute on Drug Abuse (Bethesda, MD, USA), and was dissolved in 0.9% sterile physiological saline and administered intravenously. The drug dose was calculated and is expressed as the salt form.
Self-administration
All animals were surgically prepared under sterile conditions with a chronic indwelling venous catheter implanted in a major vein (femoral or jugular) and attached to a subcutaneous vascular access port, as previously described (Howell and Wilcox 2001). The apparatus and SA procedure were previously described by Howell and Wilcox (2001). Animals were trained to respond under a fixed ratio (FR) 20 schedule of drug delivery. Subjects had the opportunity to self-administer methamphetamine during 60-min sessions once a day, 3–7 days/week in the morning (starting between 8–10am). The animals were positioned in a primate chair (Primate Products) and placed in a sound-attenuating experimental chamber for the duration of the session and maintained in their home-cages for the remainder of the day. During the test session, the operant panel was illuminated with a white light which served as a discriminative stimulus. Completion of the FR 20 resulted in a change in the stimulus light from white to red for 15s and a methamphetamine infusion (0.03 mg/kg in 0.5 ml infused over 3s). This infusion was followed by a 60-s timeout. At the end of the timeout, the white light was presented again to signal the opportunity to complete another FR. Methamphetamine intake was determined as the number of infusions received on a given session times the unit dose (0.03 mg/kg/inf).
Daytime and nighttime activity
Actiwatch sensors (Mini Mitter, Bend, OR, USA) were used to assess daytime and nighttime activity, as previously described (Andersen et al. 2010, 2013). Subjects had been adapted to wearing the activity monitors and trained to cooperate with the attachment of the sensor in their collars. Daytime activity data generated activity counts/hour. Nighttime activity data generated the following sleep-like behavior parameters: sleep efficiency (i.e., the percentage of the dark phase spent sleeping); sleep latency (i.e., the time between the lights-off time and the first sleep bout); fragmentation index (i.e., the number of immobile bouts during the dark phase that lasted less than 1 min during the sleep recording period). Sleep-like measures were solely derived from behavioral measures. All parameters were calculated using the Actiware Sleep 3.4 software program (Mini-Mitter, Bend, OR, USA).
Protocol design
Before the beginning of drug SA, Actiwatches were attached to the monkeys’ collars and baseline sleep-like behavior was measured for 1 week. Activity recording continued for the duration of the experiments. Methamphetamine SA was carried out following 3 different protocols: 14 consecutive days of SA (14 protocol); 5 days per week during 3 weeks, with a 2-day interval between 5-day blocks of SA, in a total of 15 days of methamphetamine SA across 21 days (5–2 protocol); 3 days per week during 3 weeks, with a 4-day interval between 3-day blocks of SA, in a total of 9 days of methamphetamine SA across 21 days (3–4 protocol). SA was discontinued after the end of each protocol and sleep-like measures continued for an additional baseline week between experimental protocols.
Data analysis
Within an experimental protocol (14 Protocol, 5–2 Protocol or 3–4 Protocol), the data were combined for the beginning (5 or 3 first days) and the end (5 or 3 last days) of the SA protocol. All data were analyzed using one-way repeated-measures (RM) analysis of variance (ANOVA) corrected for multiple comparisons using Dunnett’s test to indicate significance. Correlational analyses were conducted using Pearson’s Correlation. All graphical data presentations were created using Prism 5 (GraphPad Software), and all statistical tests were performed using PASW Statistics 18 (SPSS Statistics Software). Significance was accepted at an alpha of 0.05.
Results
Methamphetamine self-administration
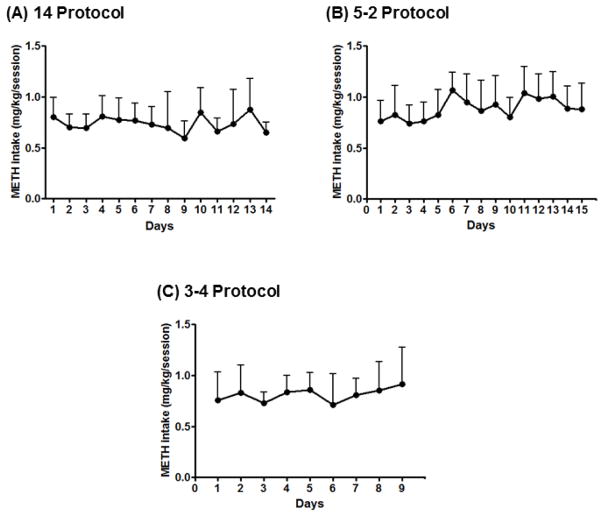
Methamphetamine (0.03 mg/kg/infusion) reliably maintained responding in all 5 subjects, with individual average drug intake ranging from 0.36 to 1.38 mg/kg/session and a group mean of 0.81±0.05 mg/kg/session. Drug intake remained stable across days within an experimental protocol, as well as between protocols. One-way RM ANOVA across the different days of methamphetamine SA showed no significant differences on methamphetamine intake (14 Protocol [F(4,69)=1.32, p>0.05], Fig. 1A; 5–2 Protocol [F(4,69)=2.57, p>0.05], Fig. 1B; 3–4 Protocol [F(4,69)=0.7, p>0.05], Fig. 1C). Pearson’s Correlation analysis showed no correlation between methamphetamine intake and sleep disruption or daytime activity levels under any of the protocols.
