Abstract
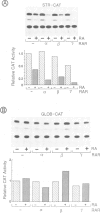
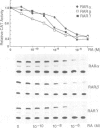
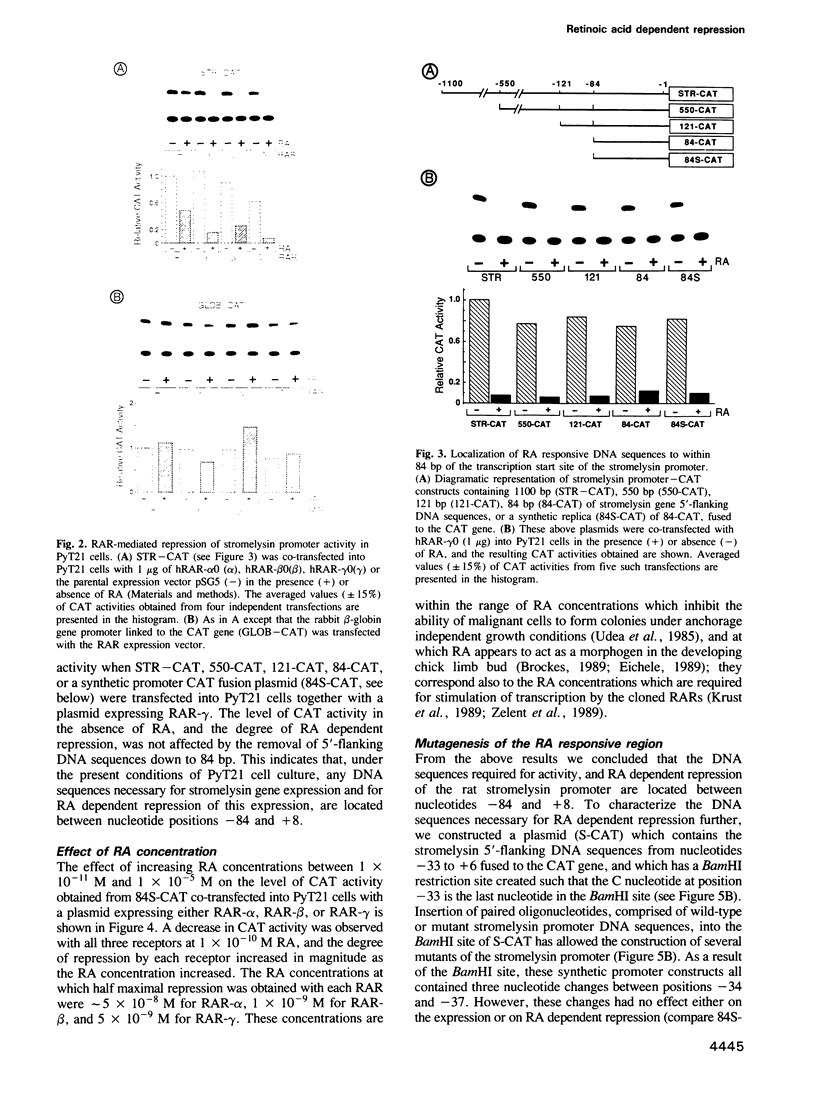
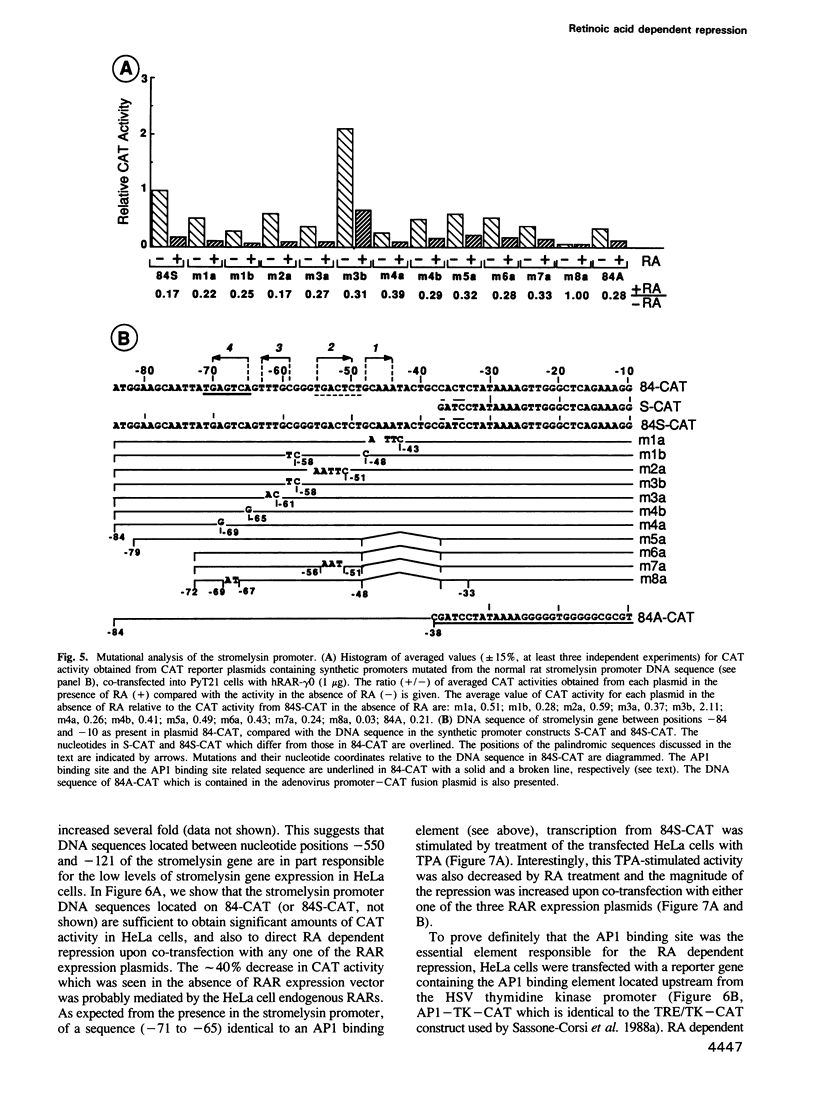
Stromelysin is a member of the metalloproteinase family which plays an important role in extracellular matrix remodelling during many normal and disease processes. We show here that in polyomavirus-transformed rat embryo fibroblast cells (PyT21), the transcription from the stromelysin gene is repressed by the vitamin A derivative retinoic acid (RA). Furthermore, expression vectors encoding the human RA receptors hRAR-alpha, hRAR-beta and hRAR-gamma repress chloramphenicol acetyltransferase (CAT) expression from stromelysin promoter-CAT gene expression vectors in RA-treated PyT21 and human HeLa cells, as determined by transient transfection assays. Through mutation and deletion analysis, we show that the RA dependent repression is mediated by a 25 bp region from nucleotide positions -72 to -48 of the rat stromelysin 5'-flanking DNA sequence. Further mutation analysis of this region indicates that the DNA sequence required for RA dependent repression colocalizes with an AP1 binding site which is essential for promoter activity. We show also that RA represses the transcriptional activity of a reporter gene containing a TPA responding AP1 binding site driving the HSV tk promoter. Thus the RAR-RA complex appears to repress transcription of the stromelysin gene by blocking activation by positive regulatory factors. However, we found no evidence supporting the possibility that the RA dependent repression could be due to RAR binding to the AP1 binding site or to the AP1 components c-fos and c-jun.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Alexander C. M., Werb Z. Proteinases and extracellular matrix remodeling. Curr Opin Cell Biol. 1989 Oct;1(5):974–982. doi: 10.1016/0955-0674(89)90068-9. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bocquel M. T., Kumar V., Stricker C., Chambon P., Gronemeyer H. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acids Res. 1989 Apr 11;17(7):2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Matrisian L. M., Gesnel M. C., Staub A., Leroy P. Sequences coding for part of oncogene-induced transin are highly conserved in a related rat gene. Nucleic Acids Res. 1987 Feb 11;15(3):1139–1151. doi: 10.1093/nar/15.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C. A., Adler R. R., Rappolee D. A., Pedersen R. A., Werb Z. Genes for extracellular-matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev. 1989 Jun;3(6):848–859. doi: 10.1101/gad.3.6.848. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Plucinska I. M., Sheldon L. A., O'Connor G. T. Half-life of synovial cell collagenase mRNA is modulated by phorbol myristate acetate but not by all-trans-retinoic acid or dexamethasone. Biochemistry. 1986 Oct 21;25(21):6378–6384. doi: 10.1021/bi00369a006. [DOI] [PubMed] [Google Scholar]
