Abstract
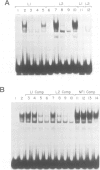
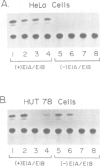
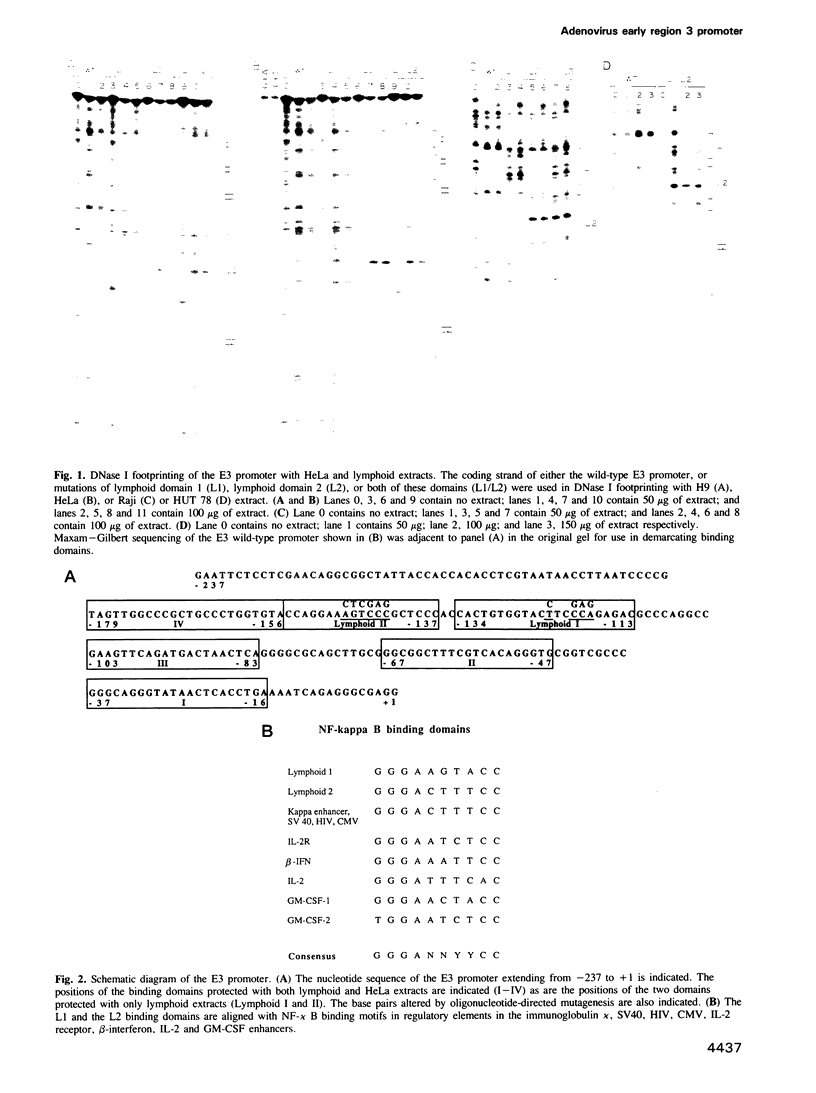
A primary site of infection by human adenoviruses is lymphoid cells. However, analysis of the viral control elements and the cellular factors that regulate adenoviral gene expression in lymphocytes has not been reported. The adenovirus early region 3 (ES) gene products are involved in the maintenance of viral persistence by complexing with the class I MHC antigens, thus preventing their cell surface expression with a resultant decrease in host immunologic destruction. To determine whether different cellular factors were involved in E3 regulation in lymphocytes as compared with HeLa cells, both DNA binding and transfection analysis with the E3 promoter in both cell types were performed. These studies detected two novel domains referred to as L1 and L2 with a variety of lymphoid but not HeLa extracts. Each of these domains possessed strong homology to motifs previously found to bind the cellular factor NF-kappa B. Transfections of E3 constructs linked to the chloramphenicol acetyltransferase gene revealed that mutagenesis of the distal NF-kappa B motif (L2) had minimal effects on promoter expression in HeLa cells, but resulted in dramatic decreases in expression by lymphoid cells. In contrast, mutagenesis of proximal NF-kappa B motif (L1) had minimal effects on gene expression in both HeLa cells and lymphoid cells but resulted in a small, but reproducible, increase in gene expression in lymphoid cells when coupled to the L2 mutation. Reversing the position and subsequent mutagenesis of the L1 and L2 domains indicated that the primary sequence of these motifs rather than their position in the E3 promoter was critical for regulating gene expression.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Atchison M. L., Perry R. P. The role of the kappa enhancer and its binding factor NF-kappa B in the developmental regulation of kappa gene transcription. Cell. 1987 Jan 16;48(1):121–128. doi: 10.1016/0092-8674(87)90362-x. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Binding of a nuclear factor to a regulatory sequence in the promoter of the mouse H-2Kb class I major histocompatibility gene. Mol Cell Biol. 1987 Jan;7(1):305–313. doi: 10.1128/mcb.7.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Two transcription factors, NF-kappa B and H2TF1, interact with a single regulatory sequence in the class I major histocompatibility complex promoter. Proc Natl Acad Sci U S A. 1988 Feb;85(3):723–727. doi: 10.1073/pnas.85.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard D. W., Böhnlein E., Lowenthal J. W., Wano Y., Franza B. R., Greene W. C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988 Sep 23;241(4873):1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Clark L., Pollock R. M., Hay R. T. Identification and purification of EBP1: a HeLa cell protein that binds to a region overlapping the 'core' of the SV40 enhancer. Genes Dev. 1988 Aug;2(8):991–1002. doi: 10.1101/gad.2.8.991. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- Davidson I., Fromental C., Augereau P., Wildeman A., Zenke M., Chambon P. Cell-type specific protein binding to the enhancer of simian virus 40 in nuclear extracts. Nature. 1986 Oct 9;323(6088):544–548. doi: 10.1038/323544a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., Wu F., Gaynor R. Upstream regulatory regions required to stabilize binding to the TATA sequence in an adenovirus early promoter. Nucleic Acids Res. 1987 Oct 26;15(20):8367–8385. doi: 10.1093/nar/15.20.