Abstract
The purpose of this study was to determine the effects of ADRB2 genotypes on muscle function (absolute power and relative power) in healthy subjects. We performed genotyping of the ADRB2 (amino acid 16) and high-intensity, steady-state exercise on 77 healthy subjects (AA = 18, AG = 25, GG = 34). There were no differences between genotype groups in age, height, weight, or BMI (age = 28.9 ± 5.7yrs, 27.9 ± 5.7yrs, 29.2 ± 5.9yrs, height = 170.7 ± 8.6cm, 174.9 ± 8.7cm, 173.4 ± 9.6cm, weight = 68.5 ± 13.0kg, 75.0 ± 12.9kg, 74.4 ± 12.9kg, and BMI = 23.4 ± 3.9, 24.4 ± 2.9, 24.7 ± 3.4, for AA, AG, and GG, respectively). The genotype groups differed significantly in watts, and watts/VO2 with heavy exercise (watts = 186.3± 54.6, 237.8 ± 54.4, 219.4 ± 79.5, watts/VO2 = 0.08 ± 0.006, 0.09 ± 0.005, 0.08 ± 0.006). There was a trend towards significance (p=0.058) for watts/kg (2.7 ± 0.4, 3.2 ± 0.5, 2.9 ± 0.8, for AA, AG, and GG, respectively). These data suggest that genetic variation of the ADRB2 may influence relative strength in healthy subjects and may become an important genetic determinant of muscular strength and functional capacity in patients with diseases that result in a loss of muscle strength.
Keywords: exercise, ADRB2 polymorphism, strength, beta-2 genotype, beta-2 receptor
INTRODUCTION
Muscular strength and power are important aspects to athletic performance. β2 agonist supplementation has been shown to increase power and increases the rates of glycolysis and glycogenolysis during sprinting in men (16). Further, Kalsen et al (2015) also demonstrated increased mean and peak power during the sprint with increased anaerobic adenosine-triphosphate (ATP) utilization following β-agonist administration. In this same study, β-agonist administration preserved whole muscle ATP concentrations with no difference in phosphocreatine breakdown. These findings suggest the β2 adrenergic receptors (ADRB2) influence anaerobic power and capacity, possibly improving anaerobic performance in power athletes.
The β2 adrenergic receptor plays a functional role in muscle size, strength and muscle regeneration (7, 30). Church et al, (2014) demonstrated reduced peak twitch force, rate of contraction, maximal force along with significantly reduced rates of regeneration in ADRB2 receptor knockout mice compared to controls. Further, supplementation with β2 agonists has demonstrated enhanced sarcoplasmic reticulum calcium release rates, maximal voluntary contraction strength and peak Wingate power in trained human and rat models (5, 14, 25). Hodge et al, (2002), also demonstrated that denervation-induced atrophy was attenuated through β2 agonist treatment in rats. These data suggest the ADRB2 regulates muscular size, strength, contractility and protects against denervation-induced atrophy, suggesting ADRB2 may play a functional role in reducing disuse atrophy associated with injury.
The mechanisms by which ADRB2 stimulation may increase muscle size, strength, and contractility are associated with its role in internal cell signalling. The activation of the ADRB2 results in the formation of adenosine 3′-5′ monophosphate (cAMP), a secondary messenger that plays many roles in the body. An increase in ADRB2 density has been correlated with increased cAMP accumulation in animal models (11, 30). Skeletal muscle cAMP signaling is shown to regulate contractility, sarcoplasmic calcium dynamics, and recovery from sustained contractile activity. The net result of cAMP activation is characterized by increased contractile force and rapid recovery of ion balance.
Further, in rodent DMD models, cAMP production was shown to slow degeneration as well as promote regeneration of skeletal muscles (2). This previous work suggests individuals with the more functional variant of the ADRB2 may have increased cAMP accumulation, resulting in improved skeletal muscle contractile activity and ability to recover as well as an attenuated degeneration of skeletal muscle. Multiple polymorphisms of the ADRB2 have been identified as including a glycine (Gly) for arginine (Arg) substitution at amino acid 16. The Gly16 polymorphism has been shown to have higher receptor density on lymphocytes, be more resistant to receptor down regulation, and functionally demonstrate higher cardiac output, stroke volume, left ventricular function, ejection fraction and vascular function when compare to the Arg16 genotype in humans (9, 13, 32, 34, 35).