- Brockes J. P. Retinoids, homeobox genes, and limb morphogenesis. Neuron. 1989 Apr;2(4):1285–1294. doi: 10.1016/0896-6273(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Jeltsch J. M., Roberts M., Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Chiocca E. A., Davies P. J., Stein J. P. The molecular basis of retinoic acid action. Transcriptional regulation of tissue transglutaminase gene expression in macrophages. J Biol Chem. 1988 Aug 15;263(23):11584–11589. [PubMed] [Google Scholar]
- Chua C. C., Geiman D. E., Keller G. H., Ladda R. L. Induction of collagenase secretion in human fibroblast cultures by growth promoting factors. J Biol Chem. 1985 May 10;260(9):5213–5216. [PubMed] [Google Scholar]
- Cohen D. R., Ferreira P. C., Gentz R., Franza B. R., Jr, Curran T. The product of a fos-related gene, fra-1, binds cooperatively to the AP-1 site with Jun: transcription factor AP-1 is comprised of multiple protein complexes. Genes Dev. 1989 Feb;3(2):173–184. doi: 10.1101/gad.3.2.173. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Dufour S., Duband J. L., Kornblihtt A. R., Thiery J. P. The role of fibronectins in embryonic cell migrations. Trends Genet. 1988 Jul;4(7):198–203. doi: 10.1016/0168-9525(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele G. Retinoids and vertebrate limb pattern formation. Trends Genet. 1989 Aug;5(8):246–251. doi: 10.1016/0168-9525(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S. M., Clark E. J., Werb Z. Coordinate regulation of stromelysin and collagenase genes determined with cDNA probes. Proc Natl Acad Sci U S A. 1987 May;84(9):2600–2604. doi: 10.1073/pnas.84.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S. M., Ruley H. E. Transcription from the stromelysin promoter is induced by interleukin-1 and repressed by dexamethasone. J Biol Chem. 1987 Dec 5;262(34):16300–16304. [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub M. P., Lutz Y., Ruberte E., Petkovich M., Brand N., Chambon P. Antibodies specific to the retinoic acid human nuclear receptors alpha and beta. Proc Natl Acad Sci U S A. 1989 May;86(9):3089–3093. doi: 10.1073/pnas.86.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halazonetis T. D., Georgopoulos K., Greenberg M. E., Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988 Dec 2;55(5):917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- Hart I. R., Goode N. T., Wilson R. E. Molecular aspects of the metastatic cascade. Biochim Biophys Acta. 1989 Jul 28;989(1):65–84. doi: 10.1016/0304-419x(89)90035-8. [DOI] [PubMed] [Google Scholar]