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Kuwabara M. D., Wu F. K., Garcia J. A., Harrich D., Briskin M., Wall R., Sigman D. S. Repeated B motifs in the human immunodeficiency virus type I long terminal repeat enhancer region do not exhibit cooperative factor binding. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9406–9410. doi: 10.1073/pnas.85.24.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980 Dec 1;152(6):1709–1719. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootenberg J. E., Ruscetti F. W., Mier J. W., Gazdar A., Gallo R. C. Human cutaneous T cell lymphoma and leukemia cell lines produce and respond to T cell growth factor. J Exp Med. 1981 Nov 1;154(5):1403–1418. doi: 10.1084/jem.154.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Greenfield C., Hiles I., Waterfield M. D., Federwisch M., Wollmer A., Blundell T. L., McDonald N. Epidermal growth factor binding induces a conformational change in the external domain of its receptor. EMBO J. 1989 Dec 20;8(13):4115–4123. doi: 10.1002/j.1460-2075.1989.tb08596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Klessig D. F. Expression of unselected adenovirus genes in human cells co-transformed with the HSV-1 tk gene and adenovirus 2 DNA. Cell. 1980 Sep;21(2):453–463. doi: 10.1016/0092-8674(80)90482-1. [DOI] [PubMed] [Google Scholar]
- Horvath J., Palkonyay L., Weber J. Group C adenovirus DNA sequences in human lymphoid cells. J Virol. 1986 Jul;59(1):189–192. doi: 10.1128/jvi.59.1.189-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath J., Weber J. M. Nonpermissivity of human peripheral blood lymphocytes to adenovirus type 2 infection. J Virol. 1988 Jan;62(1):341–345. doi: 10.1128/jvi.62.1.341-345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst H. C., Jones N. C. Identification of factors that interact with the E1A-inducible adenovirus E3 promoter. Genes Dev. 1987 Dec;1(10):1132–1146. doi: 10.1101/gad.1.10.1132. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno M., Fromental C., Staub A., Ruffenach F., Davidson I., Chambon P. The SV40 TC-II(kappa B) and the related H-2Kb enhansons exhibit different cell type specific and inducible proto-enhancer activities, but the SV40 core sequence and the AP-2 binding site have no enhanson properties. EMBO J. 1989 Dec 20;8(13):4205–4214. doi: 10.1002/j.1460-2075.1989.tb08606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Scheidereit C., Roeder R. G. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-kappa B) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornuc M., Kliewer S., Garcia J., Harrich D., Li C., Gaynor R. Adenovirus early region 3 promoter regulation by E1A/E1B is independent of alterations in DNA binding and gene activation of CREB/ATF and AP1. J Virol. 1990 May;64(5):2004–2013. doi: 10.1128/jvi.64.5.2004-2013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Ostberg L., Persson H., Philipson L., Peterson P. A. Molecular association between transplantation antigens and cell surface antigen in adenovirus-transformed cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5674–5678. doi: 10.1073/pnas.75.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpe O., Bellgrau D., Hammerling U., Lind P., Päbo S., Severinsson L., Peterson P. A. Complex formation of class I transplantation antigens and a viral glycoprotein. J Biol Chem. 1983 Sep 10;258(17):10594–10598. [PubMed] [Google Scholar]
