Abstract
Objective/Background
Poor sleep quality is common in pregnancy and associated with increased psychological distress, which has adverse consequences for families. Emerging theory suggests that mindfulness-based interventions may help reduce cognitive and emotional reactivity to stressful events. The current study examines the effects of a mindfulness-based intervention on the relationship between poor sleep quality and increased depression symptom severity and perceived stress during pregnancy. Additionally, we explored the prevalence of poor sleep quality in this unique sample and the impact of intervention on sleep quality.
Participants
Participants were 215 ethnically diverse, overweight and obese, predominantly low-income pregnant women drawn from a study examining the impact of an 8-week mindfulness-based program (Mindful Moms Training; MMT) to reduce excessive gestational weight gain, stress, and depression compared to treatment as usual (TAU).
Methods
Participants reported global sleep quality, depressive symptoms, and perceived stress at baseline and post-intervention.
Results
Most participants (63%) were categorized as poor sleepers at baseline. MMT participants did not experience significantly greater improvement in sleep quality compared to TAU participants. Baseline poor global sleep quality predicted increased depression symptom severity for all participants. Baseline poor global sleep quality predicted increased perceived stress for the TAU group only; this association was not evident in the MMT group.
Conclusions
Poor sleep quality is prevalent in overweight and obese predominantly low-income pregnant women. Poor sleep quality was associated with worsening psychological distress, but mindfulness training significantly attenuated the influence of poor sleep on perceived stress.
Keywords: Women’s Sleep, Depression, Stress, Pregnancy, Mindfulness
Introduction
Psychological distress is associated with negative consequences for pregnant women and their offspring (Stein et al., 2014). For example, antenatal depression is associated with adverse obstetric outcomes, more negative parenting behaviors, and long-lasting psychological consequences for offspring such as internalizing and externalizing disorders (Goodman, Broth, Hall, & Stowe, 2008; Goodman et al., 2011; Grote et al., 2010). Thus, there have been increased efforts to target risk factors to prevent psychological distress during pregnancy. Mounting evidence in both general and pregnant samples indicates that poor sleep quality may be one such risk factor. To our knowledge, the current study is the first to examine the impact of an intervention on the association between poor overall sleep quality and increasing psychological distress during pregnancy.
Hormonal changes and physical symptoms of pregnancy, such as nausea, increased urination, back pain, and heartburn may impair sleep quality (Balserak & Lee, 2011). As many as 76% of pregnant women experience poor global sleep quality, a comprehensive measure that includes duration, disturbance, latency, efficiency and quality (Mindell, Cook, & Nikolovski, 2015). Poor sleep quality is related to increased depression and perceived stress during pregnancy. Cross-sectional data has shown that depression symptom severity is significantly associated with worse global sleep quality and sleep disturbance in the third trimester (Chang, Brown, Nitzke, Smith, & Eghtedary, 2015; Volkovich, Tikotzky, & Manber, 2015), and depressed pregnant women report more sleep disturbance in the second and third trimesters compared to non-depressed pregnant women (Field et al., 2007). Furthermore, prospective research indicates that poor sleep predicts higher depression symptom severity and worsening during pregnancy (Skouteris, Germano, Wertheim, Paxton, & Milgrom, 2008), consistent with patterns found within general populations (Baglioni et al., 2011).
Research on the relationship between sleep and perceived stress, another aspect of psychological distress associated with adverse outcomes for pregnant women (Dunkel Schetter, 2011), is limited and more mixed. Some cross-sectional research indicates significant relationships between sleep disturbances and higher perceived stress (Blair, Porter, Leblebicioglu, & Christian, 2015), while others have found no associations between perceived stress and sleep duration, disturbance, quality, or latency (Chang et al., 2015). Prospective research points to a bidirectional relationship. For example, chronic stress predicts sleep disturbance (Hall et al., 2015), and in the other direction, poor sleepers have been found to have increased subjective and physiologic stress responses to both mild and severe stressors (Minkel et al., 2012; Wu et al., 2015).
Increasing evidence suggests that mindfulness-based interventions may improve well-being during pregnancy. A recent meta-analysis of 17 studies indicated that mindfulness-based interventions were associated with decreased depression, anxiety, and stress at post-intervention among pregnant and postpartum women (Lever Taylor, Cavanagh, & Strauss, 2016). Ong and colleagues have theorized that mindfulness-based interventions could improve clinical insomnia by targeting maladaptive cognitive and emotional reactivity related to sleep (Ong, Ulmer, & Manber, 2012). Evidence from a randomized controlled trial demonstrates the efficacy of both mindfulness-based stress reduction and mindfulness-based therapy adapted for insomnia for patients with chronic insomnia (Ong et al., 2014). Mindfulness-based interventions may also improve sleep in individuals without insomnia. For example, Kanen and colleagues conducted a meta-analysis of 16 mostly uncontrolled studies of mindfulness-based interventions including a broad range of populations (Kanen, Nazir, Sedky, & Pradhan, 2015). They found significant improvements in subjective, but not objective, reports of sleep in the general population, and effect sizes ranged from small to large. Evidence is also mixed for populations whose sleep may be disturbed by physical complaints, such as women with breast cancer (Lengacher et al., 2015). Of particular relevance for the current study, a small pilot study (N=15) found that mindful yoga was associated with improved sleep among pregnant participants who began the study during their second trimester (n=7), but not for women in their third trimester (Beddoe, Lee, Weiss, Kennedy, & Yang, 2010). In the current study, we explored the impact of mindfulness training on poor sleep quality during pregnancy compared to a treatment as usual control. Our examination of the impact of mindfulness training on sleep was exploratory for two reasons. First, the mindfulness-based intervention utilized in the current study did not focus on improving sleep. Specifically, participants were drawn from a study examining an 8-week mindfulness-based program to reduce stress, depression, and excessive gestational weight gain (ClinicalTrials.Gov identifier NCT01307683). Second, our examination of the impact of mindfulness training on sleep was exploratory because we were uncertain about the extent to which poor global sleep quality can be improved during pregnancy.