Although not specifically studied previously, demographic data from previous studies demonstrate that subjects with the Gly16 polymorphism tend to have a greater body mass index, despite higher fitness levels, possibly suggesting greater muscle mass. Therefore, the Gly16 polymorphism may not only have a protective effect on the cardiac muscles, but may also attenuate skeletal muscle degeneration through other down stream mechanisms.
Although the clinical implications of ADRB2 genotypes on muscular development and strength are important, the influence of ADRB2 genotype on performance may identify novel supplementation for improvement of sports performance. Currently, little research has investigated the direct effect of ADRB2 genotype on skeletal muscle function. Therefore, the purpose of this study was to determine the effects of ADRB2 genotypes on muscle function (absolute power and relative power) in healthy subjects.
METHODS
Experimental Approach to the Problem
This study used a one way ANOVA to compare genotype groups for indices of intensity, power (watts, watts/kg, and watts/kg), and perceived intensity (watts/hr) at high intensity cycling for healthy, untrained subjects. Peak measures were compared for genotype groups for dependent variable measures. Subjects attended three sessions of testing with 72 hours between sessions one and two on a controlled low-sodium diet and at least 24 hours between sessions two and three.
Subjects
Data analyzed for this manuscript were part of a larger study on ADRB2 genotypes and cardiopulmonary function at rest and during exercise but the data have not been assessed as presented in the present study (32, 33). This study received Institutional Board approval for the research and appropriate consent has been obtained pursuant to law and the subjects were informed of the benefits and risks of the investigation prior to signing an institutionally approved informed consent document to participate. Seventy-seven untrained subjects, ages 20 to 40, agreed to participate and were genotyped for Arg16Gly polymorphisms of the ADRB2. Individuals who were homozygous for arginine (ArgArg, n = 18), glycine (GlyGly, n = 34) or heterozygous (ArgGly, n = 25) at codon 16 agreed to participate in the study. All subjects were healthy non-smokers and not on medication.
Procedures
Subjects underwent baseline screening tests including pulmonary function testing, an incremental cycle ergometry test to exhaustion on a lode cycle ergometer, a blood draw for a complete blood count (to rule out anemia) and, in women, a pregnancy test. The baseline exercise study served as an initial familiarization session, was used to determine work intensities for subsequent sessions, and acted as a screening study to rule out myocardial ischemia and abnormal arrhythmias. Following these initial tests, subjects met with the Clinical Research Center (CRC) nutritionist and were put on a controlled sodium diet (3450 mg day–1) for 3 days with a 24-h urine collection to confirm sodium intake. This controlled sodium diet was used because previous studies have suggested that the ADRB2 may be sensitive to changes in dietary sodium (31, 18). Subjects subsequently returned to the CRC on two occasions for exercise testing while maintaining a salt neutral diet.
The next session consisted of a cycle ergometry test similar to the first visit but with the additional measurement of Q using a previously validated open-circuit acetylene uptake method (15). This session served as a further familiarization with the measurements to be made on the final study day and also allowed for confirmation of workloads for the final visit.
On the last study visit, resting measurements of Q, HR, SV and arterial BP were made. Subjects then exercised for 9 min at ~40% and 9 min at ~75% of their peak workload achieved during the initial exercise studies while measurements were repeated every 2–3 min. Nine minutes of exercise was performed because pilot data suggested that this was an adequate time frame to obtain three sets of measures and brought the subjects close to exhaustion with the higher workload. All visits were conducted in the morning to account for testing variability.
ADRB2 Genotyping
β2 adrenergic receptor genotyping was PCR-based according to methods of Bray et al (2000). Buffy coat, obtained from whole blood collected on EDTA, was extracted using the Gentra Puregene DNA Isolation Kit (Gentra Systems Inc., Minneapolis, MN, USA). The PCR reaction was conducted according to standard methods, using the following primer sequences (e.g. for Arg16Gly): (forward) 5′-AGC CAG TGC GCT TAC CTG CCA GAC-3′ (at −32) and (reverse) 3′-CA TGG GTA CGC GGC CTG GTG CTG CAG TGC-5′, resulting in a PCR product 107 base pairs in length. The reaction included 30 ng of DNA, 1.5 mM magnesium chloride, 0.5 U taq polymerase (Invitrogen, Carlsbad, CA, USA), 8.5% DMSO and standard concentrations of nucleotides and buffer in a 20 μl reaction volume. After initial denaturation at 94°C for 4 min, the fragments were amplified by 35 cycles of 1 min at 94°C, 1 min at 61°C, 1 min at 72°C, followed by 5 min at 72°C and 5 min at 98°C. The amplicons were then digested by exposure to 5 U of the restriction enzyme KpnI, followed by electrophoretic separation on 3% aragose gels, staining with ethidium bromide and visualization using UV light. The ArgArg homozygous genotype is represented by a single 107 bp band, the ArgGly group is represented by 25, 82 and 107 bp bands, and the GlyGly homozygous group by 82 and 25 bp bands.