Figure 1.
Methamphetamine intake (mg/kg/session) across days of methamphetamine self-administration (0.03 mg/kg/infusion, i.v.) for the 14 Protocol (A), the 5–2 Protocol (B) and the 3–4 Protocol (C). Data are expressed as Mean ± SEM.
Sleep-wake pattern
Sleep-like measures
Sleep-like measures are presented as normalized data (percentage of baseline). Individual-subject baseline sleep-like parameters are shown in Table 1.
Table 1.
Individual-subject baseline sleep parameters
| Subject | Sleep Efficiency (%) | Sleep Latency (min) | Fragmentation Index |
|---|---|---|---|
| ROf8 (M) | 80.76±1.4 | 25.15±7.65 | 29.06±1.66 |
| RVm8 (F) | 70.20±0.42 | 66.47±1.27 | 59.37±0.45 |
| RLk4 (M) | 68.24±2.24 | 33.5±6.5 | 48.84±1.38 |
| RZs9 (F) | 46.93±3.51 | 126.75±65.98 | 83.95±2.78 |
| RJl8 (M) | 84.37±1.9 | 20.47±7.72 | 24.31±2.73 |
M = male rhesus monkey; F = female rhesus monkey. Data are expressed as mean ± SEM.
Sleep efficiency
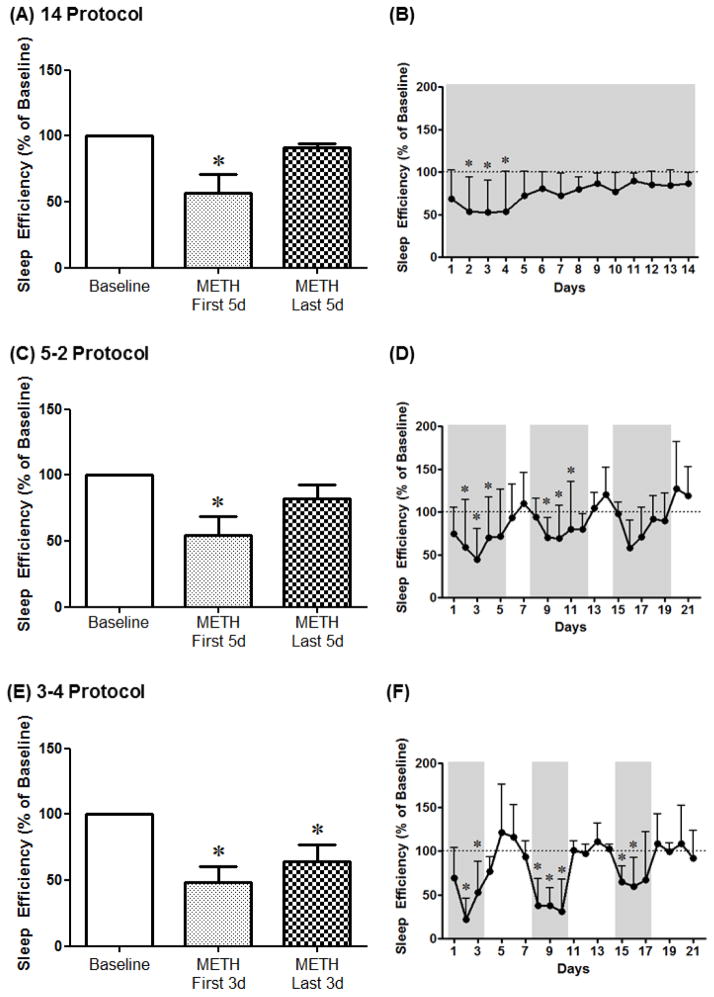
For the 14 Protocol, 1-way RM ANOVA corrected for multiple comparisons using Dunnett’s test showed a significant decrease in sleep efficiency on the first 5 days, but not the last 5 days, of methamphetamine SA compared to baseline [F(4,14)=7.93, p<0.05] (Fig. 2A). Analysis of the different days of methamphetamine SA indicated a significant decrease in sleep efficiency on days 2–4 of SA compared to baseline [F(4,74)=2.33, p<0.05] (Fig. 2B). For the 5–2 Protocol, 1-way RM ANOVA corrected for multiple comparisons using Dunnett’s test showed a significant decrease in sleep efficiency on the first 5 days, but not the last 5 days, of methamphetamine SA compared to baseline [F(4,14)=8.95, p<0.01] (Fig. 2C). Analysis of the different days of methamphetamine SA indicated a significant decrease in sleep efficiency on days 2–4 and 9–11 of SA compared to baseline [F(4,74)=2.33, p<0.05] (Fig. 2D). For the 3–4 Protocol, 1-way RM ANOVA corrected for multiple comparisons using Dunnett’s test showed a significant decrease in sleep efficiency for all methamphetamine SA blocks compared to baseline [F(4,14)=10.7, p<0.001] (Fig. 2E). Analysis of the different days of methamphetamine SA indicated a significant decrease in sleep efficiency on days 2–3, 8–10 and 15–16 of SA compared to baseline [F(4,74)=4.91, p<0.0001] (Fig. 2F).