Despite evidence linking poor sleep and psychological distress in pregnancy, there is a dearth of research investigating interventions for ameliorating deleterious effects of poor sleep on distress. Mindfulness-based interventions may hold promise. Although poor sleep quality may place pregnant women at risk for greater psychological distress, an improved ability to respond to the experience of sleeping poorly may attenuate its impact on psychological distress. Women who receive mindfulness training may be less psychologically impacted by poor sleep quality through the mechanisms of decreased cognitive and emotional reactivity and increased acceptance of difficult and sometimes unchangeable experiences. Accordingly, we hypothesized that mindfulness training would change the relationship between poor sleep quality and increased psychological distress during pregnancy; specifically that pregnant women trained in mindfulness would show a weaker association between sleep quality and distress than would control participants.
The current study focuses on a sample of ethnically diverse, overweight or obese, predominantly low-income pregnant women. Participants were drawn from a study examining the effects of an 8-week mindfulness-based program on stress, depression, and excessive gestational weight gain compared to treatment as usual. Primary outcome results indicate that the intervention led to significant reductions in perceived stress and depression symptom severity as well as improvements in the ability to accept negative states, which were explicit targets of the intervention, but not in the percentage of women who gained excessive weight during pregnancy (Epel et al., in prep). Of interest for the current paper, previous research indicates that women in this sample (i.e., predominantly low income, and overweight or obese) may be at increased risk for poor sleep (Jarosz et al., 2014; Mindell et al., 2015; Okun, Tolge, & Hall, 2014; Rich-Edwards et al., 2006). The first goal of the study was to descriptively examine the prevalence of poor global sleep quality in this unique high-risk sample. Second, we explored whether participants in the mindfulness group experienced significantly greater improvements in global sleep quality compared to treatment as usual participants. Finally, we investigated whether the 8-week mindfulness-based intervention buffered the detrimental relationship between poor sleep quality and psychological distress (i.e., depression symptom severity and perceived stress).
Methods
Participants and Procedures
Participants were drawn from a study examining an 8-week mindfulness-based program to reduce stress, depression, and excessive gestational weight gain in overweight and obese predominantly low-income pregnant women. The study protocol was approved by the institutional review boards at University of California San Francisco, California Pacific Medical Center, University of California Berkeley, and Contra Costa Regional Medical Center and Health Centers. Participants were recruited between August 2011 and June 2013 from hospital-based clinics, community health centers, Supplemental Nutrition Program for Women, Infants and Children offices, organizations providing services to pregnant women, and through online advertisements. Details of our recruitment strategy have been published previously (Coleman-Phox et al., 2013). All participants provided written informed consent.
Inclusion criteria for all participants included 1) English speaking, 2) pregnant, 3) age 18–45 years, 4) self-reported pregravid body mass index 25–41 kg/m2, and 5) low- to moderate-income. Moderate-income for this study was defined as below the median family income for San Francisco in 2011: $73,563 (Noss, 2013). Exclusionary criteria included 1) medical conditions that might affect gestational weight gain (e.g. diabetes, abnormal glucose screen in early pregnancy, hypertension, and eating disorders), 2) current mindfulness meditation practice more than once per week, 3) multiple gestation, 4) currently taking weight loss drugs, medications for diabetes, antidepressants, antipsychotics, opiate drugs, or corticosteroids, and 5) history of gastric bypass surgery. Women with singleton pregnancies 12–19 weeks gestation at the start of the intervention and able to attend 8 weekly 2-hour intervention classes were eligible to enroll in the intervention group (Mindful Moms Training, MMT). Women unable to attend MMT classes or with gestational age 20–24 weeks at enrollment who otherwise met the study criteria were eligible for the treatment as usual (TAU) group. All participants were asked to complete study questionnaires at baseline and 8–10 weeks later (post-intervention). The parent study was designed according to the ORBIT model for developing behavioral treatments (Czajkowski et al., 2015). Specifically, the MMT intervention was being tested in an early proof-of-concept pilot study of efficacy. Randomized assignment was not utilized during the current phase of research because the main goal was to examine whether MMT achieved benefit on gestational weight gain before proceeding to pilot testing that utilizes more rigorous and costly methods such as randomized assignment.
Intervention
Mindful Moms Training (MMT)
MMT aimed to reduce overeating through mindful coping with stress. MMT included elements to support healthier eating and encourage physical activity, and focused on increasing attention to and awareness of physical sensations, thoughts and emotions, and accepting negative experiences versus ruminating about them. MMT was adapted from a Mindful Motherhood intervention that has been shown to significantly reduce anxiety and negative affect among pregnant women compared to a waitlist control (Vieten, 2009; Vieten & Astin, 2008), as well as from the Mindfulness-Based Eating Awareness Training that teaches skills to differentiate hunger cues and emotion responses, and to detect and respond to satiety cues (Daubenmier et al., 2011; Kristeller & Hallett, 1999; Kristeller & Wolever, 2011). MMT consisted of 8 consecutive 2-hour weekly group sessions facilitated by two experienced mindfulness teachers who received weekly supervision in MMT. Each class included a mindful movement practice, participant sharing in response to facilitator questions, discussion of an awareness/acceptance topic, mindful eating practice, and ended with a sitting mindful awareness/stress reduction practice. Participants were encouraged to complete daily home practice.
