Abstract
Rationale
Increasing evidence indicates that alterations of the cerebral microcirculation may play a role in Alzheimer’s disease (AD), the leading cause of late-life dementia. The amyloid-β peptide (Aβ), a key pathogenic factor in AD, induces profound alterations in neurovascular regulation through the innate immunity receptor CD36, which, in turn, activates a Nox2-containing NADPH oxidase leading to cerebrovascular oxidative stress. Brain perivascular macrophages (PVM) located in the perivascular space, a major site of brain Aβ collection and clearance, are juxtaposed to the wall of intracerebral resistance vessels and are a powerful source of reactive oxygen species (ROS).
Objective
We tested the hypothesis that PVM are the main source of ROS responsible for the cerebrovascular actions of Aβ, and that CD36 and Nox2 in PVM are the molecular substrates of the effect.
Methods and Results
Selective depletion of PVM using intracerebroventricular injection of clodronate abrogates the ROS production and cerebrovascular dysfunction induced by Aβ applied directly to the cerebral cortex, administered intravascularly or overproduced in the brain of transgenic mice expressing mutated forms of the amyloid precursor protein (Tg2576 mice). In addition, using bone marrow chimeras we demonstrate that PVM are the cells expressing CD36 and Nox2 responsible for the dysfunction. Thus, deletion of CD36 or Nox2 from PVM abrogates the deleterious vascular effects of Aβ, whereas wild-type PVM reconstitute the vascular dysfunction in CD36-null mice.
Conclusions
The data identify PVM as a previously unrecognized effector of the damaging neurovascular actions of Aβ and unveil a new mechanism by which brain-resident innate immune cells and their receptors may contribute to the pathobiology of AD.
Keywords: Vascular biology, cerebrovascular disease, oxidative stress, endothelium, perivascular macrophages
Subject Terms: Animal Models of Human Disease, Basic Science Research, Vascular Biology, Endothelium/Vascular Type/Nitric Oxide, Oxidant Stress
INTRODUCTION
Alzheimer’s disease (AD) is the leading cause of cognitive impairment in the elderly, characterized pathologically by extracellular deposition of the amyloid-β peptide (Aβ) in amyloid plaques and intracellular aggregates of the microtubule associated protein tau (neurofibrillary tangles)1. Due to demographic shifts and lack of effective treatments, the prevalence of AD is estimated to rise to epidemic proportions in the next few decades and disease-modifying therapeutic interventions are sorely needed2.
Several lines of evidence suggest that vascular factors play a pathogenic role in AD3, 4. While vascular risk factors increase the risk of AD5–7, cerebrovascular function is altered in pre-symptomatic individuals who will develop AD8–10, suggesting an early involvement in the disease process. The brain is highly dependent on a continuous and well-regulated delivery of cerebral blood flow (CBF), and alterations in cerebral perfusion lead to brain dysfunction and cognitive impairment11–13. A large body of work indicates that Aβ disrupts key mechanisms regulating the cerebral microcirculation13–15. For example, Aβ suppresses the increase in CBF evoked by synaptic activity, a vital homeostatic response that matches oxygen and glucose delivery to the energy needs of the active brain, and disrupts endothelial function16–21. These neurovascular alterations are mediated by the innate immunity receptor CD36, polymorphisms of which are linked to AD susceptibility22. CD36 binds Aβ and leads to Nox2-dependent production of reactive oxygen species (ROS)23–27. However, CD36 and Nox2 are expressed in many cells, including microglia, circulating myeloid cells and endothelium28, and the relevant cell type(s) remain to be identified.
The perivascular space (Virchow-Robin space), limited by the glial basement membrane (glia limitans) and the vascular basement membrane29, has emerged as a major conduit for brain Aβ clearance, as well as a preferential site of Aβ accumulation30, 31. Brain Aβ, released extracellularly during synaptic activity32, reaches the perivascular space and exits the brain through different routes33. Brain perivascular macrophages (PVM), a unique population of brain myeloid cells distinct from macrophages in heart and systemic vessels, are closely apposed to the wall of cerebral resistance arteries in the perivascular space, and, as all macrophages, can produce large amounts of ROS34–36. Therefore, PVM are exposed to Aβ and are uniquely poised to induce cerebrovascular oxidative stress, but their participation in the vascular effects of Aβ has not been explored.
We examined the role of PVM in the cerebrovascular actions of Aβ. We found that depletion of PVM abrogates the vascular oxidative stress and neurovascular dysfunction induced by Aβ, either administered to wild type (WT) mice or produced endogenously in the brain of transgenic mice expressing a mutated form of amyloid precursor protein (APP) (Tg2576). Furthermore, using bone marrow (BM) chimeras we demonstrated that expression of CD36 and Nox2 in PVM is absolutely required for vascular effects of Aβ. The findings implicate for the first time PVM in the neurovascular dysfunction of Aβ, and suggest a novel mechanism by which brain-resident innate immune cells may promote AD.
METHODS
Mice
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medicine. Experiments were performed in 3–4 month-old transgenic mice overexpressing the Swedish mutation of the amyloid precursor protein (APP) (Tg2576)37 or age-matched WT littermates, referred to as WT mice. In BM chimera experiments CD36−/− and Nox2−/− mice were used as BM donors. In some studies, GFP+ mice (JAX Stock #006567) were used as BM donors. All mice were males and derived from in house colonies23–26.
Intracerebroventricular injection of clodronate or dextran
Liposomes containing clodronate or phosphate buffered saline (PBS) were administered intracerebroventricularly (icv) as previously described34, 38. Isoflurane-anesthetized mice were placed in a stereotaxic frame. Ten μl of clodronate-liposomes (7 mg/ml) or PBS-liposomes (vehicle) were injected into the cerebral ventricles with a glass micropipette through a burr hole drilled on the right parietal bone. Mice were used in the experiments 5–7 days later, when PVM depletion is well developed and stable34, 38. In some experiments, PVM were identified by their ability to phagocytize dextran34, 39. For dextran injections, 10 μl of Alexa Fluor® 680 dextran (10,000 MW, anionic, fixable, ThermoFisher Scientific, D34680; 2.5 mg/ml) in saline or saline alone were injected and PVM labeling was examined 24 hrs later.
Labeling cortical blood vessels with DiO
Cortical blood vessels were labeled with the lipophilic dye DiO [DiOC18(3) (3,3′-Dioctadecyloxacarbocyanine Perchlorate)], as described40. Briefly, mice were anesthetized (5% isoflurane) and transcardially perfused with PBS (2 ml) followed by DiO (1:50, V-22886, Molecular Probes; 5ml/mouse) and then by 4% paraformaldehyde (PFA). Brains were harvested and post-fixed in 4% PFA overnight, and then cut (thickness 150 μm) using a vibratome and examined under the confocal microscope (Leica SP5).