Figure 2.
Sleep efficiency averaged across the first days (METH First 5d and METH First 3d) or the last days (METH Last 5d and METH Last 3d) of methamphetamine self-administration (0.03 mg/kg/infusion, i.v.) as well as across protocol days for the 14 Protocol (A, B), the 5–2 Protocol (C, D) and the 3–4 Protocol (E, F). Data are expressed as Mean ± SEM. Dotted lines represent baseline sleep efficiency (100%). *p<0.05 compared with baseline.
Sleep Latency
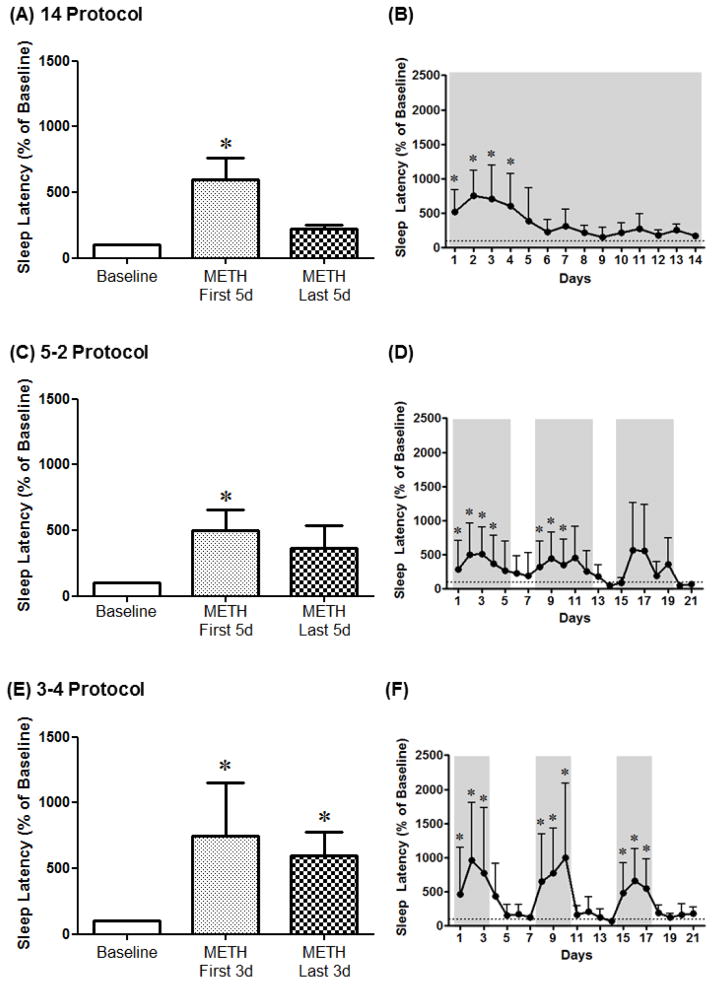
For the 14 Protocol, 1-way RM ANOVA corrected for multiple comparisons using Dunnett’s test showed a significant increase in sleep latency on the first 5 days, but not the last 5 days, of methamphetamine SA compared to baseline [F(4,14)=7.65, p<0.01] (Fig. 3A). Analysis of the different days of methamphetamine SA indicated a significant increase in sleep latency on days 1–4 of SA compared to baseline [F(4,74)=4.9, p<0.0001] (Fig. 3B). For the 5–2 Protocol, 1-way RM ANOVA corrected for multiple comparisons using Dunnett’s test showed a significant increase in sleep latency on the first 5 days, but not the last 5 days, of methamphetamine SA compared to baseline [F(4,14)=4.03, p<0.05] (Fig. 3C). Analysis of the different days of methamphetamine SA indicated a significant increase in sleep latency on days 1–4 and 8–10 of SA compared to baseline [F(4,74)=4.16, p<0.0001] (Fig. 3D). For the 3–4 Protocol, 1-way RM ANOVA corrected for multiple comparisons using Dunnett’s test showed a significant increase in sleep latency for all methamphetamine SA blocks compared to baseline [F(4,14)=3.79, p<0.05] (Fig. 3E). Analysis of the different days of methamphetamine SA indicated a significant increase in sleep latency on days 1–3, 8–10 and 15–17 of SA compared to baseline [F(4,74)=2.67, p<0.001] (Fig. 3F).
Figure 3.
Sleep latency averaged across the first days (METH First 5d and METH First 3d) or the last days (METH Last 5d and METH Last 3d) of methamphetamine self-administration (0.03 mg/kg/infusion, i.v.) as well as across protocol days for the 14 Protocol (A, B), the 5–2 Protocol (C, D) and the 3–4 Protocol (E, F). Data are expressed as Mean ± SEM. Dotted lines represent baseline sleep latency (100%). *p<0.05 compared with baseline.