Treatment as usual (TAU)
No restrictions were placed on the mental health care TAU participants received during the study period, and participants with elevated depression symptom severity scores were referred to mental health providers and allowed to remain in the trial. TAU participants completed study measures at baseline and approximately 8–10 weeks later (“post-intervention”).
Measures
Demographics
A study-designed questionnaire was used to assess demographic variables such as age, household income, household size, education, race, and pregravid body mass index (BMI=weight (kilograms)/height (meters2)).
Depression symptom severity
The Patient Health Questionnaire (PHQ) was administered at baseline and post-intervention to assess depression symptom severity in the previous two weeks (Kroenke, Spitzer, & Williams, 2001). The PHQ has been previously validated in a sample of pregnant, predominantly low-income women (Sidebottom, Harrison, Godecker, & Kim, 2012). The PHQ contains nine items, but one item assessing sleep was removed for the current analyses. Thus, possible scores in the current study range from 0–24, with higher scores indicating higher depression symptom severity. Cronbach’s alphas were > 0.80 at both timepoints.
Perceived stress
The 10-item Perceived Stress Scale (PSS) was used to measure the extent to which situations in the last month were subjectively appraised as stressful (Cohen, Kamarck, & Mermelstein, 1983), and was administered at baseline and post-intervention. Possible scores range from 0–40, with higher scores indicating higher perceived stress. Cronbach’s alphas were > 0.85 at both timepoints.
Global sleep quality
The Pittsburgh Sleep Quality Index (PSQI) is a 19-item measure comprised of seven components assessing sleep duration, disturbance, latency, efficiency, quality, days of dysfunction due to sleepiness, and needing medication to sleep (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). Each component score ranges from 0–3, and the components are summed to create a PSQI global sleep quality score ranging from 0–21. Higher scores indicate worse global sleep quality, and scores above 5 indicate poor sleep. Cronbach’s alphas were > 0.71 at both timepoints.
Data Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at University of California San Francisco (Harris et al., 2009). REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies. All data analyses were performed using SPSS version 23. Item mean substitution was used for participants missing less than or equal to 30% of data on the PSS or PHQ (n=10 at baseline and post-intervention for PSS; n= 8 at baseline and n=6 at post-intervention for PHQ). Component mean substitution was used for participants missing one PSQI component (n=15 at baseline; n=15 at post-intervention). Participants were excluded from analyses if missing >30% of data on the PSS (n=12 baseline, n=44 post-intervention) or PHQ (n=6 baseline, n=44 post-intervention), or for missing >1 component on the PSQI (n=52 baseline, n=79 post-intervention, including 35 who were missing data at each time point due to an administration error).
To examine sleep quality, we present means and standard deviations of PSQI global sleep quality scores and the percentage of participants experiencing poor global sleep quality (i.e., PSQI global sleep quality score > 5) at each time point. We also report the percentage of participants who had an increased PSQI global sleep quality score from baseline to post-intervention.
We explored the impact of mindfulness training on PSQI global sleep quality in two ways. First, we used a chi-square test of homogeneity to examine whether the proportion of participants categorized as poor sleepers at post-intervention was significantly different between groups (MMT vs TAU). Second, we employed repeated measures analyses of variance to test group (MMT, TAU) by time (baseline, post-intervention) interactions for PSQI global sleep quality scores.
Finally, we examined the impact of mindfulness training on the relationship between global sleep quality and psychological distress. First, we ran Pearson’s product-moment correlations to assess the relationships between PSQI global sleep quality, depression symptom severity, and perceived stress at baseline and post-intervention by group. Second, hierarchical multiple regression models were used to examine the impact of mindfulness training on the relationship between baseline global sleep quality and increasing distress. To identify demographic covariates to include in the hierarchical multiple regression models, we examined the relationships between race/ethnicity, poverty, partner status, education, age at enrollment, and pregravid BMI with each dependent variable. Age at enrollment was significantly associated with post-intervention depression symptom severity, and thus was adjusted for accordingly. Given the recruitment strategy that women in TAU tended to be later in their pregnancy than women in MMT, we adjusted for gestational age at enrollment. No other demographic variables were significantly associated with the dependent variables. Finally, hierarchical multiple regression models were used to examine the interaction of group and baseline PSQI global sleep quality on post-intervention depression symptom severity and perceived stress. In other words, we tested whether enrollment in a mindfulness-based intervention modified the prospective contribution of global sleep quality on changes in psychological distress in our sample. We entered baseline depression symptom severity (or perceived stress), group, gestational age at enrollment, and baseline PSQI global sleep quality in the first step, and the interaction between baseline PSQI global sleep quality and group in the second step.