Immunohistochemistry
Mice were anesthetized with sodium pentobarbital (120 mg/kg, i.p.) and perfused transcardially with PBS followed by 4% PFA in PBS. Brains were removed, stored overnight, and sectioned in a vibratome (section thickness: 40 μm). Free-floating brain sections were permeabilized with 0.5% Triton X-100 and non-specific binding was blocked with 1% of normal donkey serum. Sections were randomly selected and incubated with the primary antibodies CD206 (clone MR5D3, rat polyclonal, 1:200, Serotec), Glut-1 (rabbit polyclonal, 1:200, Calbiochem), Iba-1 (rabbit polyclonal, 1:500, Wako Chemicals), or GFAP (mouse monoclonal, 1:1000, Sigma) overnight at 4°C. After washing, brain sections were incubated with a Cy5- or a FITC-conjugated secondary antibody (1:200; Jackson ImmunoResearch Laboratories), mounted on slides and imaged with a confocal microscope (Leica SP5). The specificity of the immunofluorescence was verified by omission of the primary and/or secondary antibody or blocking of the antigen. All quantifications were performed by investigators blinded to the treatment on randomly selected fields within the somatosensory cortex.
Identification and quantification of PVM in somatosensory cortex
PVM were identified by well-established criteria, including expression of CD206, ability to phagocytize dextran and perivascular location34, 38, 41 (fig 1). The association with cortical blood vessels was confirmed by co-labeling with the endothelial marker Glut-1 (rabbit polyclonal, 1:200, Calbiochem), the smooth muscle marker α-actin or DiO40. For CD206+ PVM, randomly selected fields (20× objective; 4 confocal images/mouse; n=5 mice/group) within the somatosensory cortex were analyzed. For dextran+ PVM, a representative coronal section from each mouse was reconstructed from tiled images taken with the confocal microscope, and the whole somatosensory cortex (n=3/group) was analyzed. ImageJ (NIH) was used for all image analyses.
Figure 1. PVM, juxtaposed to penetrating cerebral blood vessels, are depleted by clodronate.
In WT mice (non-transgenic littermates) injected with PSB-liposome (vehicle), PVM identified by phagocytized dextran (green) are closely apposed to penetrating neocortical vessels, identified by the lipophilic dye DiO (red) (A). PVM in Tg2576 mice injected with vehicle have numbers and distributions similar to WT mice (B). Intracerebroventricular (icv) administration of clodronate-containing liposomes depletes PVM both in WT and Tg2576 mice 5 days later (C,D). Quantification of the number of PVM in WT and tg2576 mice after icv injection of liposomes containing PBS or clodronate (E). No differences between WT and Tg2576 mice are observed. *p<0.05 from PBS; n=5/group; Analysis of variance and Tukey’s test.
Cerebrovascular ROS measurement
ROS production was assessed in vivo by hydroethidine (HE) microfluorography23–25, 34, 42. To assess ROS production in PVM, mice were first injected icv with clodronate- or PBS-liposome and 5–7 days later with dextran (see above). The day after dextran injection, in WT mice HE (2 μM in Ringer; Invitrogen) with or without Aβ (5 μM) was superfused over the somatosensory cortex. In tg2576 mice, HE (10 mg/kg) was injected into the jugular vein. BM-transplanted mice were injected with icv dextran one day before ROS measurement. Sixty minutes after HE administration, mice were injected with DiO to label cerebral blood vessels as described above. Coronal brain sections were then cut through the cortex underlying the cranial window, and ROS dependent fluorescence associated with blood vessels or PVM was quantified as described previously23–25, 34, 42.
Bone marrow transplant
Procedures for BM transplant have been previously described34, 43 and are only summarized. Whole-body irradiation was performed in 7 weeks-old mice (Nordion Gammacell 40 Exactor). Eighteen hours later, mice were transplanted with BM cells (2×106, i.v.) isolated from the donor CD36−/−, Nox2−/−, and WT controls. Mice were housed in cages with sulfamethoxazole (0.12%; w/v) and trimethoprim (0.024%) added to drinking water for the first 2 weeks. Reconstitution of BM cells was verified 5 weeks after irradiation by testing the percentage of positive CD36 or Nox2 genomic DNA in isolated blood leukocytes43. Chimerism was >95% for both CD36−/− and Nox2−/− BM chimeras. For studies of PVM number and distribution after BM transplant in Tg2576 mice, the BM of transgenic mice expressing GFP was transplanted into irradiated Tg2576 mice or littermates.
General surgical procedures for CBF measurement
As described in detail elsewhere24, 25, 42, mice were anesthetized with isoflurane (induction, 5%; surgery, 2%) and maintained with urethane (750 mg/kg; i.p.) and α-chloralose (50 mg/kg; i.p.). A femoral artery was cannulated for recording of arterial pressure and collection of blood samples for blood gas analysis. The trachea was intubated and mice were artificially ventilated with a mixture of N2 and O2. Arterial blood pressure (80–90 mmHg), blood gases (pO2, 120–140 mmHg; pCO2, 30–40 mmHg; pH, 7.3–7.4), and rectal temperature (37°C) were monitored and controlled. Throughout the experiment the level of anesthesia was monitored by testing motor responses to tail pinch. The somatosensory cortex was exposed through a small craniotomy (2×2 mm). The dura was removed, and the exposed cortex was continuously bathed with a modified Ringer’s solution (36–37°C; pH: 7.3–7.4)(see ref.44 for composition). CBF was continuously monitored at the site of superfusion with a laser-Doppler probe (Vasamedic, St. Paul, MN) positioned stereotaxically on the neocortical surface and connected to a computerized data acquisition system. CBF values were expressed as percent increase relative to the resting level. Resting CBF is reported as arbitrary laser-Doppler perfusion units (LDU). Zero values for CBF were obtained after the heart was stopped by an overdose of isoflurane at the end of the experiment.
Experimental protocol for CBF experiments
CBF recordings were started after arterial pressure and blood gases were in a steady state. CBF responses to whisker stimulation were recorded while gently stroking the whiskers with a cotton-tipped applicator for 60 sec. All pharmacological agents were dissolved in a modified Ringer’s solution. To test endothelium-dependent responses, the endothelial nitric oxide (eNOS)-dependent vasodilator acetylcholine (10 μM, Sigma), the Ca2+ ionophore A23187 (3 μM; Sigma) or bradykinin (50 μM; Sigma) was topically superfused for 3–5 min and the evoked CBF increases recorded. To test smooth muscle reactivity, CBF response to adenosine (400 μM, Sigma) or the NO donor S-Nitroso-N-acetyl-DL-penicillamine (SNAP; 50 μM, Sigma) were examined24, 25, 42. The increase in CBF produced by hypercapnia was tested by introducing 5% CO2 in the ventilator to increase arterial pCO2 up to 50–60 mmHg. Once a stable increase in CBF was obtained for 3–5 min, pCO2 was returned to normocapnia.
Measurement of Aβ
Aβ was measured using an ELISA-based assay, as described previously23, 24, 26. Briefly, the left hemispheres from the mice used for CBF studies were sonicated in 1% SDS with protease inhibitors and centrifuged. The supernatant contained SDS-soluble Aβ peptides. The pellet was sonicated in 70% formic acid and centrifuged as above. The formic acid extract was neutralized by a 1:20 dilution into 1 M Tris phosphate buffer (pH 8.0). Aβ1-40 and Aβ1-42 concentrations (pM) were determined in supernatant (SDS-soluble) and in the formic acid extract of the pellet (SDS-insoluble) using the BAN-50/BA-27 and BAN-50/BC005 sandwich ELISA assay.