Statistical Analyses
All statistical comparisons were made using a statistical software package (SPSS; SPSS Inc; Chicago, IL, version 19). Group demographics were compared with a one-way analysis of variance (ANOVA) using an α level of 0.05 to determine significance. Genotype differences in performance were compared with an ANOVA using a Tukey post hoc test to detect differences among the specific genotype groups. An α level of 0.05 was used for the ANOVA and post hoc analyses.
RESULTS
Subject Characteristics
There was no difference between genotype groups in age, weight, height, body mass index (BMI), or body surface area (BSA) (Table 1).
Table 1.
Subject characteristics (mean ± standard deviation, N or p-value)
| Study Demographics | ||||
|---|---|---|---|---|
|
| ||||
| N | Mean | SD | p-value | |
| Age (years) | ||||
| Arg/Arg | 18 | 28.9 | 5.67 | 0.708 |
| Arg/Gly | 24 | 27.9 | 5.74 | |
| Gly/Gly | 34 | 29.2 | 5.94 | |
| Total | 76 | 28.7 | 5.76 | |
|
| ||||
| Height (cm) | ||||
| Arg/Arg | 18 | 170.8 | 8.61 | 0.341 |
| Arg/Gly | 24 | 175 | 8.79 | |
| Gly/Gly | 34 | 173.5 | 9.67 | |
| Total | 76 | 173.3 | 9.17 | |
|
| ||||
| Weight (kg) | ||||
| Arg/Arg | 18 | 68.5 | 13.06 | 0.214 |
| Arg/Gly | 24 | 75.1 | 12.96 | |
| Gly/Gly | 34 | 74.4 | 12.93 | |
| Total | 76 | 73.2 | 13.07 | |
|
| ||||
| BMI (kg/m2) | ||||
| Arg/Arg | 18 | 23.4 | 3.85 | 0.448 |
| Arg/Gly | 24 | 24.4 | 2.91 | |
| Gly/Gly | 34 | 24.7 | 3.39 | |
| Total | 76 | 24.3 | 3.35 | |
|
| ||||
| BSA (m2) | ||||
| Arg/Arg | 18 | 1.79 | 0.19 | 0.189 |
| Arg/Gly | 24 | 1.90 | 0.20 | |
| Gly/Gly | 34 | 1.88 | 0.19 | |
| Total | 76 | 1.87 | 0.20 | |
|
| ||||
| VO2 (mL/min) | ||||
| Arg/Arg | 18 | 2257.83 | 761.19 | 0.152 |
| Arg/Gly | 24 | 2712.12 | 629.37 | |
| Gly/Gly | 34 | 2613.41 | 872.19 | |
| Total | 76 | 2562.34 | 784.55 | |
|
| ||||
| VO2/KG | ||||
| Arg/Arg | 18 | 32.35 | 5.98 | 0.200 |
| Arg/Gly | 24 | 36.29 | 5.61 | |
| Gly/Gly | 34 | 35.11 | 8.50 | |
| Total | 76 | 34.85 | 7.18 | |
Arg/Arg = genotype (homozygous for ADRB2 resulting in arginine at amino acid 16), Arg/Gly = genotype (heterozygous for ADRB2 resulting in one arginine and one glycine at amino acid 16), and Gly/Gly = genotype (homozygous for ADRB2 resulting in glycine at amino acid 16). BMI = body mass index; BSA = body surface area; VO2 = maximal oxygen consumption; VO2/KG = maximal oxygen consumption corrected for kilogram. There were no statistically significant differences in demographic data.