Results
Participant Flow and Descriptives
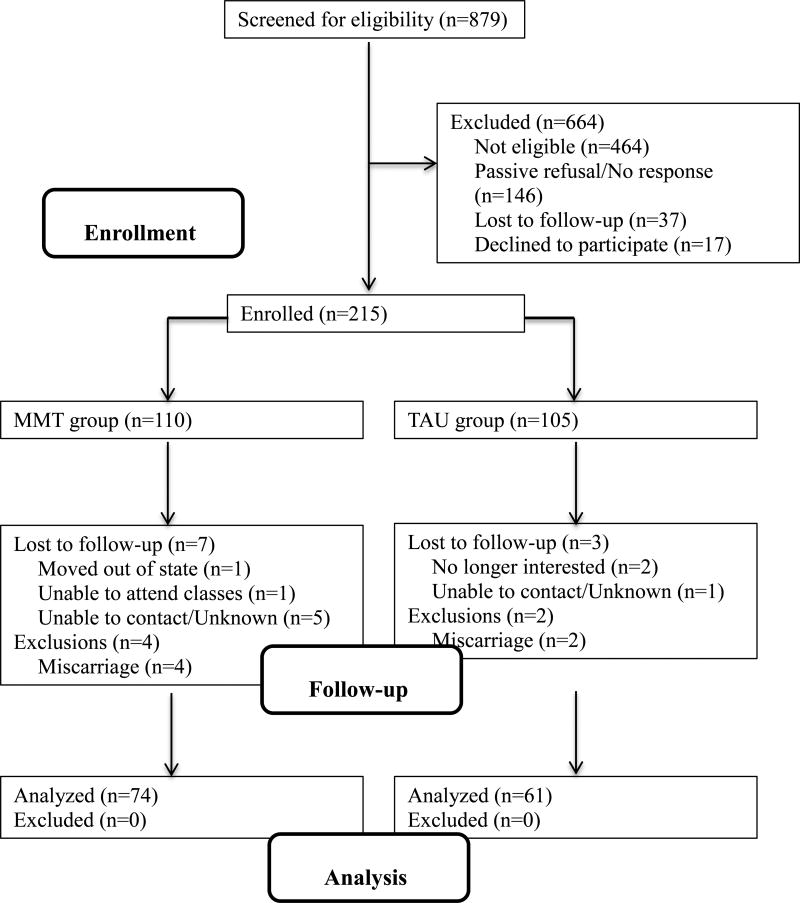
Participant flow is presented in a CONSORT diagram in Figure 1. Of the 879 screened, 269 (31%) were eligible, and 215 (80%) were enrolled among those eligible. Sample sizes for analyses vary due to missing data. The analysis sample size presented in Figure 1 is for the Group × Baseline PSQI interaction for depression symptom severity. The n for the Group × Baseline PSQI for perceived stress is 134 (n=73 MMT). The n for the change in global sleep quality analysis is 116 (n=64 MMT). Baseline sociodemographic characteristics by assignment group is presented in Table 1. There were no significant between group differences on age, race, education, federal poverty level, or marital status at baseline. When testing between group differences in gestational age at enrollment, a preliminary Levene’s test for equality of variances indicated that the variances of the two groups were significantly different, so an independent samples t-test that does not assume equal variances was used to examine between-group differences. This indicated that TAU participants had a significantly later gestational age at enrollment compared to MMT participants (t(166.75) = 10.22, p < .001) due to the recruitment strategy employed
Figure 1.
CONSORT participant flow diagram. MMT=Mindful Moms Training; TAU = treatment as usual. The n presented in the analysis boxes is for the Group × Baseline PSQI for depression symptom severity. Sample size for remaining analyses vary due to missing data (see text).
Table 1.
Participant Baseline Sociodemographic Characteristics By Group, Reported as M (SD) or % (n)
| Characteristic | TAU | MMT |
|---|---|---|
| Age (years) | 27.96 (5.99) | 27.85 (5.72) |
| Gestational age at enrollment (weeks)* | 19.99 (4.45) | 14.81 (2.62) |
| Race/ethnicity | ||
| African American | 44.76% (47) | 35.78% (39) |
| Latina | 25.71% (27) | 32.11% (35) |
| Other/Multiracial | 15.24% (16) | 19.27% (21) |
| White | 14.29% (15) | 12.84% (14) |
| High school education or equivalent | 84.76% (89) | 90.91% (100) |
| Below 100% poverty level | 52.58% (51) | 47.12% (49) |
| Married, in a committed relationship, or engaged | 69.23% (72) | 67.27% (74) |
| Number of children | 1.01 (1.31) | 0.87 (1.05) |
Due to the recruitment strategy employed, TAU participants had a significantly later gestational age compared to MMT participants, p<.001.
Descriptive data for baseline and post-intervention depression symptom severity, perceived stress, and sleep quality by group are presented in Table 2. There were no significant between group differences for these measures at baseline. At both time points, the majority of TAU and MMT participants were categorized as poor sleepers, as defined by a PSQI total score > 5. About a third of participants in each group had an increased PSQI global sleep quality score from baseline to post-intervention (36.54% and 35.94% for TAU and MMT participants, respectively).
Table 2.