Statistics
Sample size was determined by power analysis based on previous published works published by our lab on CBF regulation. Animals were randomly assigned to treatment or control group and analysis was performed in a blinded fashion. Group difference was analyzed using Student’s t test (paired or unpaired) or analysis of variance (ANOVA) with Tukey’s test, as appropriate. Data are expressed as mean±SEM and statistical difference was considered significant at p<0.05.
RESULTS
Brain PVM depletion ameliorates the neurovascular dysfunction induced by Aβ
PVM, identified by CD206 immunochemistry, their perivascular location and their ability to phagocytize dextran, were observed in close apposition to somatosensory cortex blood vessels (Figure 1; Online Figure I). In particular, PVM were juxtaposed to penetrating arterioles identified by the smooth muscle cell marker α-actin (Online Figure I), attesting to their association with cerebral resistance vessels. PVM also expressed CD36 (Online Figure II), the innate immunity receptor involved in the cerebrovascular effects of Aβ25, 26. Consistent with previous observations34, PVM were not observed in vessels smaller than 10 μm (precapillary arterioles and capillaries), due to the fusion of the glial and vascular basement membranes and obliteration of the perivascular space29.
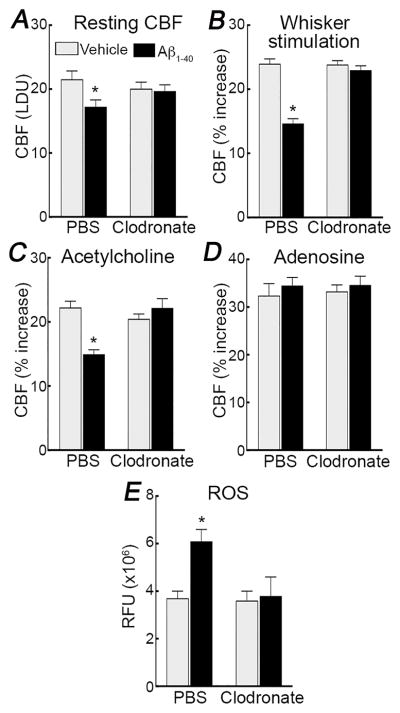
To deplete brain PVM we used icv injection of liposome-encapsulated clodronate and assessed the extent of the depletion 5 days later, when the effect is most pronounced and stable34, 38. Clodronate, but not control liposomes, deplete PVM and meningeal macrophages in the somatosensory cortex (>80%). In agreement with previous observations in adult mice34, clodronate did not deplete microglia, smooth muscle cells, astrocytes, or endothelium (Figure 1; Online Figures. I–III). In WT mice treated with control liposomes, superfusion of Aβ1-40 (5μM) on the somatosensory cortex reduced resting CBF and attenuated the CBF increase induced by whisker stimulation (functional hyperemia; −40±3%), by neocortical application of the endothelium-dependent vasodilators ACh (−31±3%), bradykinin (−46±1%), and Ca2+ ionophore A23187 (−34±6%), or by the NO donor SNAP (−29±3% ) (Figure 2; Online Figure IVA–C) (p<0.05). As before23–26, 42, the CBF response to neocortical application of the smooth muscle relaxant adenosine or to hypercapnia, a potent cerebrovasodilatatory stimulus45, 46, was not affected (Figure 2D; Online Figure IVD) (p>0.05). However, we cannot rule out that a reduction would be observed with a milder hypercapnic challenge producing smaller CBF increases. PVM depletion by clodronate, did not alter CBF responses, but prevented in full the Aβ-induced attenuation in functional hyperemia, endothelium-dependent responses, and response to SNAP (Figure 2A–C; Online Figure IVA–C). Clodronate also attenuated the increase in cerebrovascular ROS induced by Aβ (Figure 2E).
Figure 2. PVM depletion prevents the cerebrovascular effects of neocortical superfusion of Aβ1-40 in WT mice.
In WT mice treated with PBS liposomes, superfusion of the somatosensory cortex with Aβ1-40 (5μM) attenuates resting CBF (A), and the increase in CBF evoked by whisker stimulation (B), or by neocortical application of ACh (10μM) (C), but not adenosine (400μM) (D). Treatment with clodronate does not affect baseline cerebrovascular responses, but rescues the attenuation in resting CBF and CBF responses to whiskers stimulation and ACh induced by Aβ1-40 superfusion. Furthermore, clodronate suppresses the Aβ-induced increase in cerebrovascular ROS in the somatosensory cortex, assessed by DHE microfluorography (E). LDU, arbitrary laser-Doppler perfusion units; RFU, relative fluorescence units; *p<0.05 from vehicle, ANOVA and Tukey’s test; n=5/group.
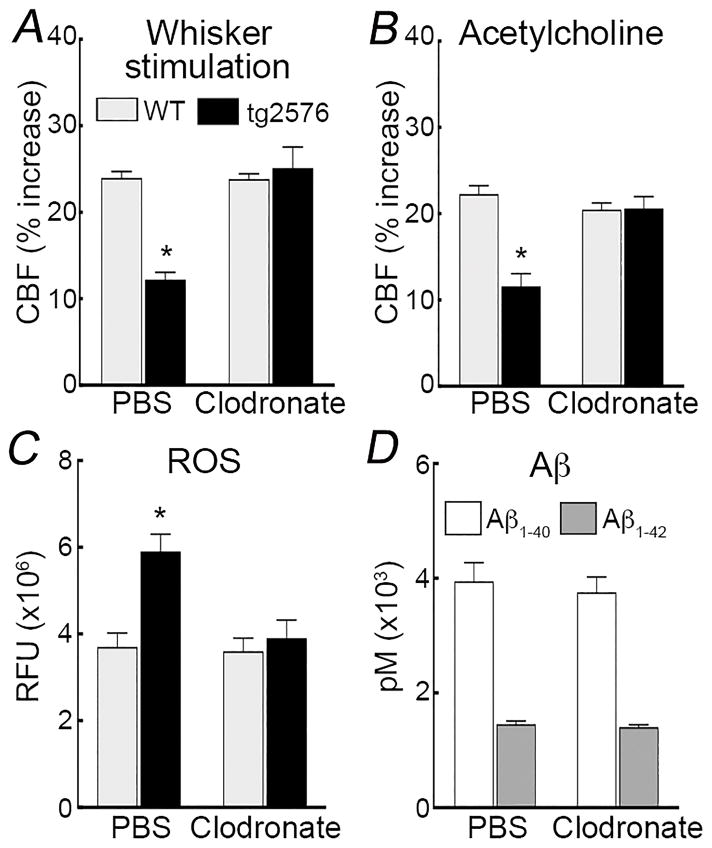
To provide further evidence that PVM participate in the neurovascular effects of Aβ, we used 3–4 month-old Tg2576 mice23, 25, 42. At this age, Tg2576 mice have elevated levels of brain Aβ but do not exhibit amyloid pathology (amyloid plaques and cerebral amyloid angiopathy)23–26, 42. The number of PVM did not differ between Tg2576 mice and WT littermates (Figure 1E), but the intensity of the CD36 immunolabel seemed greater in CD206+ PVM of Tg2576 mice (Online Figure IIB), as reported25, 26. Clodronate depleted PVM in Tg2576 mice as effectively as in WT mice (Figure 1; Online Figure IIB), without altering brain Aβ1-40 and Aβ1-42 (Figure 3D). However, in agreement with the results with topical Aβ, clodronate treatment prevented the neurovascular dysfunction and vascular oxidative stress in Tg2576 mice (Figure 3A,B; Online Figure IVE–I). These observations indicate that PVM depletion ameliorates the cerebrovascular dysfunction induced by exogenous Aβ and observed in Tg2576 mice.