- Horton W. E., Yamada Y., Hassell J. R. Retinoic acid rapidly reduces cartilage matrix synthesis by altering gene transcription in chondrocytes. Dev Biol. 1987 Oct;123(2):508–516. doi: 10.1016/0012-1606(87)90409-x. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Olashaw N. E., Matrisian L. M. Transforming growth factor beta 1 and cAMP inhibit transcription of epidermal growth factor- and oncogene-induced transin RNA. J Biol Chem. 1988 Nov 15;263(32):16999–17005. [PubMed] [Google Scholar]
- Klein-Hitpass L., Ryffel G. U., Heitlinger E., Cato A. C. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988 Jan 25;16(2):647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Clerc R. G., Brenowitz M., Sharp P. A. The Oct-2 protein binds cooperatively to adjacent octamer sites. Genes Dev. 1989 Oct;3(10):1625–1638. doi: 10.1101/gad.3.10.1625. [DOI] [PubMed] [Google Scholar]
- Lee R. F., Concino M. F., Weinmann R. Genetic profile of the transcriptional signals from the adenovirus major late promoter. Virology. 1988 Jul;165(1):51–56. doi: 10.1016/0042-6822(88)90657-5. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T., Krieg P., Fürstenberger G., Briand J. P., Leroy P., Breathnach R. The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9413–9417. doi: 10.1073/pnas.83.24.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Leroy P., Ruhlmann C., Gesnel M. C., Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol Cell Biol. 1986 May;6(5):1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Rautmann G., Magun B. E., Breathnach R. Epidermal growth factor or serum stimulation of rat fibroblasts induces an elevation in mRNA levels for lactate dehydrogenase and other glycolytic enzymes. Nucleic Acids Res. 1985 Feb 11;13(3):711–726. doi: 10.1093/nar/13.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A. Matrix regulation of cell shape and gene expression. Curr Opin Cell Biol. 1989 Oct;1(5):995–999. doi: 10.1016/0955-0674(89)90071-9. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordacq J. C., Linzer D. I. Co-localization of elements required for phorbol ester stimulation and glucocorticoid repression of proliferin gene expression. Genes Dev. 1989 Jun;3(6):760–769. doi: 10.1101/gad.3.6.760. [DOI] [PubMed] [Google Scholar]
- Morrison-Graham K., Weston J. A. Mouse mutants provide new insights into the role of extracellular matrix in cell migration and differentiation. Trends Genet. 1989 Apr;5(4):116–121. doi: 10.1016/0168-9525(89)90042-5. [DOI] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Bretz U., Baggiolini M. Partial purification of collagenase and gelatinase from human polymorphonuclear leucocytes. Analysis of their actions on soluble and insoluble collagens. Biochem J. 1982 Apr 1;203(1):209–221. doi: 10.1042/bj2030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985 Mar 10;260(5):3079–3083. [PubMed] [Google Scholar]