Descriptive Data for Depression Symptom Severity, Perceived Stress, and Sleep Quality by Group and Timepoint, Reported as M (SD) or % (n)
| Characteristic | TAU | MMT | ||
|---|---|---|---|---|
|
| ||||
| Baseline | Post- intervention |
Baseline | Post- intervention |
|
| Depression Symptom Severity (PHQ) | 5.68 (4.39) | 4.84 (3.93) | 6.16 (4.82) | 3.52 (3.52) |
| Perceived Stress (PSS) | 18.71 (6.65) | 17.05 (7.35) | 18.78 (6.27) | 15.64 (5.82) |
| Global Sleep Quality (PSQI) | 6.58 (3.51) | 6.77 (3.25) | 6.96 (3.71) | 6.40 (3.58) |
| Poor Sleep Quality (PSQI > 5) | 64.38% (47) | 69.35% (43) | 62.22% (56) | 56.72% (38) |
Note. PHQ = Patient Health Questionnaire, sleep item removed; PSQI = Pittsburgh Sleep Quality Index; PSS = Perceived Stress Scale
Exploratory analysis: Change in Global Sleep Quality by Group
The between-group difference in the proportion of participants categorized as poor sleepers at post-intervention was not statistically significant (Χ2 = 2.21, p = .14; see Table 2 for percentages). Additionally, the Group × Time interaction for PSQI global sleep quality was not significant, F(1, 114) = 0.039, p =.84, partial eta2 <.001. There were no main effects of group (F(1, 114) = 0.15, p = .70, partial eta2 = .001) or time (F(1, 114) = 2.05, p = .16, partial eta2 = .018).
Impact of Intervention on relationship between Sleep and Distress
At baseline, PSQI global sleep quality was significantly correlated with depression symptom severity (MMT r = 0.41, p < .001; TAU r = 0.47, p < .001) and perceived stress (MMT r = 0.28, p = .01; TAU r = .42, p < .001) in both groups. At post-intervention, PSQI global sleep quality was significantly correlated with depression symptom severity in both groups (MMT r = 0.27, p = .03; TAU r = 0.40, p = .001). Post-intervention PSQI global sleep quality was significantly correlated with perceived stress only for TAU participants (MMT r = 0.13, p = .30; TAU r = 0.50, p < .001).
Next, we tested whether enrollment in a mindfulness-based intervention modified the prospective contribution of global sleep quality on changes in depression symptom severity and perceived stress. There was no significant interaction between intervention group and baseline PSQI global sleep quality for post-intervention depression (b = −0.12, SE = 0.16, p=0.46), after adjusting for the effects of baseline depression symptom severity, gestational age at enrollment, and age. Examining the simple slopes revealed a statistically significant positive linear relationship between baseline PSQI global sleep quality, indicative of poorer overall sleep quality, and post-intervention depression symptom severity for TAU participants (b = 0.28, SE = 0.13, p = .025) and a non-significant positive relationship for MMT participants (b = 0.17, SE = 0.11, p = .11), after adjusting for the effects of baseline depression symptom severity, gestational age at enrollment, and age. See Table 3.
Table 3.
Hierarchical Regression Analyses Predicting Post-Intervention Depression Symptom Severity (Top Panel, n=135) and Perceived Stress (Bottom Panel, n=134)
| Post-intervention Depression Symptom Severity
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1
|
Model 2
|
|||||||
| Predictor | B | SE | t | B | SE | t | ||
| Constant | 3.31 | 2.26 | 1.46 | 3.06 | 2.29 | 1.33 | ||
| Baseline PHQ | 0.37 | 0.07 | 5.04** | 0.36 | 0.07 | 4.96** | ||
| Group | −1.63 | 0.74 | −2.20* | −0.89 | 1.25 | −0.71 | ||
| Gestational age | −0.04 | 0.08 | −0.50 | −0.05 | 0.08 | −0.59 | ||
| Age | −0.04 | 0.05 | −0.81 | −.004 | 0.05 | −0.83 | ||
| Baseline PSQI | 0.22 | 0.08 | 2.58* | 0.28 | 0.13 | 2.27* | ||
| Baseline PSQI*Group | −0.12 | 0.16 | −0.74 | |||||
| R2 | 0.31** | |||||||
| ΔR2 | 0.03 | |||||||
| Cohen’s f2 | 0.45 | 0.52 | ||||||
|
| ||||||||
| Post-intervention Perceived Stress | ||||||||
|
|
||||||||
| Model 1 | Model 2 | |||||||
|
|
|
|||||||
| Predictor | B | SE | t | B | SE | t | ||
|
| ||||||||
| Constant | 5.16 | 3.26 | 1.58 | 4.04 | 3.26 | 1.24 | ||
| Baseline PSS | 0.47 | 0.08 | 5.88** | 0.45 | 0.08 | 5.66** | ||
| Group | −1.43 | 1.26 | −1.13 | 2.14 | 2.12 | 1.01 | ||
| Gestational age | −0.01 | 0.13 | −0.08 | −0.05 | 0.13 | −0.34 | ||
| Baseline PSQI | 0.47 | 0.14 | 3.32* | 0.81 | 0.21 | 3.80** | ||
| Baseline PSQI*Group | −0.56 | 0.27 | −2.09* | |||||
| R2 | 0.36** | |||||||
| ΔR2 | 0.02 | |||||||
| Cohen’s f2 | 0.56 | 0.38 | ||||||
Note. PHQ = Patient Health Questionnaire, sleep item removed; PSQI = Pittsburg Sleep Quality Index; PSS = Perceived Stress Scale.