Sleep Fragmentation
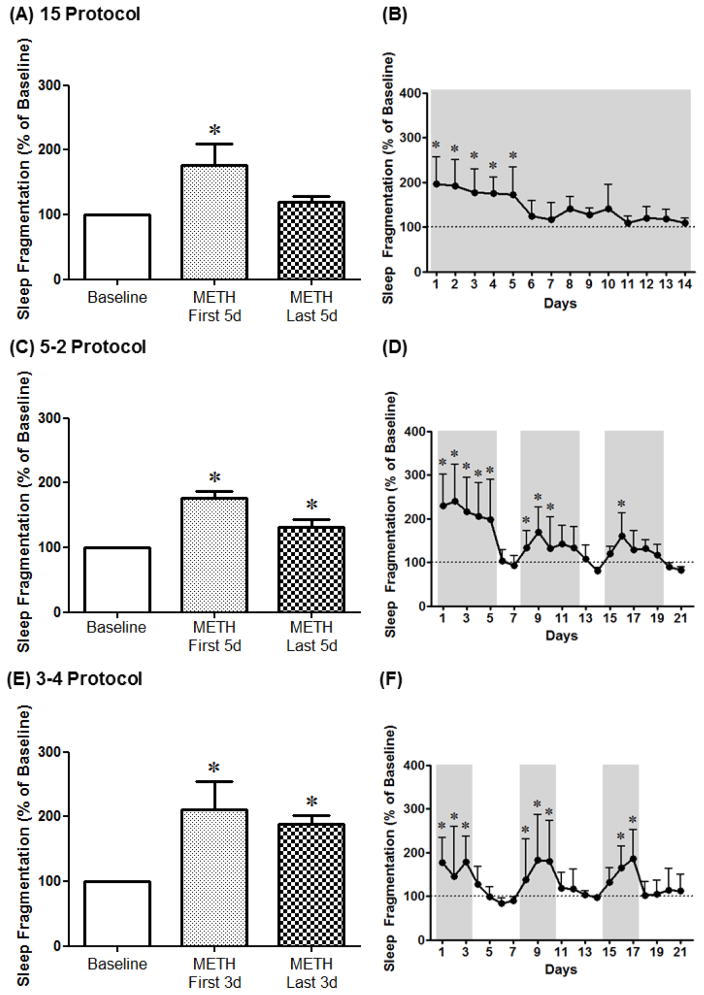
For the 14 Protocol, 1-way RM ANOVA corrected for multiple comparisons using Dunnett’s test showed a significant increase in sleep fragmentation on the first 5 days, but not the last 5 days, of methamphetamine SA compared to baseline [F(4,14)=3.75, p<0.05] (Fig. 4A). Analysis of the different days of methamphetamine SA indicated a significant increase in sleep fragmentation on days 1–5 of SA compared to baseline [F(4,74)=4.48, p<0.0001] (Fig. 4B). For the 5–2 Protocol, 1-way RM ANOVA corrected for multiple comparisons using Dunnett’s test showed a significant increase in sleep fragmentation for all methamphetamine SA blocks compared to baseline [F(4,14)=23.56, p<0.0001] (Fig. 4C). Analysis of the different days of methamphetamine SA indicated a significant increase in sleep fragmentation on days 1–5, 8–10 and 16 of SA compared to baseline [F(4,74)=6.03, p<0.0001] (Fig. 4D). For the 3–4 Protocol, 1-way RM ANOVA corrected for multiple comparisons using Dunnett’s test showed a significant increase in sleep fragmentation for all methamphetamine SA blocks compared to baseline [F(4,14)=6.76, p<0.05] (Fig. 4E). Analysis of the different days of methamphetamine SA indicated a significant increase in sleep fragmentation on days 1–3, 8–10 and 16–17 of SA compared to baseline [F(4,74)=2.59, p<0.01] (Fig. 4F).
Figure 4.
Sleep fragmentation averaged across the first days (METH First 5d and METH First 3d) or the last days (METH Last 5d and METH Last 3d) of methamphetamine self-administration (0.03 mg/kg/infusion, i.v.) as well as across protocol days for the 14 Protocol (A, B), the 5–2 Protocol (C, D) and the 3–4 Protocol (E, F). Data are expressed as Mean ± SEM. Dotted lines represent baseline sleep fragmentation (100%). *p<0.05 compared with baseline.
Daytime activity measures
Averaged daytime activity data (activity counts/hour) were combined across the 12h daytime period (7h–19h) and are presented as normalized data (percentage of baseline). Individual-subject baseline daytime activity are expressed as mean±SEM; individual subject codes followed by corresponding values were: ROf8: 6399±190; RVm8: 8595±478; RLk4: 12107±383; RZs9: 14545±508; RJl8: 11964±100. Daytime activity data are also presented as activity counts across the hours of the day after the SA sessions and before lights off (12h–18h).