Power Measures
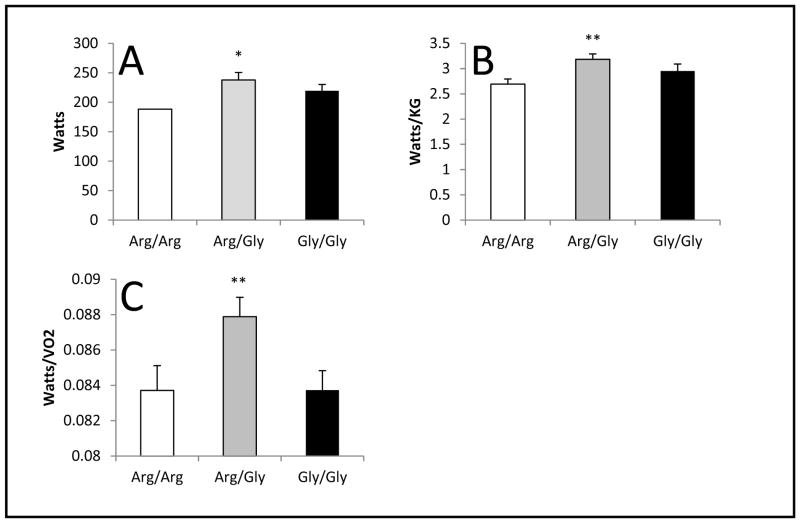
There were no differences in any of the power parameters (watts, watts/VO2, watts/kg) with light exercise. With heavy exercise, there were significant effects of genotype on power parameters (pANOVE<0.05) (Figure 1). Specifically, following post-hoc analysis, it was determined that the Arg/Gly group achieved significantly higher watts (p=0.04) than the Arg/Arg group (237.8 ± 54.4, 186.3 ± 54.6, for AG and AA respectively, SE=20.67, 95% CI (2.00, 100.82)). Additionally, the Arg/Gly group had significantly greater relative power as measured by watts/kg (p=0.046) than the Arg/Arg group (3.2 ± 0.5, 2.7 ± 0.4, for AG and AA respectively, SE=0.202, 95% CI (0.007, 0.97)). Further, the Arg/Gly group demonstrated significantly greater watts/VO2 (p=0.034) than the Gly/Gly group (0.09 ± 0.005, 0.08 ± 0.006, for AG and GG respectively, SE=0.002, 95% CI (0.0003, 0.008)). There was no difference in watts/VO2 between the Arg/Gly and Arg/Arg groups, nor were there any differences in VO2 between genotype groups.
Figure 1.
Watts, watts/kg and watts/VO2: Arg/Arg vs Arg/Gly vs Gly/Gly during peak exercise
Panels depict the maximum values during peak exercise for A) Watts, B) Relative power (Watts/KG), and C) Muscular efficiency (Watts/VO2). The error bars represent the SE of the mean.
*p<0.05 for Arg/Arg vs Arg/Gly pairwise comparison.
**p<0.05 for Arg/Gly vs Gly/Gly pairwise comparison.
Intensity Measures
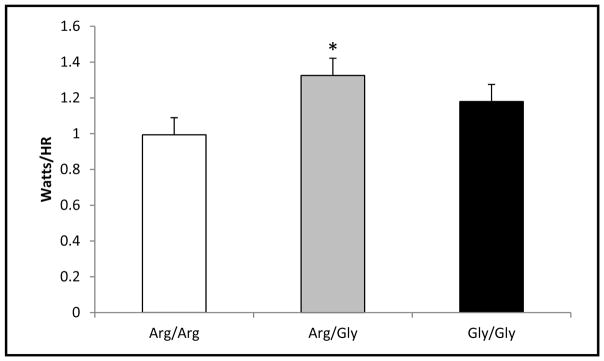
Similar to power indices, there were significant genotype differences in indices of relative and perceived intensity. The Arg/Gly group demonstrated significantly higher watts/hr (p=0.019) than the Arg/Arg group (1.3 ± 0.3, 0.99 ± 0.3, for AG and AA respectively, SE=0.119, 95% CI (0.046, 0.62)) (Table 2). This is likely due to the ability of the Arg/Gly group to exercise at a lower relative intensity (higher peak watts while maintaining lower heart rates) (p=0.038; 181 ± 9.7, 189 ± 9.9, for AG and AA respectively, SE-2.97, 95% CI (0.35, 14.57)). Further, the Arg/Gly group reported lower RPEs (p=0.032) than the Arg/Arg group (18.6 ± 0.51, 18.9 ± 0.24, for AG and AA respectively, SE=0.15, 95% CI (0.03, 0.74)) despite producing higher watts (Figure 2).
Table 2.