p < .05;
p < .001
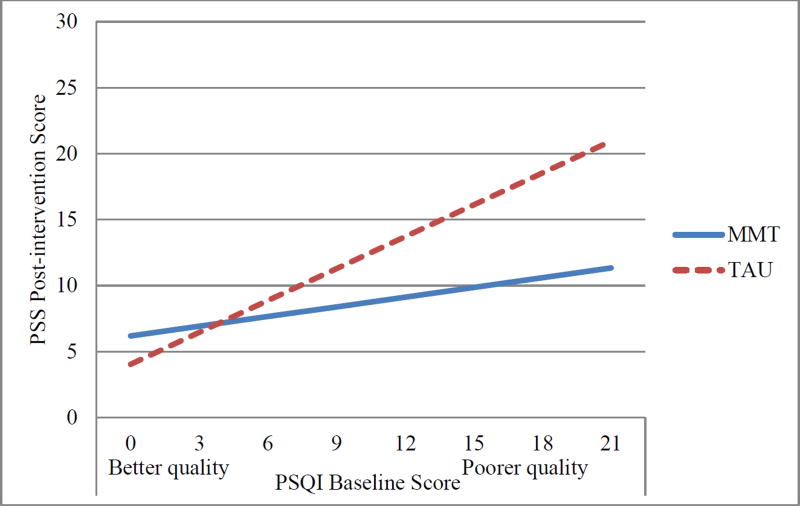
Hierarchical multiple regression revealed that intervention group moderated the effect of baseline PSQI global sleep quality on post-intervention perceived stress (Table 3). Tests of the simple slopes revealed there was a statistically significant positive linear relationship between baseline PSQI global sleep quality and post-intervention perceived stress for TAU participants (b = 0.81, SE = 0.21, p < .001), such that poorer PSQI global sleep quality at baseline was associated with higher perceived stress at post-intervention, after adjusting for the effects of baseline perceived stress and gestational age at enrollment. This association was nonsignificant for mindfulness participants (b = 0.25, SE = 0.18, p=0.17). See Figure 2.
Figure 2.
Interaction of Baseline PSQI × Group for post-intervention perceived stress. Depicted are simple slopes adjusting for baseline perceived stress and gestational age. As depicted here, a baseline PSQI global sleep quality score of 15 was associated with a score of approximately 16 on the PSS at post-intervention for TAU participants, and 10 for MMT participants. MMT=Mindful Moms Training; TAU = treatment as usual.
Discussion
The current study represents an important contribution to the small but growing body of research demonstrating the role of poor sleep quality in worsening distress during pregnancy. The major findings indicate that a mindfulness intervention for pregnant women disrupted the association between poor global sleep quality and perceived stress. Poor sleep quality at baseline predicted increases in perceived stress over the two-month study period in treatment as usual participants. Intriguingly, for women who received mindfulness training, overall sleep quality at baseline was not related to increased stress, suggesting that even women who slept poorly at baseline were protected from experiencing higher perceived stress at post-intervention. Evidence for reductions in the impact of poor sleep quality on perceived stress is important due to potential benefits of averting the negative consequences of stress during pregnancy. Antenatal stress has been associated with risk for adverse obstetric outcomes, as well as potential brain and behavior consequences for offspring (Bock, Wainstock, Braun, & Segal, 2015; Staneva, Bogossian, Pritchard, & Wittkowski, 2015; Weinstock, 2008).
More research is needed to replicate the buffering effect and to elucidate the mechanisms by which mindfulness training may attenuate (or disrupt) the relationship between poor sleep quality and perceived stress. One of the main skills taught in the Mindful Mothers Training program was increased acceptance of and decreased reactivity to stressful experiences. Thus, although poor sleep quality may be inevitable for many women during pregnancy, the ability to respond with compassion and acceptance may prevent a downward spiral into increased stress and depression. Consistent with this idea, MMT participants experienced significantly greater improvements in attitudes of acceptance (versus avoidance) about distressing experiences compared to TAU participants (Epel et al., in prep).
We did not observe a buffering effect of mindfulness training for depression symptom severity. Women who slept poorly at baseline suffered from increasing depression symptom severity regardless of intervention group. This may be because the MMT program focused on skills to reduce stress versus skills to respond to mood changes. These findings replicate prior research supporting the role of poor sleep in worsening depression among the general population and during pregnancy specifically (Baglioni et al., 2011; Skouteris et al., 2008), and extends it to a unique sample of ethnically diverse, predominantly low-income, overweight or obese pregnant women. Further research is greatly needed to examine whether treating poor sleep quality during pregnancy can help prevent depression sand its wide-ranging negative associations for obstetric outcomes, infants, and children (Goodman et al., 2008; Goodman et al., 2011; Grote et al., 2010).
MMT participants in the current study did not demonstrate improvements in self-reported global sleep quality for at least three possible reasons. First, the MMT program focused on increasing mindful eating and decreasing stress, and did not specifically target sleep in women with an insomnia diagnosis. Second, sleep quality during pregnancy may be more refractory to intervention due to normative physiological changes that disrupt sleep (Lee, Zaffke, & McEnany, 2000). Finally, we used a single self-report measure of global sleep quality, and it is possible that other measures of insomnia severity or of objective sleep parameters may have been able to detect changes in sleep.
The results of the current study indicate that poor sleep quality is common in this sample of predominantly low-income, ethnically diverse, obese and overweight pregnant women. Prior research has suggested that this population may be at increased risk for poor sleep quality, and indeed, the majority of women (over 60%) in the current study were categorized as poor sleepers. However, compared to a large sample of pregnant, predominantly Caucasian women, participants in the current study had better global sleep quality by 1–2 points (Mindell et al., 2015). Further research is needed to discern who is most at risk for poor sleep during pregnancy and most likely to benefit from intervention.