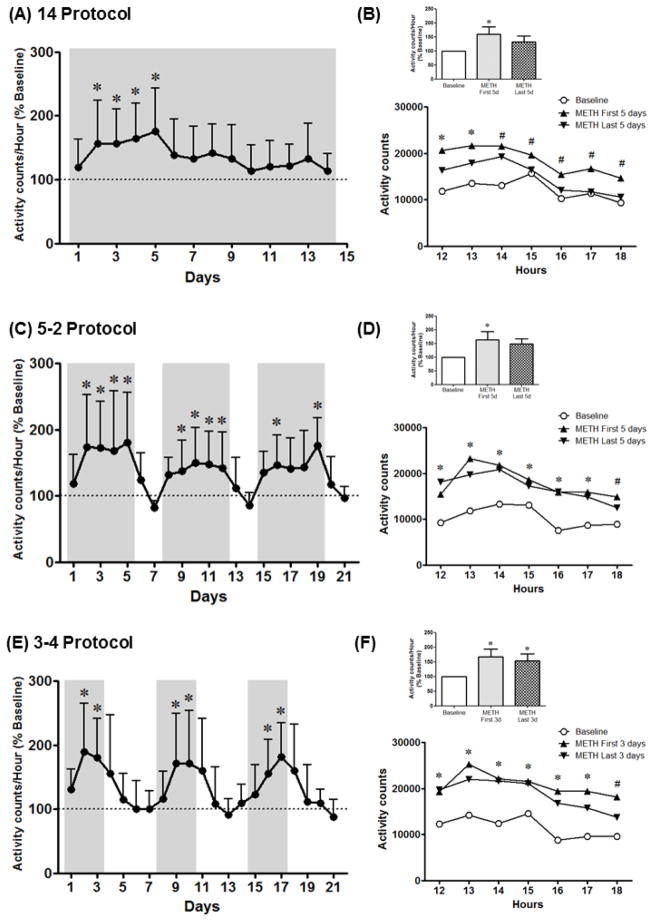
For the 14 Protocol, analysis of the different days of methamphetamine SA indicated a significant increase in activity counts/hour on days 2–5 of SA compared to baseline [F(4,74)=3.92, p<0.001] (Fig. 5A). One-way RM ANOVA corrected for multiple comparisons using Dunnett’s test of the averaged daytime activity data showed a significant increase in daytime activity on the first 5 days, but not the last 5 days, of methamphetamine SA compared to baseline [F(4,14)=4.35, p<0.05] (Fig. 5B). Two-way RM ANOVA of the activity counts at each hour during daytime indicated a significant effect of time [F(6,96)=11.67, p<0.0001] and treatment [F(4,16)=3.89, p<0.05], but no interaction. From hours 12–13, activity counts were higher for all methamphetamine SA blocks compared to baseline, while from hours 15–18 only activity counts for the first 5 days of methamphetamine SA were higher compared to baseline (Fig. 5B).
Figure 5.
Daytime activity across protocol days for the 14 Protocol (A), the 5–2 Protocol (C) and the 3–4 Protocol (E). Daytime activity averaged across the first days (METH First 5d and METH First 3d) or the last days (METH Last 5d and METH Last 3d) of methamphetamine self-administration (0.03 mg/kg/infusion, i.v.) for the daytime period (7h–19h) as well as across daytime hours (12h–18h) for the 14 Protocol (B), the 5–2 Protocol (D) and the 3–4 Protocol (F). Data are expressed as Mean ± SEM. Dotted lines represent baseline daytime activity (100%). *p<0.05 compared with baseline for *all data points or # METH First 5 days.
For the 5–2 Protocol, analysis of the different days of methamphetamine SA indicated a significant increase in activity counts/hour on days 2–5, 9–12, 16 and 19 of SA compared to baseline [F(4,74)=3.4, p<0.0001] (Fig. 5C). One-way RM ANOVA corrected for multiple comparisons using Dunnett’s test of the averaged daytime activity data showed a significant increase in daytime activity on the first 5 days, but not the last 5 days, of methamphetamine SA compared to baseline [F(4,14)=4.46, p<0.05] (Fig. 5D). Two-way RM ANOVA of the activity counts at each hour during daytime indicated a significant effect of time [F(6,96)=25.19, p<0.0001] and treatment [F(4,16)=3.9, p<0.05], but no interaction. From hours 12–17, activity counts were higher for all methamphetamine SA blocks compared to baseline, while for hour 18 only activity counts for the first 5 days of methamphetamine SA were higher compared to baseline (Fig. 5D).
For the 3–4 Protocol, analysis of the different days of methamphetamine SA indicated a significant increase in activity counts/hour on days 2–3, 9–10 and 16–17 of SA compared to baseline [F(4,74)=5.12, p<0.0001] (Fig. 5E). One-way RM ANOVA corrected for multiple comparisons using Dunnett’s test of the averaged daytime activity data showed a significant increase in daytime activity for all methamphetamine SA blocks compared to baseline [F(4,14)=6.0, p<0.05] (Fig. 5F). Two-way RM ANOVA of the activity counts at each hour during daytime indicated a significant effect of time [F(6,96)=9.65, p<0.0001] and treatment [F(4,16)=6.88, p<0.05], but no interaction. From hours 12–17 activity counts were higher for all methamphetamine SA blocks compared to baseline, while for hour 18 only activity counts for the 9 days and first 3 days of methamphetamine SA were higher compared to baseline (Fig. 5F).
Discussion
Results from the present study demonstrate that intravenous methamphetamine SA markedly disrupted sleep-like measures and increased daytime activity in rhesus monkeys. Tolerance developed to these effects with repeated methamphetamine intake exceeding 5 consecutive days. Inclusion of washout periods (2 or 4 days) between blocks of methamphetamine SA attenuated the development of tolerance over the days of methamphetamine SA, with longer breaks from methamphetamine intake being more effective in maintaining the sleep-disrupting and stimulant effects of methamphetamine.