Indices of intensity (mean ± standard deviation, N or p-value) during peak exercise
| Indices of Intensity | ||||
|---|---|---|---|---|
|
| ||||
| N | Mean | SD | p-value | |
| HR (beats/min) | ||||
| Arg/Arg | 18 | 188.5 | 9.89 | 0.024* |
| Arg/Gly | 24 | 181.0 | 9.68 | |
| Gly/Gly | 34 | 186.9 | 9.43 | |
| Total | 76 | 185.4 | 9.98 | |
|
| ||||
| RPE | ||||
| Arg/Arg | 18 | 18.94 | 0.24 | 0.039* |
| Arg/Gly | 24 | 18.56 | 0.51 | |
| Gly/Gly | 34 | 18.76 | 0.55 | |
| Total | 76 | 18.74 | 0.49 | |
|
| ||||
| RER | ||||
| Arg/Arg | 18 | 1.16 | 0.015 | 0.836 |
| Arg/Gly | 24 | 1.15 | 0.010 | |
| Gly/Gly | 34 | 1.15 | 0.010 | |
| Total | 76 | 1.15 | 0.006 | |
|
| ||||
| RR (breaths/min) | ||||
| Arg/Arg | 18 | 43.17 | 2.10 | 0.295 |
| Arg/Gly | 24 | 40.84 | 1.38 | |
| Gly/Gly | 34 | 39.74 | 1.21 | |
| Total | 76 | 40.90 | 0.86 | |
HR = heart rate; RPE = rating of perceived exertion; RER = respiratory exchange ratio; RR = respiratory rate.
p< 0.05
Figure 2.
Watts/hr: Arg/Arg vs Arg/Gly vs Gly/Gly during peak exercise
Depicts maximal watts corrected for heart rate at peak exercise. The error bars represent the SE of the mean.
*p<0.05 for Arg/Arg vs Arg/Gly pairwise comparison.
DISCUSSION
In the present study we demonstrate that genetic variations of the ADRB2 are associated with differences in muscular power, efficiency and intensity. Individuals with one arginine and one glycine allele (Arg/Gly) demonstrated significantly higher peak power (watts), relative power (watts/kg), muscular efficiency (watts/VO2) and exercise intensity (watts/hr) during heavy, steady-state exercise. Interestingly, despite producing higher peak watts, the Arg/Gly group also reported significantly lower rating of perceived exertion (RPE). Similar to previous observations, although not statistically significant, individuals with at least one glycine allele (Arg/Gly and Gly/Gly) were heavier than those homozygous for arginine (68.5 ± 13.1, 75.1 ± 12.9, 74.4 ± 12.9, for AA, AG and GG respectively). These findings may be due to the regulation of several downstream mechanisms by the ADRB2.
There are several pathways by where activation of the ADRB2 may regulate skeletal muscle size and strength. One such pathway is through phosphorylation by catecholamines. ADRB2s are g-coupled protein receptors where upon binding of a catecholamine to the receptor stimulates a dissociation of the guanine protein which phosphorylates adenylyl cyclase (AC). Adenylyl cyclase produces cAMP which phosphorylates protein kinase A (PKA) into its active form (2).
The first process whereby ADRB2 activation may increase muscular size and strength is binding of a catecholamine to the ADRB2 resulting in dissociation of the guanine-linked subunits. Further, previous work suggests the Gαi-linked Gβγ subunits activate the phosphoinositide 3-kinase-protein kinase-B (PI3K-AKT) signaling pathway (28). The PI3K-AKT signaling pathway has been shown to regulate protein synthesis, gene transcription, cell proliferation, and cell survival (3, 12). Although there are three distinct isoforms of AKT, the predominant skeletal muscle isoform is AKT1. It has been demonstrated that AKT1 inhibits the forkhead box O transcription factors (FOXO) (36). This is significant because FOXO has been implicated in muscle atrophy (17). Thus, by phosphorylating and inactivating FOXO, AKT1 blocks the induction of FOXO-mediated atrophy signaling. Additionally, ADRB2 activation has been found to reduce the expression of FOXO-mediated atrophy signaling in skeletal muscle from denervated and hindlimb-suspended rats (17). This suggests that the ADRB2 plays a functional role in attenuating skeletal muscle atrophy in addition to promoting skeletal muscle growth. Further study of the genotypes in disease models is certainly warranted.