Several limitations must be noted. First, the current study did not employ random assignment to group because the MMT study was an early proof of concept pilot study of efficacy. MMT and TAU participants differed at baseline only on gestational age, but considering observational research documenting that global sleep quality and depression change naturally over the course of pregnancy, future research should include random assignment (Gavin et al., 2005; Mindell et al., 2015). Second, our assessment of sleep consisted of a single subjective measure of sleep. Although there is some research to suggest that subjective quality of sleep is associated more strongly with depression than objective measures (Dorheim, Bondevik, Eberhard-Gran, & Bjorvatn, 2009), future research would benefit from the use of a multi-method approach such as objective sleep measurement with actigraphy. Third, although we view the diversity of our sample as an important and novel strength, the uniqueness of this sample may limit generalizability to other samples. Fourth, participants were not screened for sleep disorders during pregnancy. Participants may have been at increased risk for sleep disordered breathing due to being overweight or obese (Wilson et al., 2013). In future work examining the impact of mindfulness training on sleep quality among pregnant women, it would be informative to investigate the extent to which outcomes depend on sleep disorder status.
In summary, we found that poor sleep quality was prevalent in this sample, and replicated previous research demonstrating that poor sleep quality predicted depression symptom worsening. The encouraging findings that mindfulness training moderated the impact of poor sleep quality on perceived stress highlight the need for further randomized controlled research in this area, particularly given potential benefits for both women and their offspring.
Acknowledgments
This research was supported with funding by the National Heart, Lung, and Blood Institute (U01 HL097973, PIs: Elissa Epel, Nancy Adler, Barbara Laraia). Jennifer Felder is supported by a University of California, San Francisco, Preterm Birth Initiative transdisciplinary post-doctoral fellowship, funded by Marc and Lynne Benioff and the Bill and Melinda Gates Foundation.
References
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Riemann D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Balserak BI, Lee K. Sleep disturbances and sleep-realted disorders in pregnancy. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Fifth. St. Louis, MO: Elsevier Saunders; 2011. pp. 1572–1586. [Google Scholar]
- Beddoe AE, Lee KA, Weiss SJ, Kennedy HP, Yang CPP. Effects of Mindful Yoga on Sleep in Pregnant Women: A Pilot Study. Biological Research for Nursing. 2010;11(4):363–370. doi: 10.1177/1099800409356320. [DOI] [PubMed] [Google Scholar]
- Blair LM, Porter K, Leblebicioglu B, Christian LM. Poor Sleep Quality and Associated Inflammation Predict Preterm Birth: Heightened Risk among African Americans. Sleep. 2015;38(8):1259–1267. doi: 10.5665/sleep.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Wainstock T, Braun K, Segal M. Stress In Utero: Prenatal Programming of Brain Plasticity and Cognition. Biological Psychiatry. 2015;78(5):315–326. doi: 10.1016/j.biopsych.2015.02.036. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index - a New Instrument for Psychiatric Practice and Research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chang MW, Brown R, Nitzke S, Smith B, Eghtedary K. Stress, Sleep, Depression and Dietary Intakes Among Low-Income Overweight and Obese Pregnant Women. Maternal and Child Health Journal. 2015;19(5):1047–1059. doi: 10.1007/s10995-014-1604-y. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Coleman-Phox K, Laraia BA, Adler N, Vieten C, Thomas M, Epel E. Recruitment and Retention of Pregnant Women for a Behavioral Intervention: Lessons from the Maternal Adiposity, Metabolism, and Stress (MAMAS) Study. Preventing Chronic Disease. 2013:10. doi: 10.5888/pcd10.120096. Unsp 120096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, Charlson ME. From Ideas to Efficacy: The ORBIT Model for Developing Behavioral Treatments for Chronic Diseases. Health Psychology. 2015;34(10):971–982. doi: 10.1037/hea0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, Epel E. Mindfulness Intervention for Stress Eating to Reduce Cortisol and Abdominal Fat among Overweight and Obese Women: An Exploratory Randomized Controlled Study. J Obes. 2011;2011:651936. doi: 10.1155/2011/651936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorheim SK, Bondevik GT, Eberhard-Gran M, Bjorvatn B. Subjective and objective sleep among depressed and non-depressed postnatal women. Acta Psychiatrica Scandinavica. 2009;119(2):128–136. doi: 10.1111/j.1600-0447.2008.01272.x. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C. Psychological Science on Pregnancy: Stress Processes, Biopsychosocial Models, and Emerging Research Issues. Annual Review of Psychology, Vol 62. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- Epel E, Laraia B, Coleman-Phox K, Leung C, Vieten C, Mellin L, Adler N. Effects of a mindfulness-based intervention on stress and weight gain for pregnant low-income women: A controlled trial. doi: 10.1007/s12529-019-09779-2. (in prep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Figueiredo B, Schanberg S, Kuhn C. Sleep disturbances in depressed pregnant women and their newborns. Infant Behavior & Development. 2007;30(1):127–133. doi: 10.1016/j.infbeh.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression - A systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106(5):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Broth MR, Hall CM, Stowe ZN. Treatment of postpartum depression in mothers: secondary benefits to the infants. Infant Mental Health Journal. 