Figure 3. PVM depletion counteracts the neurovascular dysfunction and vascular oxidative stress in Tg2576 mice.
PVM depletion by clodronate counteracts the attenuation in the CBF response to whisker stimulation (A) or neocortical application of ACh (B), as well as the increase in cerebrovascular ROS (C) in Tg2576 mice. Clodronate does not alter brain concentrations of SDS-soluble Aβ1-40 and Aβ1-42 in tg2576 mice. RFU, relative fluorescence units; *p<0.05 from WT, ANOVA and Tukey’s test; n=5–6/group.
Deletion of CD36 or Nox2 in PVM ameliorates the neurovascular dysfunction
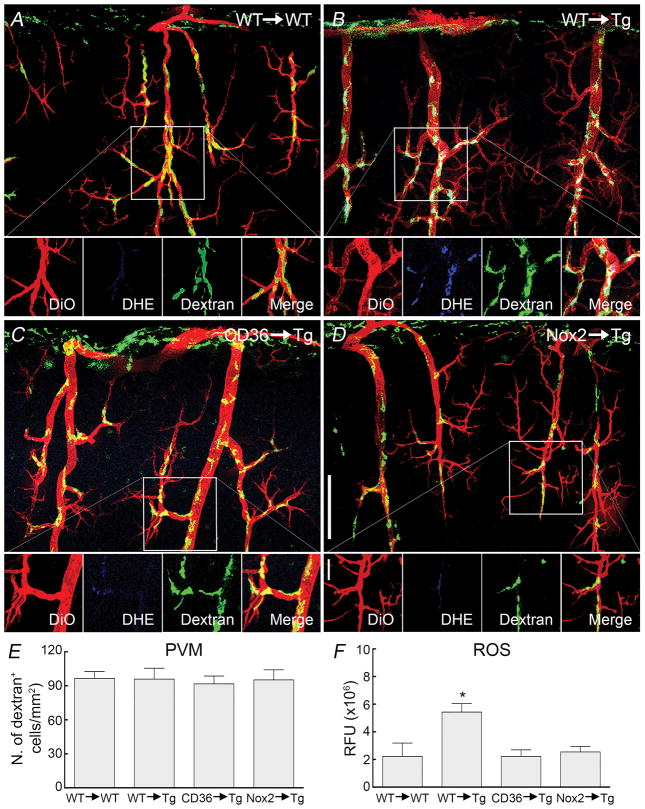
We used CD36−/− or Nox2−/− BM chimeras to replace PVM with PVM lacking CD36 or Nox2 in WT and Tg2576 mice. After lethal irradiation, PVM are eliminated and slowly replaced with BM-derived macrophages, but microglia and other resident brain cells are not affected, at least in young WT and APP mice34, 47. This approach has been previously used for gene targeting in PVM34, 47. Since immune cell trafficking may be altered in APP-expressing mice36, 47, 48, we first investigated whether the repopulation of the perivascular space by PVM after BM transplant was comparable in WT and Tg2576 mice. GFP+ BM was transplanted in WT and Tg2576 mice and, 2 months later, perivascular GFP+ cells were examined in both groups. We found that the number of PVM and their perivascular distribution did not differ between WT and Tg2576 mice (Online Figure V). Furthermore, to investigate if PVM repopulation is altered by lack of CD36 or Nox2 in macrophages, we quantified PVM in tg2676 mice transplanted with CD36−/− or Nox2−/− BM, and there were no differences in number and distribution of PVM (Figure 4A–E).
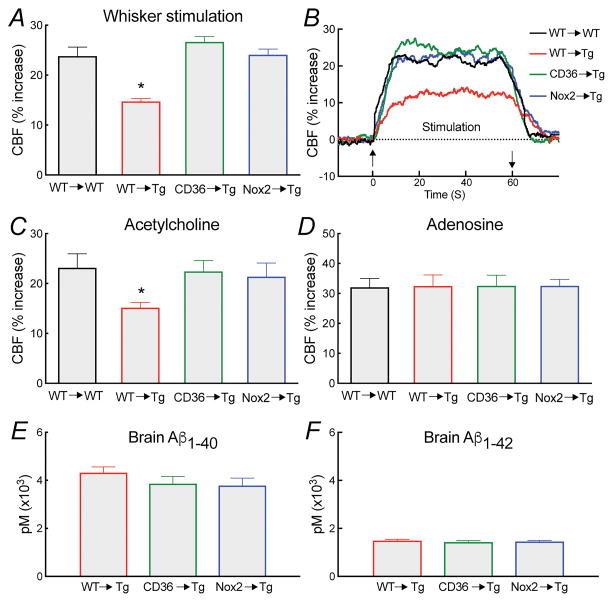
Figure 4. Effect of transplantation of CD36−/− or Nox2−/− BM in Tg2576 mice on PVM number and ROS production.
Vessels are labeled with DiO (red), ROS production is detected with DHE (blue) and PVM are labeled with dextran (green). In WT mice transplanted with WT BM (WT→WT) PVM have numbers and distribution comparable to those of naïve WT mice (A). Similarly, Tg2576 mice transplanted with WT BM (WT→Tg) exhibited number and distribution similar to those of naïve Tg2576 mice (B). However, ROS production in PVM was observed in WT→Tg mice (DHE insert in B). Transplant of CD36−/− (CD36→Tg ) or Nox2−/− (Nox2→Tg) BM in Tg2576 mice does not alter PVM number or distribution, but suppresses PVM ROS production (DHE inserts in C and D). Quantification of the numbers of PVM in the BM chimeras studied (E). Quantification of cerebrovascular ROS in the BM chimeras showing that ROS production is suppressed in CD36→Tg and Nox2→Tg mice (F). RFU, relative fluorescent units; * p<0.05 from WT→WT, CD36→Tg, or Nox2→Tg; ANOVA and Tukey’s test; n=5/group.