Another process by which ADRB2 stimulation may increase muscular size and strength is by phosphorylation of PKA via cAMP. PKA phosphorylation into its active state results in dissociation of the PKA subunits. It has been demonstrated that the free C-subunits of PKA diffuse passively into the nucleus, where they have the capability for direct phosphorylation of multiple regulator genes of the cAMP response element binding protein (CREB) (6). CREB is a nuclear transcription factor that is universally expressed and has many processes, including cell proliferation, differentiation, adaptation, and survival (21). Current research suggests CREB plays a role in mediating the activity of the transcription factor myocyte enhancer factor-2 (MEF2), a family of transcription factors that play a key role in the differentiation of muscle cells (1). ADRB2 activation is also associated with an increased expression of neuron-derived orphan receptor-1 (NOR-1) (24). Pearen et al (2006) also demonstrated siRNA-mediated inhibition of NOR-1 expression was associated with a significant increase in the levels of myostatin mRNA. Myostatin is a member of the transforming growth factor-β superfamily and the primary negative regulator of muscle mass (24). This suggest the ADRB2 plays a functional role in the regulation of muscle mass through increased NOR-1 expression resulting in decreased myostatin levels promoting skeletal muscle growth. Collectively, these data suggest the ADRB2 plays multiple roles in regulating skeletal muscle growth through downstream signaling.
In addition to the mediation of internal cell signaling, stimulation of the ADRB2 can also regulate calcium-mediated proteolysis. Both cAMP and phosphorylated PKA can either directly or indirectly inhibit calpain activity. Calpains are calcium-mediated proteases degrade myofibrils and by inhibiting calpain activity, myofibril size and integrity are preserved. This would decrease muscle damage and loss due to increased calcium flux from exercise. Research has shown using a nonhydrolyzable cAMP analog and activation of ADRB2 to inhibit protein degradation in both rats and chicks, suggesting cAMP may directly phosphorylate calpains to inhibit activity (22, 23). Research also suggests PKA demonstrates the ability to phosphorylate calpains, which is important in the context of this study because increased ADRB2 activation attributed to the Gly16 genotype would result in increased PKA concentrations and decreased calpain activity and myofibril degradation. Studies in rat models have demonstrated a phosphorylation site at serine 369 which would restrict domain movement and keep m-calpain in an inactive state, suggesting direct phosphorylation of calpain by PKA to have a negative-control effect on calpain activation (27, 29). The ability of cAMP and PKA to modulate calpain concentrations and activity suggests a mechanism whereby the Gly16 genotype may influence muscular size and strength.
Furthermore, ADRB2 stimulation may also regulate calpastatin activity, a calpain-specific inhibitor, thereby decreasing calpain concentrations and activity. Recent research has demonstrated that the calpastatin promoter sequence between nt −1653 and +130 contains a single cAMP binding site located at nt −76 (8). This suggests a direct pathway whereby cAMP signaling can lead to increased calpastatin gene transcription reducing calpain-mediated protein degradation. Further, multiple phosphorylation sites have been identified on calpastatin, particularly those found in the L and XL domain coded by exon 6, suggesting cAMP to have the ability to directly phosphorylate calpastatin (37). In addition to its ability to phosphorylate calpains, research suggests the C-domain of PKA can also directly phosphorylate calpastatin (25). These data suggest another pathway whereby ADRB2 stimulation may inhibit calpain activity and regulate muscle size and strength.
Hence, there are multiple pathways whereby ADRB2 activation may increase muscle size, strength, and contractility as well as protect against disuse atrophy. These implications suggest ADRB2 stimulation as a viable supplementation site, thus improving sport performance and attenuating muscular loss resulting from injury. In the current study, the Arg/Gly polymorphism demonstrated significantly higher power measures, muscular efficiency, and exercise intensity. This suggests the more functional polymorphism of the ADRB2 may have an effect on these measures and that ADRB2 stimulation may improve muscular size, strength, and contractility.
PRACTICAL APPLICATIONS
There are many pathways by which the ADRB2 can influence muscular development and strength. This study suggests that genetic variations of the ADRB2 are associated with muscular power and efficiency. We hypothesize the improved muscular function in the Arg/Gly group could be due to increased lymphocyte density and resistance to downregulation associated with the Gly16 polymorphism resulting in increased accumulation of downstream products which have been implicated in the regulation of muscle size and strength. These findings may imply a novel, safe approach to the attenuation of muscle degradation associated with disuse atrophy resulting from injury and to the improvement of muscular power and efficiency in athletes.