During the first 3–5 days of methamphetamine SA, sleep-like behavior was disrupted in all 3 protocols, an effect that immediately dissipated after methamphetamine discontinuation (Protocols 5–2 and 3–4). These data are consistent with previous studies on the effects of stimulants on sleep. Studies from our group have shown that acute noncontingent amphetamine treatment disrupted sleep in rhesus monkeys (Murnane et al. 2013). We have also demonstrated that 5-day blocks of methamphetamine SA induced sleep disruption in rhesus monkeys, with sleep normalizing immediately after drug discontinuation (Andersen et al. 2013). Studies in healthy humans have also shown decreases in subjective and objective sleep measures after acute methamphetamine administration (Comer et al. 2001; Kirkpatrick et al. 2012a), with sleep returning to normal baseline levels (no evidence of residual effects) following active methamphetamine (Comer et al. 2001). Importantly, studies conducted in experienced methamphetamine users also found marked decreases in sleep measures after acute methamphetamine administration (Perez et al. 2008; Kirkpatrick et al. 2012b). These results indicate that frequent use does not blunt the acute drug effects on sleep, corroborating the present findings in monkeys with a long history of exposure to methamphetamine.
Methamphetamine SA also had marked stimulant effects on daytime activity. Subjects showed an increase in general activity from days 2 through 3–5 of drug intake. Although subjects did not express significantly higher daytime activity compared to baseline on the first day of methamphetamine SA, this effect was sensitized, with an increase in general daytime activity from days 2 through 3–5 of drug intake. The development of sensitization to the behavioral effects of psychostimulants has been extensively described in rodents, and is considered to be a hallmark of plasticity associated with drug abuse (Bradberry 2007). Our findings corroborate extensive literature on repeated and single dose-induced locomotor sensitization to amphetamines in rodents (Saito et al. 2014; Fukushiro et al. 2012; Chinen et al. 2006). The literature on behavioral sensitization to psychostimulants in nonhuman primates, on the other hand, is sparse and has relied mainly on the evaluation of behavioral measures progressing from lower to higher doses (Bradberry 2007). Our results add to the literature by showing the development of a rapid locomotor sensitization to the stimulant effects of methamphetamine in rhesus monkeys.
According to our results, tolerance developed to both the sleep-disrupting and daytime stimulant effects of methamphetamine with repeated drug intake exceeding 5 consecutive days (14 Protocol). A decrease in sleep disruption occurred despite stable intake, indicating that attenuated effects on sleep-like behavior and daytime activity were not a result of lower methamphetamine consumption. In addition, although Protocol 3–4 resulted in less methamphetamine exposures (9 exposures compared to 14 and 15 for Protocols 14 and 5–2, respectively), that is not sufficient to explain the lack of tolerance under Protocol 3–4. On the 9th day of methamphetamine SA (day 9 of the 14 Protocol, day 14 of the 5–2 Protocol and day 17 of the 3–4 Protocol), the effects of methamphetamine on sleep-like behavior were still present only when animals were submitted to the 3–4 Protocol. Our findings corroborate previous clinical studies showing tolerance to methamphetamine-induced sleep disruption (Comer et al. 2001; Kirkpatrick et al. 2012a) and daytime stimulant effects (Kelly et al. 1991) following repeated administration of low single oral doses in humans. Studies have also shown tolerance to other behavioral effects of amphetamine-type stimulants, such as decreased anorexic effects (Bittner et al. 1981; Kraeuchi et al. 1985; White et al. 2010), and decreased effectiveness in children chronically treated for ADHD (Ross et al. 2002).
The development of tolerance to the anorexic and attention-related effects of amphetamines can be prevented by increasing the drug dosage (Ross et al. 2002; White et al. 2010). The same seems to apply to the sleep-disrupting and stimulant effects of amphetamines. A previous study conducted in children with ADHD has shown that when subjects were given amphetamine daily with dose increasing weekly for 3 weeks, amphetamine treatment had marked long-lasting dose-dependent effects on sleep, with no signs of tolerance (Santisteban et al. 2014). Mitler and colleagues (1993) have shown that treatment with methamphetamine also caused a dose-dependent stimulant effect on daytime behavior and improvement in performance tasks in both narcoleptics and control individuals with no signs of tolerance. Because our experiments were conducted with contingent methamphetamine administration, we were not able to evaluate the effects of increasing drug dosage on the development of tolerance to the effects of methamphetamine. Nevertheless, our data provide primary evidence that inclusion of washout periods between methamphetamine SA also prevents the development of tolerance to both the sleep-disrupting and the daytime stimulant effects of methamphetamine over the days of drug SA.