2008;29(5):492–513. doi: 10.1002/imhj.20188. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal Depression and Child Psychopathology: A Meta-Analytic Review. Clinical Child and Family Psychology Review. 2011;14(1):1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A Meta-analysis of Depression During Pregnancy and the Risk of Preterm Birth, Low Birth Weight, and Intrauterine Growth Restriction. Archives of General Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Casement MD, Troxel WM, Matthews KA, Bromberger JT, Kravitz HM, Buysse DJ. Chronic Stress is Prospectively Associated with Sleep in Midlife Women: The SWAN Sleep Study. Sleep. 2015;38(10):1645–1654. doi: 10.5665/sleep.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz PA, Davis JE, Yarandi HN, Farkas R, Feingold E, Shippings SH, Williams D. Obesity in Urban Women: Associations with Sleep and Sleepiness, Fatigue and Activity. Womens Health Issues. 2014;24(4):E447–E454. doi: 10.1016/j.whi.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Kanen JW, Nazir R, Sedky K, Pradhan BK. The effects of mindfulness-based interventions on sleep disturbance: a meta-analysis. Adolescent Psychiatry. 2015;5:105–115. [Google Scholar]
- Kristeller JL, Hallett CB. An Exploratory Study of a Meditation-based Intervention for Binge Eating Disorder. J Health Psychol. 1999;4(3):357–363. doi: 10.1177/135910539900400305. [DOI] [PubMed] [Google Scholar]
- Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eat Disord. 2011;19(1):49–61. doi: 10.1080/10640266.2011.533605. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstetrics and Gynecology. 2000;95(1):14–18. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- Lengacher CA, Reich RR, Paterson CL, Jim HS, Ramesar S, Alinat CB, Kip KE. The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: a randomized controlled trial. Psycho-Oncology. 2015;24(4):424–432. doi: 10.1002/pon.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever Taylor B, Cavanagh K, Strauss C. The Effectiveness of Mindfulness-Based Interventions in the Perinatal Period: A Systematic Review and Meta-Analysis. Plos One. 2016;11(5):e0155720. doi: 10.1371/journal.pone.0155720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA, Cook RA, Nikolovski J. Sleep patterns and sleep disturbances across pregnancy. Sleep Medicine. 2015;16(4):483–488. doi: 10.1016/j.sleep.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, Dinges DF. Sleep Deprivation and Stressors: Evidence for Elevated Negative Affect in Response to Mild Stressors When Sleep Deprived. Emotion. 2012;12(5):1015–1020. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noss A. Household Income: 2012. 2013 Retrieved from: https://www.census.gov/prod/2013pubs/acsbr12-02.pdf.
- Okun ML, Tolge M, Hall M. Low Socioeconomic Status Negatively Affects Sleep in Pregnant Women. Jognn-Journal of Obstetric Gynecologic and Neonatal Nursing. 2014;43(2):160–167. doi: 10.1111/1552-6909.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JC, Manber R, Segal Z, Xia YL, Shapiro S, Wyatt JK. A Randomized Controlled Trial of Mindfulness Meditation for Chronic Insomnia. Sleep. 2014;37(9):1553–U1186. doi: 10.5665/sleep.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JC, Ulmer CS, Manber R. Improving sleep with mindfulness and acceptance: A metacognitive model of insomnia. Behaviour Research and Therapy. 2012;50(11):651–660. doi: 10.1016/j.brat.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Kleinman K, Abrams A, Harlow BL, McLaughlin TJ, Joffe H, Gillman MW. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. Journal of Epidemiology and Community Health. 2006;60(3):221–227. doi: 10.1136/jech.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidebottom AC, Harrison PA, Godecker A, Kim H. Validation of the Patient Health Questionnaire (PHQ)-9 for prenatal depression screening. Archives of Womens Mental Health. 2012;15(5):367–374. doi: 10.1007/s00737-012-0295-x. [DOI] [PubMed] [Google Scholar]
- Skouteris H, Germano C, Wertheim EH, Paxton SJ, Milgrom J. Sleep quality and depression during pregnancy: a prospective study. Journal of Sleep Research. 2008;17(2):217–220. doi: 10.1111/j.1365-2869.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Staneva A, Bogossian F, Pritchard M, Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women and Birth. 2015;28(3):179–193. doi: 10.1016/j.wombi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Pariante CM. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):20p. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Vieten C. Mindful Motherhood: Practical Tools for Staying Sane During Pregnancy and Your Child’s First year. Oakland, CA: New Harbinger Publications, Inc.; 2009. [Google Scholar]
- Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: results of a pilot study. Archives of Womens Mental Health. 2008;11(1):67–74. doi: 10.1007/s00737-008-0214-3. [DOI] [PubMed] [Google Scholar]
- Volkovich E, Tikotzky L, Manber R. Objective and subjective sleep during pregnancy: links with depressive and anxiety symptoms. Arch Womens Ment Health. 2015 doi: 10.1007/s00737-015-0554-8. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews. 2008;32(6):1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wilson DL, Walker SP, Fung AM, O’Donoghue F, Barnes M, Howard M. Can we predict sleep-disordered breathing in pregnancy? The clinical utility of symptoms. Journal of Sleep Research. 2013;22(6):670–678. doi: 10.1111/jsr.12063. [DOI] [PubMed] [Google Scholar]
- Wu ZH, Stevens RG, Tennen H, North CS, Grady JJ, Holzer C. Sleep Quality Among Low-Income Young Women in Southeast Texas Predicts Changes in Perceived Stress Through Hurricane Ike. Sleep. 2015;38(7):1121–1128. doi: 10.5665/sleep.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]