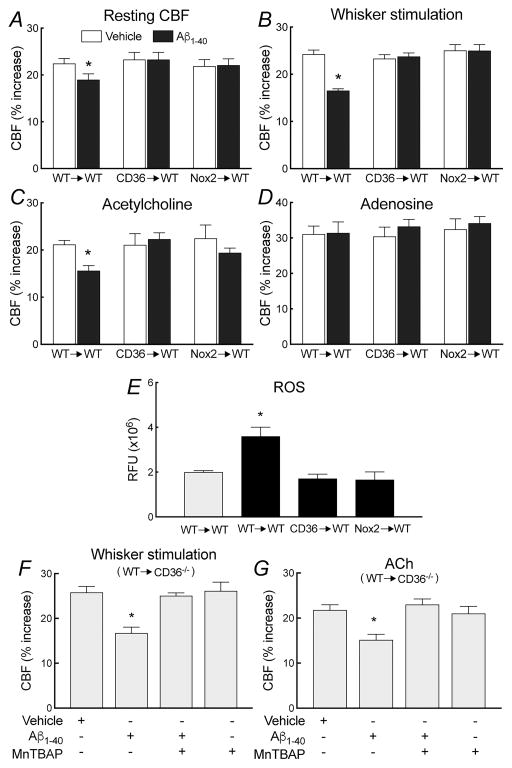
Next, we studied the cerebrovascular dysfunction induced by Aβ in BM chimeras. Functional hyperemia, endothelium-dependent CBF responses, and responses to adenosine, SNAP or hypercapnia in WT mice receiving WT BM (WT→WT) were identical to those observed in naïve WT mice (Figure 5A–D; Online Figure VIA–D), demonstrating that the BM transplantation procedure did not alter baseline cerebrovascular responses. However, the neurovascular dysfunction and vascular oxidative stress induced by topical application of Aβ1-40 were not observed in CD36−/−→WT or Nox2−/−→WT chimeras (Figure 5A–E; Online Figure VIA–D). Importantly, transplant of WT (CD36+/+) BM in CD36−/− mice was able to re-establish the neurovascular dysfunction induced by Aβ1-40 (Figure 5F–G). The effect was counteracted by neocortical superfusion of the ROS scavenger MnTBAP (Figure 5F–G; Online Figure VIE–F), consistent with the involvement of ROS in the dysfunction induced by the BM-derived PVM repopulating the cerebral vasculature after transplant.
Figure 5. Deletion of CD36 or Nox2 in PVM ameliorates the neurovascular dysfunction and vascular oxidative stress induced by Aβ1-40 superfusion in WT mice.
In WT→WT mice, cerebrovascular response to whisker stimulation, ACh, and adenosine do not differ from those of naïve WT mice (A–D). Furthermore, Aβ1-40 superfusion attenuates resting CBF and CBF responses to whisker stimulation and ACh, but not adenosine (A–D). However, these cerebrovascular effects of Aβ1-40 are not observed in CD36→Tg and Nox2→Tg chimeras (A–D). Aβ1-40 increases cerebrovascular ROS production WT→WT, but not in CD36→Tg and Nox2→Tg mice (E). Transplant of WT (CD36+/+) BM is able to re-establish the neurovascular dysfunction induced by Aβ in CD36−/− mice (WT→CD36−/−), an effect counteracted by neocortical superfusion of the ROS scavenger MnTBAP (50μM) (F,G). RFU, relative fluorescence units; *p<0.05 from vehicle (A–D), CD36→WT, or Nox2→WT (F,G); ANOVA and Tukey’s test; n=5–6/group.
In agreement with the Aβ superfusion findings, transplantation with CD36−/− or Nox2−/− BM rescued the neurovascular dysfunction in Tg2576 mice, without altering brain Aβ levels (Figure 6; Online Figure VII). Transplant of WT BM in Tg2576 mice did not suppress ROS production in PVM, but transplantation of CD36−/− or Nox2−/− BM abolished it (Figure 4), attesting to the role of CD36 and Nox2 in PVM in the Aβ-induced ROS production.
Figure 6. Deletion of CD36 or Nox2 in PVM counteracts the neurovascular dysfunction and vascular oxidative stress in Tg2576 mice.
Tg2576 mice receiving WT BM (WT→Tg) exhibit attenuation in CBF responses to whisker stimulation (A,B) and ACh (C) similar to those observed in Tg2576 mice not subjected to BM transplantation. Transplant of CD36−/− or Nox2−/− BM rescues the neurovascular dysfunction in Tg2576 mice (A–D), without altering brain Aβ1-40 and Aβ1-42 (E,F). *p<0.05 from WT→WT, CD36→Tg, or Nox2→Tg; ANOVA and Tukey’s test; n=5–6/group.
PVM depletion ameliorates the neurovascular dysfunction induced by circulating Aβ
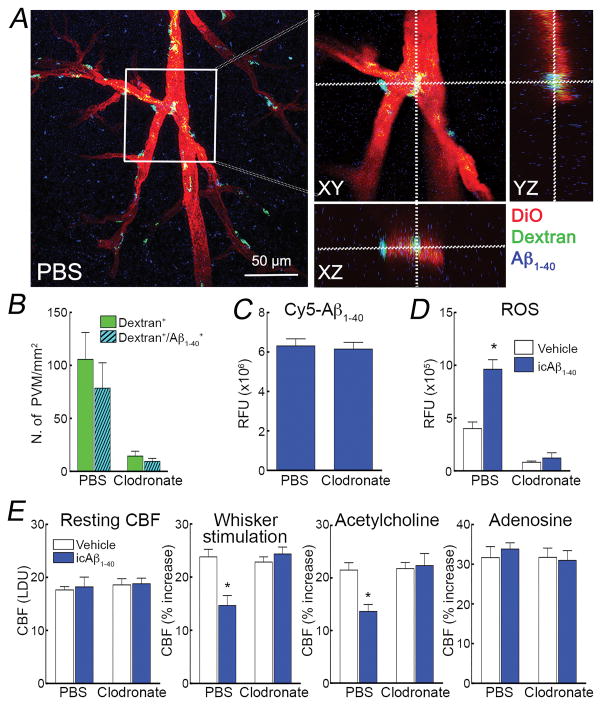
Circulating and brain Aβ both have a role in neurovascular dysfunction, an effect mediated by ROS25, 49. Circulating Aβ is thought to cross the BBB by engaging the receptor for advanced glycation end-products (RAGE)50. Therefore, we sought to determine if PVM also contribute to the neurovascular effects of circulating Aβ. To this end, first we sought to establish if blood borne Aβ enters the perivascular space and reaches PVM. Intracarotid administration of cy5-labelled Aβ1-40, at a concentration and infusion rate that produce a steady state increase in plasma Aβ comparable to that of Tg2576 mice25, 49, was able to cross the vascular wall, enter the perivascular space, and reach PVM (Figure 7A,B; Online Figure VIII). Clodronate depleted PVM without affecting Cy5-labelled Aβ1-40 (Figure 7C,D). As previously reported25, 49, Aβ1-40 infusion resulted in attenuation of the CBF increase produced by whisker stimulation and by endothelium-dependent vasodilators in the somatosensory cortex ipsilateral to the infusion, while endothelium-independent responses were not affected (Figure 7F). PVM depletion prevented in full the neurovascular dysfunction induced by circulating Aβ1-40, an effect associated with suppression of vascular oxidative stress (Figure 7E,F).
Figure 7. Circulating Aβ1-40 reaches PVM and mediates oxidative stress and neurovascular dysfunction.