- Lavery D., Fu S. M., Lufkin T., Chen-Kiang S. Productive infection of cultured human lymphoid cells by adenovirus. J Virol. 1987 May;61(5):1466–1472. doi: 10.1128/jvi.61.5.1466-1472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Nelsen B., Hellman L., Sen R. The NF-kappa B-binding site mediates phorbol ester-inducible transcription in nonlymphoid cells. Mol Cell Biol. 1988 Aug;8(8):3526–3531. doi: 10.1128/mcb.8.8.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiyama H., Fromental C., Xiao J. H., Chambon P. Cell-specific activity of the constituent elements of the simian virus 40 enhancer. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7881–7885. doi: 10.1073/pnas.84.22.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondek B., Shepard A., Herr W. Discrete elements within the SV40 enhancer region display different cell-specific enhancer activities. EMBO J. 1987 Apr;6(4):1017–1025. doi: 10.1002/j.1460-2075.1987.tb04854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Jansson M., Philipson L. Synthesis and genomic site for an adenovirus type 2 early glycoprotein. J Mol Biol. 1980 Feb 5;136(4):375–394. doi: 10.1016/0022-2836(80)90396-4. [DOI] [PubMed] [Google Scholar]
- Persson H., Signäs C., Philipson L. Purification and characterization of an early glycoprotein from adenovirus type 2-infected cells. J Virol. 1979 Mar;29(3):938–948. doi: 10.1128/jvi.29.3.938-948.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Lenardo M., Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Read-Connole E., Gallo R. C. T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet. 1984 Dec 22;2(8417-8418):1472–1473. doi: 10.1016/s0140-6736(84)91666-0. [DOI] [PubMed] [Google Scholar]
- Päbo S., Nilsson T., Peterson P. A. Adenoviruses of subgenera B, C, D, and E modulate cell-surface expression of major histocompatibility complex class I antigens. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9665–9669. doi: 10.1073/pnas.83.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Baeuerle P. A. NF-kappa B as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol Cell Biol. 1990 Mar;10(3):1281–1286. doi: 10.1128/mcb.10.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Signäs C., Katze M. G., Persson H., Philipson L. An adenovirus glycoprotein binds heavy chains of class I transplantation antigens from man and mouse. Nature. 1982 Sep 9;299(5879):175–178. doi: 10.1038/299175a0. [DOI] [PubMed] [Google Scholar]
- Wirth T., Baltimore D. Nuclear factor NF-kappa B can interact functionally with its cognate binding site to provide lymphoid-specific promoter function. EMBO J. 1988 Oct;7(10):3109–3113. doi: 10.1002/j.1460-2075.1988.tb03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. K., Garcia J. A., Harrich D., Gaynor R. B. Purification of the human immunodeficiency virus type 1 enhancer and TAR binding proteins EBP-1 and UBP-1. EMBO J. 1988 Jul;7(7):2117–2130. doi: 10.1002/j.1460-2075.1988.tb03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Garcia J., Mitsuyasu R., Gaynor R. Alterations in binding characteristics of the human immunodeficiency virus enhancer factor. J Virol. 1988 Jan;62(1):218–225. doi: 10.1128/jvi.62.1.218-225.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano O., Kanellopoulos J., Kieran M., Le Bail O., Israël A., Kourilsky P. Purification of KBF1, a common factor binding to both H-2 and beta 2-microglobulin enhancers. EMBO J. 1987 Nov;6(11):3317–3324. doi: 10.1002/j.1460-2075.1987.tb02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]