LIMITATIONS
There are inherent limitations regarding a genetics study including sample size and genotype distribution. Limited statistical power because of the modest sample size and different genotype distribution in the present study (N = 77) may have played a role in limiting the significance of some of the statistical comparisons conducted. A post hoc power analysis revealed the power to detect statistically significant differences between groups for watts, watts/kg, and watts/VO2 to be .84, .95, and 1.00 respectively at α = 0.05, suggesting sufficient sample size for the present study. Additionally, time of day and time of year for testing as well as training background were not controlled for in the present study, which may affect test-retest reliability. An intraclass correlation coefficient analysis revealed the test-retest reliability for watts, watts/kg, and watts/VO2 to be 0.52, 0.49, and 0.59 respectively, suggesting fair test-retest reliability. Therefore, we cannot rule out the influence of uncontrolled for variables on these measures.
Acknowledgments
We are sincerely grateful to the subjects who donated both their time and effort to be a part of this study. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by NSCA. This study was funded by NIH grants: HL108962-06. The authors have no conflict of interest to disclose.
References
- 1.Berdeaux R, Goebel N, Banaszynski L, et al. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med. 2007;13:597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- 2.Berdeaux R, Stewart R. cAMP signaling in skeletal muscle adaptation: Hypertrophy, metabolism, and regeneration. Am J Physiol. 2012;303(1):E1–E17. doi: 10.1152/ajpendo.00555.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 4.Bray MS, Krushkal J, Li L, et al. Positional genomic analysis identifies the β2-adrenergic receptor gene as a susceptibility locus for human hypertension. Circulation. 2000;101:2877–82. doi: 10.1161/01.cir.101.25.2877. [DOI] [PubMed] [Google Scholar]
- 5.Cairns SP, Borrani F. β-Adrenergic modulation of skeletal muscle contraction: Key role of excitation-contraction coupling. J Physiol. 2015;593(21):4713–27. doi: 10.1113/JP270909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Church JE, Trieu J, Sheorey R, et al. Functional β-adrenoceptors are important for early muscle regeneration in mice through effects on myoblast proliferation and differentiation. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cong M, Goll DE, Antin PB. cAMP responsiveness of the bovine calpastatin gene promoter. Biochim Biophys Acta. 1998;1443(1–2):186–92. doi: 10.1016/s0167-4781(98)00203-6. [DOI] [PubMed] [Google Scholar]
- 9.Eisenach JH, Barnes SA, Pike TL, et al. Arg16/Gly β2-adrenergic receptor polymorphism alters the cardiac output response to isometric exercise. J Appl Physiol. 2005;99(5):1776–81. doi: 10.1152/japplphysiol.00469.2005. [DOI] [PubMed] [Google Scholar]
- 11.George ST, Berrios M, Hadcock JR, Wang H, Malbon CC. Receptor density and cAMP accumulation: Analysis in CHO cells exhibiting stable expression of a cDNA that encodes the beta 2-adrenergic receptor. Biochem and Biophys Res Commun. 1988;150(2):665–72. doi: 10.1016/0006-291x(88)90443-3. [DOI] [PubMed] [Google Scholar]
- 12.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37(10):1974–84. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33(47):9414–9. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 14.Hinkle RT, Hodge KM, Cody DB, Sheldon RJ, Kobilka BK, Isfort RJ. Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the beta2-adrenergic receptor. Muscle Nerve. 2002;25(5):729–34. doi: 10.1002/mus.10092. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BD, Beck KC, Joyner MJ, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: Comparison with direct fick. J Appl Physiol. 2000;88(5):1650–8. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- 16.Kalsen A, Hostrup M, Soderlund K, Karlsson S, Backer V, Bangsbo J. Inhaled beta2-agonist increases power output and glycolysis during sprinting in men. Med Sci Sports Exerc. 2016;48(1):39–48. doi: 10.1249/MSS.0000000000000732. [DOI] [PubMed] [Google Scholar]