A recent study that seems to be the first report of methamphetamine pharmacokinetic parameters in rhesus monkeys indicated that the half-life of methamphetamine is of approximately 3.2h in rhesus monkeys when given intramuscularly, while plasma levels of the methamphetamine metabolite amphetamine peak at 5h post-methamphetamine (Banks et al. 2016). On the other hand, methamphetamine is known to have a half-life of approximately 12h in humans for smoked, intravenous and oral drug administration, with a peak in amphetamine levels 10–24 hours after methamphetamine and still detectable after 48 hours in humans (Cook et al. 1992, 1993). Although there are no reports of intramuscular methamphetamine pharmacokinetics in humans for comparison, human studies with intravenous methamphetamine administration suggest that the effects of methamphetamine and/or its metabolite amphetamine under our experimental conditions could be longer than those observed by Banks and colleagues (2016). It is important to note, however, that although the development of tolerance to the sleep-disrupting effects of methamphetamine was accompanied by tolerance to the daytime stimulant effects of the drug, these effects were not a function of drug intake in the present study. Further studies are necessary in order to investigate the mechanisms underlying the wake-promoting and sleep-disrupting effects of methamphetamine.
In summary, the present study provides evidence of the development of tolerance to the stimulant and sleep-disrupting effects of methamphetamine, and that inclusion of washout periods between blocks of drug intake can prevent this phenomenon. It has been previously reported that the average frequency of use of methamphetamine among chronic users is about 5 times per week, being lower in males (about 2 times per week) than in females (about 6 times per week) (Suwannachom et al. 2015). In another study conducted with methamphetamine users, 75.6% out of 189 participants reported using methamphetamine no more frequently than 9 times per month (Ding et al. 2014). Preclinical evidence from the present study indicate that the pattern and frequency of use of methamphetamine in humans would be associated with long-term effects on daytime activity and sleep. Because sleep impairment is considered a risk factor for drug relapse and for the development of drug abuse (Ford and Kamerow 1989; Brower and Perron 2010; Wong et al. 2010; Hasler et al. 2012), treatment strategies should focus on sleep as a contributing factor for methamphetamine abuse.
Acknowledgments
The authors thank Juliet Brown, Lisa Neidert and Melis Odabas-Geldiay for their excellent assistance with the experiments. This research was supported by USPHS Grants DA10344 (LLH), DA031246 (LLH) and ODP51OD11132 (Yerkes), AFIP and FAPESP Grant 2015/25482-3 (LFB).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Andersen ML, Diaz MP, Murnane KS, Howell LL. Effects of methamphetamine self-administration on actigraphy-based sleep parameters in rhesus monkeys. Psychopharmacology (Berl) 2013;227:101–7. doi: 10.1007/s00213-012-2943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology (Berl) 2010;210:439–48. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Smith DA, Kisor DF, Poklis JL. Relationship between discriminative stimulus effects and plasma methamphetamine and amphetamine levels of intramuscular methamphetamine in male rhesus monkeys. Pharmacol Biochem Behav. 2016;141:58–65. doi: 10.1016/j.pbb.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barateau L, Lopez R, Dauvilliers Y. Management of Narcolepsy. Curr Treat Options Neurol. 2016;18:43. doi: 10.1007/s11940-016-0429-y. [DOI] [PubMed] [Google Scholar]
- Berro LF, Andersen ML, Tufik S, Howell LL. Actigraphy-based sleep parameters during the reinstatement of methamphetamine self-administration in rhesus monkeys. Exp Clin Psychopharmacol. 2016;24:142–6. doi: 10.1037/pha0000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner SE, Wagner GC, Aigner TG, Seiden LS. Effects of a high-dose treatment of methamphetamine on caudate dopamine and anorexia in rats. Pharmacol Biochem Behav. 1981;14:481–6. doi: 10.1016/0091-3057(81)90306-3. [DOI] [PubMed] [Google Scholar]
- Bonnefond A, Tassi P, Roge J, Muzet A. A critical review of techniques aiming at enhancing and sustaining worker’s alertness during the night shift. Ind Health. 2004;42:1–14. doi: 10.2486/indhealth.42.1. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology (Berl) 2007;191:705–17. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- Briars L, Todd T. A Review of Pharmacological Management of Attention-Deficit/Hyperactivity Disorder. J Pediatr Pharmacol Ther. 2016;21:192–206. doi: 10.5863/1551-6776-21.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med Hypotheses. 2010;74:928–933. doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen CC, Faria RR, Frussa-Filho R. Characterization of the rapid-onset type of behavioral sensitization to amphetamine in mice: role of drug-environment conditioning. Neuropsychopharmacology. 2006;31:151–9. doi: 10.1038/sj.npp.1300789. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Effects of repeated oral methamphetamine administration in humans. Psychopharmacology (Berl) 2001;155:397–404. doi: 10.1007/s002130100727. [DOI] [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-metham-phetamine hydrochloride. Drug Metab Dispos. 1993;21:717–723. [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Sadler BM, Hill JM, Voyksner RD, Pugh DE, White WR, Perez-Reyes M. Pharmacokinetics of oral methamphetamine and effects of repeated daily dosing in humans. Drug Metab Dispos. 