In WT mice treated with PBS, Cy5-labelled Aβ1-40 infused into the carotid artery (Cy5-Aβ1-40) (1μM, 150 μl/hr) (blue) colocalizes with dextran-labelled PVM (green) surrounding cerebral blood vessels labelled with DiO (red), and resulting in the cyan color in the merged image (A). Orthogonal projections illustrating co-localization of dextran-labelled PVM (green) and Cy5-labelled Aβ (blue) around blood vessels (red) (B). Cy5-labelled Aβ is colocalized with more than 80% of dextran-positive PVM (C). In mice treated with clodronate, the signal from Cy5-labelled Aβ1-40 is still present in the brain and not different from PBS treated mice (D), but is no longer associated with PVM, which are depleted (Online Figure VIII). ROS are increased in PVM of mice receiving the i.c. infusion of Aβ1-40 (icAβ1-40), which is suppressed by clodronate treatment (E). icAβ1-40 attenuates the increase in CBF induced by whisker stimulation or ACh, an effect reversed by clodronate (F). Resting CBF or the CBF response to adenosine is not affected by icAβ (F). LDU, arbitrary laser-Doppler perfusion units; RFU relative fluorescence units; *p<0.05 from vehicle, ANOVA and Tukey’s test; n=5–6/group.
DISCUSSION
Several studies have shown that Aβ leads to vascular dysfunction through oxidative stress16, 17, 20, but the effector cells exposed to Aβ and responsible for the ROS production were not identified. Since brain PVM can produce large amounts of ROS34 and are located in the perivascular space, a major site of brain Aβ trafficking and accumulation31, 33, we sought to determine whether PVM are involved in the neurovascular effects of the peptide. We found that selective depletion of PVM abrogates the vascular oxidative stress and neurovascular dysfunction induced by Aβ, either applied directly to the neocortex of WT mice or produced endogenously in Tg2576 mice. The effect could not be attributed to depletion of microglia, to non-specific alterations in vascular structure and reactivity resulting in vasoparalysis, or to reductions in brain Aβ in Tg2576 mice. Elevated levels of circulating Aβ have been implicated in vascular alterations in AD and other dementias51–54, and lead to cerebrovascular disturbances in animal models49. Therefore, we asked if PVM also contribute to the deleterious neurovascular effects of circulating Aβ. We found that intravascular Aβ crosses the vascular wall, enters the perivascular space, reaches PVM, induced ROS production, and alters neurovascular function. These novel observations establish PVM as the main source of the vascular ROS responsible for the CBF alterations induced by parenchymal and plasma Aβ.
Next, we sought to establish whether CD36 and Nox2 in PVM are required for the deleterious cerebrovascular effects of Aβ. To this end, we used BM chimeras in which CD36 or Nox2 was deleted in PVM by transplantation of CD36- or Nox2-deficient BM. First, we verified that the extent of PVM replacement by blood-borne myeloid cells was comparable between the two mouse lines and was not altered by lack of CD36 or Nox2. Then, we tested the cerebrovascular effects of Aβ in the chimeras. The BM transplant procedure did not alter CBF regulation in WT mice or the suppression of neurovascular responses in Tg2576 mice. However, transplantation of CD36- or Nox2-deficient BM abrogated the cerebrovascular dysfunction and vascular oxidative stress in WT mice exposed to exogenous Aβ or in Tg2576 mice. Furthermore, WT BM was able to re-establish Aβ-induced vascular dysfunction in CD36-deficient mice, suggesting that the CD36 signaling pathway in PVM is absolutely necessary for the harmful vascular effects of Aβ. The findings, collectively, identify for the first time CD36 and Nox2 localized to PVM as the molecular substrates underlying the neurovascular dysfunction induced by Aβ.
Although cerebral endothelial cells in culture have the potential to produce ROS in response to Aβ via CD3642, depletion of PVM completely abrogates vascular oxidative stress and neurovascular dysfunction, suggesting an essential role of PVM in the neurovascular effects of Aβ in vivo. Therefore, previous results in which Aβ vasoactivity was not observed in CD36-null mice25 have to be attributed to lack of CD36 in PVM and not endothelial cells. This conclusion is also supported by our finding that WT BM transplant in CD36−/− mice, in which endothelial cells lack CD36, re-establishes the ROS-dependent neurovascular dysfunction induced by Aβ. Therefore, WT PVM are able to drive the neurovascular dysfunction even if endothelial cells lack CD36. Aβ has also been reported to increase ROS in isolated cerebral blood vessels25, 55. However, these vascular preparations are likely to also include PVM which remain attached to the outer vessel wall during the isolation procedure.
There has been an increasing appreciation for the role of PVM in the brain’s own innate immunity system35, 36. PVM, a unique population of brain myeloid cells distinct from microglia, have been implicated in hypothalamic-adrenal axis and neurohumoral activation in the setting of myocardial infarction, emotional stress or systemic inflammation 56–58. PVM may also have a role in neuroimmune trafficking in brain infections 38, 58, and in the structural and functional cerebrovascular alterations of hypertension34, 59, as well as in tumor angiogenesis and cerebrovascular repair60. In models of cerebral amyloidosis, PVM depletion reduces Aβ accumulation in cerebral blood vessels61. The present results expand our understanding of the pathobiology of these cells in AD by revealing a key role of PVM in Aβ-induced neurovascular dysfunction.
The perivascular space and associated innate immune cells have recently attracted much attention in the mechanisms of AD33, 62. The brain’s perivascular space is a major conduit for clearance of toxic molecules from the brain, including Aβ33, 63. In AD, tortuous perivascular spaces may impede Aβ clearance from the brain and promote the harmful interaction between Aβ and cerebral blood vessels, as well as cerebral amyloid angiopathy30, 31, 64. Our results provide the first demonstration that PVM are the cells in the perivascular space driving the neurovascular dysfunction induced by Aβ. On the other hand, increasing evidence suggests a role of innate immunity cells and receptors in AD pathogenesis62. While microglial cells, resident brain immune cells, may degrade Aβ and shield the brain from the deleterious effects of amyloid62, 65, innate immunity receptors, including CR1, TREM2, CD33, and CD36, among others, have been linked to increased AD susceptibility in genome-wide association studies22, 62. However, it remained unclear how innate immunity exerts its harmful effects in AD36. Our findings identify PVM as critical cells initiating Aβ-induced neurovascular dysfunction and suggest a new mechanism by which innate immune cells in the perivascular space may contribute to AD.
A limitation of the present study is that, by using presymptomatic Tg2576 mice, we could not assess the impact of PVM on the cognitive deficits associated with Aβ accumulation. This is for the following reasons. Considering that the effect of clodronate is limited to a few weeks38, we could not determine if PVM depletion protects Tg2576 mice from cognitive deficits, which develop over several months24, 26, 37. We also explored the possibility of using BM chimeras to delete Nox2 and CD36 in PVM. However, this approach proved not to be feasible because, as the Tg2576 BM chimeras age, the brain parenchyma is infiltrated by BM-derived cells with the morphological characteristics of microglia (unpublished observations)47. Therefore, the specific contribution of PVM replacement could not be reliably assessed under these conditions. These limitations notwithstanding, considering the well-established link between neurovascular dysfunction and cognitive impairment in this mouse model24, 26, it is reasonable to postulate that PVM depletion may result in improved cognition. However, this assumption needs to be verified experimentally. Future studies using more suitable methods to selectively target PVM in older APP mice could assess the role of PVM on cognitive dysfunction, as well in the phosphorylation of tau, a major pathogenic factor in AD which is influenced by endothelial NO 66.