- 17.Kline WO, Panaro FJ, Yang H, Bodine SC. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J Appl Physiol. 2007;102(2):740–7. doi: 10.1152/japplphysiol.00873.2006. [DOI] [PubMed] [Google Scholar]
- 18.Kotanko P, Hoglinger O, Skrabal F. Beta-2 adrenoreceptor density in fibroblast culture correlates with human NaCl sensitivity. Am J Physiol. 1992;263(3):623–7. doi: 10.1152/ajpcell.1992.263.3.C623. [DOI] [PubMed] [Google Scholar]
- 21.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 22.Navegantes LC, Resano NM, Migliorini RH, Kettelhut IC. Catecholamines inhibit Ca2+-dependent proteolysis in rat skeletal muscle through beta(2)-adrenoceptors and cAMP. Am J Physiol. 2001;281(3):E449–54. doi: 10.1152/ajpendo.2001.281.3.E449. [DOI] [PubMed] [Google Scholar]
- 23.Navegantes LC, Machado CR, Resano NM, Migliorini RH, Kettelhut IC. Beta2-agonists and cAMP inhibit protein degradation in isolated chick (gallus domesticus) skeletal muscle. Brit Poult Sci. 2003;44(1):149–54. doi: 10.1080/0007166031000085355. [DOI] [PubMed] [Google Scholar]
- 24.Pearen MA, Ryall JG, Maxwell MA, Ohkura N, Lynch GS, Muscat GE. The orphan nuclear receptor NOR-1 is a target of β-adrenergic signaling in skeletal muscle. Endocrinology. 2006;147(11):5217–27. doi: 10.1210/en.2006-0447. [DOI] [PubMed] [Google Scholar]
- 25.Reiken S, Lacampagne A, Zhou H, et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: Defective regulation in heart failure. J Cell Biol. 2003;160(6):919–28. doi: 10.1083/jcb.200211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salamino F, Detullio R, Michetti M, Mengotta P, Melioni E, Pontremoli S. Modulation of calpastatin specificity in rat tissues by reversible phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1994;199(3):1326–32. doi: 10.1006/bbrc.1994.1376. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt P, Holsboer F, Spendler D. B2-adrenergic receptors potentiate glucocorticoid receptor transactivation via G protein βγ-subunits and the phosphoinositide 3-kinase pathway. Mol Endocrinol. 2001;15(4):553–64. doi: 10.1210/mend.15.4.0613. [DOI] [PubMed] [Google Scholar]
- 29.Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Bio. 2002;22(8):2716–27. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva MT, Wensing LA, Brum PC, Camara NO, Miyabara EH. Impaired structural and functional regeneration of skeletal muscles from β2-adrenoceptor knockout mice. Acta Physiol Scand. 2014;211(4):617–33. doi: 10.1111/apha.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skrabal F, Kotanko B, Loft FC. Inverse regulation of alpha-2 and beta-2 adrenoceptors in salt-sensitive hypertension: An hypothesis. Life Sci. 1989;45(22):2061–76. doi: 10.1016/0024-3205(89)90071-4. [DOI] [PubMed] [Google Scholar]
- 32.Snyder EM, Beck KC, Dietz NM, et al. Arg16Gly polymorphism of the β2-adrenergic receptor is associated with differences in cardiovascular function at rest and during exercise in humans. J Physiol. 2006;571(1):121–30. doi: 10.1113/jphysiol.2005.098558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder EM, Beck KC, Dietz NM, Joyner MJ, Turner ST, Johnson BD. Influence of β2-adrenergic receptor genotype on airway function during exercise in healthy adults. Chest. 2006;129(3):762–70. doi: 10.1378/chest.129.3.762. [DOI] [PubMed] [Google Scholar]
- 34.Snyder EM, Hulsebus ML, Turner ST, Joyner MJ, Johnson BD. Genotype related differences in [beta]2 adrenergic receptor density and cardiac function. Med Sci Sports Exerc. 2006;38(5):882–6. doi: 10.1249/01.mss.0000218144.02831.f6. [DOI] [PubMed] [Google Scholar]
- 35.Tang W, Devereux RB, Kitzman DW, et al. The Arg16Gly polymorphism of the beta2-adrenergic receptor and left ventricular systolic function. Am J Hypertens. 2003;16(11):945–51. doi: 10.1016/s0895-7061(03)01001-x. [DOI] [PubMed] [Google Scholar]
- 36.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO’s road. Sci STKE. 2003;2003(172):RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 37.Tullio RD, Cantoni C, Broggio C, et al. Involvement of exon 6-mediated calpastatin intracellular movements in the modulation of calpain activation. Biochim Biophys Acta. 2009;1790(3):182–7. doi: 10.1016/j.bbagen.2008.11.002. [DOI] [PubMed] [Google Scholar]