1992;20:856–862. [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Ding Y, Lin H, Zhou L, Yan H, He N. Adverse childhood experiences and interaction with methamphetamine use frequency in the risk of methamphetamine-associated psychosis. Drug Alcohol Depend. 2014;142:295–300. doi: 10.1016/j.drugalcdep.2014.06.042. [DOI] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Fukushiro DF, Josino FS, Saito LP, Costa JM, Zanlorenci LH, Berro LF, Fernandes-Santos L, Morgado F, Mári-Kawamoto E, Frussa-Filho R. Differential effects of intermittent and continuous exposure to novel environmental stimuli on the development of amphetamine-induced behavioral sensitization in mice: implications for addiction. Drug Alcohol Depend. 2012;124:135–41. doi: 10.1016/j.drugalcdep.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Girotto E, Mesas AE, de Andrade SM, Birolim MM. Psychoactive substance use by truck drivers: a systematic review. Occup Environ Med. 2014;71:71–6. doi: 10.1136/oemed-2013-101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck DM, Brecht ML, Lovinger K. Mortality, causes of death, and health status among methamphetamine users. J Addict Dis. 2015;34:88–100. doi: 10.1080/10550887.2014.975610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Intravenous drug self-administration in nonhuman primates. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; Boca Raton, FL: 2001. pp. 91–110. [Google Scholar]
- Huang YS, Tsai MH, Guilleminault C. Pharmacological treatment of ADHD and the short and long term effects on sleep. Curr Pharm Des. 2011;17:1450–8. doi: 10.2174/138161211796197179. [DOI] [PubMed] [Google Scholar]
- Huang YS, Tsai MH. Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge. CNS Drugs. 2011;25:539–54. doi: 10.2165/11589380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Fischman MW. The effects of repeated amphetamine exposure on multiple measures of human behavior. Pharmacol Biochem Behav. 1991;38:417–26. doi: 10.1016/0091-3057(91)90301-h. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Levin FR, Foltin RW, Hart CL. Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology (Berl) 2012a;219:191–204. doi: 10.1007/s00213-011-2390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2012b;219:109–22. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuchi K, Rudolph K, Wirz-Justice A, Feer H. Similarities in feeding behavior of chronic methamphetamine treated and withdrawn rats to VMH lesioned rats. Pharmacol Biochem Behav. 1985;23:917–20. doi: 10.1016/0091-3057(85)90092-9. [DOI] [PubMed] [Google Scholar]
- Mahoney JJ, 3rd, De La Garza R, 2nd, Jackson BJ, Verrico CD, Ho A, Iqbal T, Newton TF. The relationship between sleep and drug use characteristics in participants with cocaine or methamphetamine use disorders. Psychiatry Res 2014. 1993;219:367–71. doi: 10.1016/j.psychres.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler MM, Hajdukovic R, Erman MK. Treatment of narcolepsy with methamphetamine. Sleep. 16:306–17. [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler TI, Kapur VK, Brown T, Swick TJ, Alessi C, Aurora RN, Boehlecke B, Chesson AL, Jr, Friedman L, Maganti R, Owens J, Pancer J, Zak R. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–11. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Andersen ML, Rice KC, Howell LL. Selective serotonin 2A receptor antagonism attenuates the effects of amphetamine on arousal and dopamine overflow in non-human primates. J Sleep Res. 2013;22:581–8. doi: 10.1111/jsr.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez AY, Kirkpatrick MG, Gunderson EW, Marrone G, Silver R, Foltin RW, Hart CL. Residual effects of intranasal methamphetamine on sleep, mood, and performance. Drug Alcohol Depend. 2008;94:258–62. doi: 10.1016/j.drugalcdep.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel N, Rohleder NH, Wagenpfeil S, Haertel-Petri R, Kesting MR. Evaluation of methamphetamine-associated socioeconomic status and addictive behaviors, and their impact on oral health. Addict Behav. 2015;50:182–7. doi: 10.1016/j.addbeh.2015.06.040. [DOI] [PubMed] [Google Scholar]
- Ross DC, Fischhoff J, Davenport B. Treatment of ADHD when tolerance to methylphenidate develops. Psychiatr Serv. 2002;53:102. doi: 10.1176/appi.ps.53.1.102. [DOI] [PubMed] [Google Scholar]
- Saito LP, Fukushiro DF, Hollais AW, Mári-Kawamoto E, Costa JM, Berro LF, Aramini TC, Wuo-Silva R, Andersen ML, Tufik S, Frussa-Filho R. Acute total sleep deprivation potentiates amphetamine-induced locomotor-stimulant effects and behavioral sensitization in mice. Pharmacol Biochem Behav. 2014;117:7–16. doi: 10.1016/j.pbb.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Santisteban JA, Stein MA, Bergmame L, Gruber R. Effect of extended-release dexmethylphenidate and mixed amphetamine salts on sleep: a double-blind, randomized, crossover study in youth with attention-deficit hyperactivity disorder. CNS Drugs. 2014;28:825–33. doi: 10.1007/s40263-014-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwannachom N, Thananchai T, Junkuy A, O’Brien TE, Sribanditmongkol P. Duration of detection of methamphetamine in hair after abstinence. Forensic Sci Int. 2015;254:80–6. doi: 10.1016/j.forsciint.2015.06.030. [DOI] [PubMed] [Google Scholar]
- White W, Hundley MB, White IM. The effects of dose and repeated administration on the longer-term hypophagia produced by amphetamine in rats. Pharmacol Biochem Behav. 2010;97:384–91. doi: 10.1016/j.pbb.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Nigg JT, Zucker RA. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcohol Clin Exp Res. 2010;34:1033–1044. doi: 10.1111/j.1530-0277.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]