In conclusion, we have demonstrated that PVM are essential for the neurovascular dysfunction induced by Aβ in three separate mouse models. PVM exert their harmful effects through the Aβ “receptor” CD36 and the ROS producing enzyme Nox2. PVM are also needed for the neurovascular dysfunction induced by circulating Aβ, an effect mediated by Aβ entering the perivascular space and reaching PVM to induce vascular oxidative stress. The findings identify PVM as the missing link between Aβ and neurovascular dysfunction, and unveil a new mechanism by which innate immune cells and receptors, long thought to play a role in the disease, contribute to the pathobiology of AD. Therefore, manipulating PVM could be a promising therapeutic strategy to stave off Aβ-induced brain dysfunction and damage.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Alzheimer’s disease (AD), the most common cause of late-life dementia, is characterized pathologically by the accumulation in brain of the amyloid-b peptide (Ab) and the microtubule associated protein tau
Alterations in the regulation of the cerebral microcirculation have recently emerged as a significant contributor to cognitive impairment in AD
The neurovascular dysfunction is mediated by reactive oxygen species (ROS) produced by the binding of Ab to the innate immunity receptor CD36 leading to activation of the superoxide producing enzyme Nox2
What New Information Does This Article Contribute?
Perivascular macrophages (PVM), myeloid cells juxtaposed to the outer wall of arteriole penetrating the substance of the brain, are the main source of the ROS mediating the neurovascular dysfunction produced by brain Ab
PVM are also responsible for neurovascular alterations mediated by plasma Ab, which crosses the blood-brain barrier and reaches these cells in the perivascular space
PVM are the cellular site of the expression of CD36 and NOX2 responsible for neurovascular dysfunction
Alterations in the regulation of cerebral microvasculature play a pathogenic role in AD. Such neurovascular dysfunction may deprive the brain of oxygen and glucose, amplifying the harmful neuronal effects of Aβ and tau pathology. The Aβ peptide in brain and plasma alters neurovascular function through CD36 and Nox2-derived ROS, but the cellular bases of the effect have not been elucidated. We report that brain PVM, a unique sub-population of resident brain macrophages exposed to Aβ draining through the perivascular space, are necessary and sufficient for the oxidative stress driving the neurovascular dysfunction in mouse models of amyloid pathology. Furthermore, we identify PVM as the cells expressing CD36 and Nox2 responsible for the vascular effects of Aβ. These observations establish PVM as the main source of the ROS responsible for the CBF alterations induced by Aβ, and identify these cells as the site of CD36 and Nox2 expression enabling the neurovascular dysfunction. The results expand our understanding of the role of PVM in brain diseases, suggest a new way in which innate immune cells may contribute to AD, and provide a new putative therapeutic target for this devastating disease.
Acknowledgments
SOURCES OF FUNDING
Supported by NIH grants R01-NS37853, R37-NS89323, R01-NS95441 and R01-NS100447. Contributions from the Feil Family Foundation and the Fondation Leducq are gratefully acknowledged.
Nonstandard Abbreviations and Acronyms
- Aβ
Amyloid-β
- ACh
Acetylcholine
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- BM
Bone marrow
- CBF
Cerebral blood flow
- CD33
Cluster of differentiation 33
- CD36
Cluster of differentiation 36
- CD206
Cluster of differentiation 206
- CR1
Complement receptor type 1
- Cy5
Cyanine 5
- CBF
Cerebral blood flow
- DiO
DiOC18(3) (3,3′-Dioctadecyloxacarbocyanine Perchlorate
- FITC
Fluorescein
- GFAP
Glial fibrillary acidic protein
- GFP
Green fluorescent protein
- Glut1
Glucose transporter 1
- Iba1
Ionized calcium binding adaptor molecule 1
- LDU
Arbitrary laser-Doppler perfusion units
- MnTBAP
Mn(III)tetrakis (4-benzoic acid) porphyrin
- Nox2
NADPH oxidase 2
- PFA
Paraformaldehyde
- PVM
Perivascular macrophages
- RFU
Relative fluorescence units
- ROS
Reactive oxygen species
- SDS
Sodium dodecyl sulfate
- SNAP
S-Nitroso-N-Acetyl-D,L-Penicillamine
- Tg2576
Mice overexpressing the Swedish mutation of the amyloid precursor protein (APPK670/671L)
- TREM2
Triggering receptor expressed on myeloid cells 2
- WT
Wild type
Footnotes
In April 2017, the average time from submission to first decision for all original research papers submitted to Circulation Research was 11.94 days.
DISCLOSURES
None.
References
- 1.De Strooper B, Karran E. The Cellular Phase of Alzheimer’s Disease. Cell. 2016;164:603–15. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9:63–75. e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–60. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 4.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer’s disease. Lancet. 2016;388:505–17. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 5.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–46. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 6.Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34:335–7. doi: 10.1161/01.str.0000054050.51530.76. [DOI] [PubMed] [Google Scholar]
- 7.Tosto G, Bird TD, Bennett DA, Boeve BF, Brickman AM, Cruchaga C, Faber K, Foroud TM, Farlow M, Goate AM, Graff-Radford NR, Lantigua R, Manly J, Ottman R, Rosenberg R, Schaid DJ, Schupf N, Stern Y, Sweet RA, Mayeux R National Institute on Aging Late-Onset Alzheimer Disease/National Cell Repository for Alzheimer Disease Family Study G. The Role of Cardiovascular Risk Factors and Stroke in Familial Alzheimer Disease. JAMA neurology. 2016;73:1231–1237. doi: 10.1001/jamaneurol.2016.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC Alzheimer’s Disease Neuroimaging I. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nature communications. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–94. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 10.Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis. 2014;42:S411–9. doi: 10.3233/JAD-141467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Silva TM, Faraci FM. Microvascular Dysfunction and Cognitive Impairment. Cell Mol Neurobiol. 2016;36:241–58. doi: 10.1007/s10571-015-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–66. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–38. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katusic ZS, Austin SA. Endothelial nitric oxide: protector of a healthy mind. Eur Heart J. 2014;35:888–94. doi: 10.1093/eurheartj/eht544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koizumi K, Wang G, Park L. Endothelial Dysfunction and Amyloid-beta-Induced Neurovascular Alterations. Cell Mol Neurobiol. 2016;36:155–165. doi: 10.1007/s10571-015-0256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han BH, Zhou ML, Abousaleh F, Brendza RP, Dietrich HH, Koenigsknecht-Talboo J, Cirrito JR, Milner E, Holtzman DM, Zipfel GJ. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J Neurosci. 2008;28:13542–50. doi: 10.1523/JNEUROSCI.4686-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–61. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 18.Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Abeta 1–40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–40. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol. 2002;283:H315–23. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- 20.Tong XK, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J Neurosci. 2012;32:4705–15. doi: 10.1523/JNEUROSCI.0169-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Eckman C, Younkin S, Hsiao KK, Iadecola C. Increased susceptibility to ischemic brain damage in transgenic mice overexpressing the amyloid precursor protein. J Neurosci. 1997;17:7655–61. doi: 10.1523/JNEUROSCI.17-20-07655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sery O, Janoutova J, Ewerlingova L, Halova A, Lochman J, Janout V, Khan NA, Balcar VJ. CD36 gene polymorphism is associated with Alzheimer’s disease. Biochimie. 2017;135:46–53. doi: 10.1016/j.biochi.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–77. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–52. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, Younkin L, Younkin SG, Van Nostrand WE, Cho S, Anrather J, Carlson GA, Iadecola C. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci U S A. 2011;108:5063–8. doi: 10.1073/pnas.1015413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park L, Zhou J, Zhou P, Pistick R, El Jamal S, Younkin L, Pierce J, Arreguin A, Anrather J, Younkin SG, Carlson GA, McEwen BS, Iadecola C. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2013;110:3089–94. doi: 10.1073/pnas.1300021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–50. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 28.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Science signaling. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–35. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez J, Berezuk C, McNeely AA, Gao F, McLaurin J, Black SE. Imaging the Perivascular Space as a Potential Biomarker of Neurovascular and Neurodegenerative Diseases. Cell Mol Neurobiol. 2016;36:289–99. doi: 10.1007/s10571-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008;18:253–66. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–22. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Menard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain--implications for Alzheimer diseaser. Nat Rev Neurol. 2016;12:248. doi: 10.1038/nrneurol.2016.36. [DOI] [PubMed] [Google Scholar]
- 34.Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, Racchumi G, Murphy M, Van Rooijen N, Anrather J, Iadecola C. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126:4674–4689. doi: 10.1172/JCI86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kierdorf K, Prinz M, Geissmann F, Gomez Perdiguero E. Development and function of tissue resident macrophages in mice. Semin Immunol. 2015;27:369–78. doi: 10.1016/j.smim.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20:136–144. doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- 37.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 38.Polfliet MM, Goede PH, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. A method for the selective depletion of perivascular and meningeal macrophages in the central nervous system. J Neuroimmunol. 2001;116:188–95. doi: 10.1016/s0165-5728(01)00282-x. [DOI] [PubMed] [Google Scholar]
- 39.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–44. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Song Y, Zhao L, Gaidosh G, Laties AM, Wen R. Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat Protoc. 2008;3:1703–8. doi: 10.1038/nprot.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016 doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park L, Wang G, Moore J, Girouard H, Zhou P, Anrather J, Iadecola C. The key role of transient receptor potential melastatin-2 channels in amyloid-beta-induced neurovascular dysfunction. Nature communications. 2014;5:5318. doi: 10.1038/ncomms6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Bonilla L, Racchumi G, Murphy M, Anrather J, Iadecola C. Endothelial CD36 Contributes to Postischemic Brain Injury by Promoting Neutrophil Activation via CSF3. J Neurosci. 2015;35:14783–93. doi: 10.1523/JNEUROSCI.2980-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iadecola C. Nitric oxide participates in the cerebrovasodilation elicited from cerebellar fastigial nucleus. Am J Physiol. 1992;263:R1156–61. doi: 10.1152/ajpregu.1992.263.5.R1156. [DOI] [PubMed] [Google Scholar]
- 45.Iadecola C. Does nitric oxide mediate the increases in cerebral blood flow elicited by hypercapnia? Proc Natl Acad Sci U S A. 1992;89:3913–6. doi: 10.1073/pnas.89.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 47.Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, Kummer MP, Quinn M, Bruck W, Bechmann I, Heneka MT, Priller J, Prinz M. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J Neurosci. 2011;31:11159–71. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Ramos J, Song S, Sava V, Catlow B, Lin X, Mori T, Cao C, Arendash GW. Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer’s mice. Neuroscience. 2009;163:55–72. doi: 10.1016/j.neuroscience.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park L, Zhou P, Koizumi K, El Jamal S, Previti ML, Van Nostrand WE, Carlson G, Iadecola C. Brain and circulating levels of Abeta1-40 differentially contribute to vasomotor dysfunction in the mouse brain. Stroke. 2013;44:198–204. doi: 10.1161/STROKEAHA.112.670976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–13. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 51.Gomis M, Sobrino T, Ois A, Millan M, Rodriguez-Campello A, Perez de la Ossa N, Rodriguez-Gonzalez R, Jimenez-Conde J, Cuadrado-Godia E, Roquer J, Davalos A. Plasma beta-amyloid 1-40 is associated with the diffuse small vessel disease subtype. Stroke. 2009;40:3197–201. doi: 10.1161/STROKEAHA.109.559641. [DOI] [PubMed] [Google Scholar]
- 52.Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, Rosand J, Growdon JH, Greenberg SM. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–9. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 53.Head E, Doran E, Nistor M, Hill M, Schmitt FA, Haier RJ, Lott IT. Plasma amyloid-beta as a function of age, level of intellectual disability, and presence of dementia in Down syndrome. J Alzheimers Dis. 2011;23:399–409. doi: 10.3233/JAD-2010-101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marti-Fabregas J, Delgado-Mederos R, Marin R, de la Ossa NP, Alonso de Lecinana M, Rodriguez-Yanez M, Sanahuja J, Purroy F, De Arce AM, Carrera D, Dinia L, Guardia-Laguarta C, Lleo A. Prognostic value of plasma beta-amyloid levels in patients with acute intracerebral hemorrhage. Stroke. 2014;45:413–7. doi: 10.1161/STROKEAHA.113.002838. [DOI] [PubMed] [Google Scholar]
- 55.Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C. Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab. 2004;24:334–42. doi: 10.1097/01.WCB.0000105800.49957.1E. [DOI] [PubMed] [Google Scholar]
- 56.Serrats J, Grigoleit JS, Alvarez-Salas E, Sawchenko PE. Pro-inflammatory immune-to-brain signaling is involved in neuroendocrine responses to acute emotional stress. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Serrats J, Schiltz JC, Garcia-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–15. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pires PW, Girgla SS, McClain JL, Kaminski NE, van Rooijen N, Dorrance AM. Improvement in middle cerebral artery structure and endothelial function in stroke-prone spontaneously hypertensive rats after macrophage depletion. Microcirculation. 2013;20:650–61. doi: 10.1111/micc.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis CE, Harney AS, Pollard JW. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell. 2016;30:18–25. doi: 10.1016/j.ccell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2009;106:1261–6. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015;16:229–36. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 63.Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: the role, regulation and restoration of LRP1. Front Aging Neurosci. 2015;7:136. doi: 10.3389/fnagi.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan P, Condello C, Keene CD, Wang Y, Bird TD, Paul SM, Luo W, Colonna M, Baddeley D, Grutzendler J. TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron. 2016;90:724–39. doi: 10.1016/j.neuron.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Austin SA, Katusic ZS. Loss of Endothelial Nitric Oxide Synthase Promotes p25 Generation and Tau Phosphorylation in a Murine Model of Alzheimer’s Disease. Circ Res. 2016;119:1128–1134. doi: 10.1161/CIRCRESAHA.116.309686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.