- Nakajima M., Lotan D., Baig M. M., Carralero R. M., Wood W. R., Hendrix M. J., Lotan R. Inhibition by retinoic acid of type IV collagenolysis and invasion through reconstituted basement membrane by metastatic rat mammary adenocarcinoma cells. Cancer Res. 1989 Apr 1;49(7):1698–1706. [PubMed] [Google Scholar]
- Nicholson R., Murphy G., Breathnach R. Human and rat malignant-tumor-associated mRNAs encode stromelysin-like metalloproteinases. Biochemistry. 1989 Jun 13;28(12):5195–5203. doi: 10.1021/bi00438a042. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Uhlén M., Josephson S., Gatenbeck S., Philipson L. An improved positive selection plasmid vector constructed by oligonucleotide mediated mutagenesis. Nucleic Acids Res. 1983 Nov 25;11(22):8019–8030. doi: 10.1093/nar/11.22.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Ostrowski L. E., Finch J., Krieg P., Matrisian L., Patskan G., O'Connell J. F., Phillips J., Slaga T. J., Breathnach R., Bowden G. T. Expression pattern of a gene for a secreted metalloproteinase during late stages of tumor progression. Mol Carcinog. 1988;1(1):13–19. doi: 10.1002/mc.2940010106. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Quinones S., Saus J., Otani Y., Harris E. D., Jr, Kurkinen M. Transcriptional regulation of human stromelysin. J Biol Chem. 1989 May 15;264(14):8339–8344. [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz R. Transcriptional repression in eukaryotes. Trends Genet. 1990 Jun;6(6):192–197. doi: 10.1016/0168-9525(90)90176-7. [DOI] [PubMed] [Google Scholar]
- Risse G., Jooss K., Neuberg M., Brüller H. J., Müller R. Asymmetrical recognition of the palindromic AP1 binding site (TRE) by Fos protein complexes. EMBO J. 1989 Dec 1;8(12):3825–3832. doi: 10.1002/j.1460-2075.1989.tb08560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez R., Nicholson R., Gesnel M. C., Matrisian L. M., Breathnach R. Structure-function relationships in the collagenase family member transin. J Biol Chem. 1988 Aug 25;263(24):11892–11899. [PubMed] [Google Scholar]
- Sassone-Corsi P., Lamph W. W., Kamps M., Verma I. M. fos-associated cellular p39 is related to nuclear transcription factor AP-1. Cell. 1988 Aug 12;54(4):553–560. doi: 10.1016/0092-8674(88)90077-3. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Lamph W. W., Verma I. M. Direct interaction between fos and jun nuclear oncoproteins: role of the 'leucine zipper' domain. Nature. 1988 Dec 15;336(6200):692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J., Meikle M. C. Neutral metallo-proteinases of rabbit bone. Separation in latent forms of distinct enzymes that when activated degrade collagen, gelatin and proteoglycans. Biochem J. 1978 May 1;171(2):493–496. doi: 10.1042/bj1710493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirum K. L., Brinckerhoff C. E. Cloning of the genes for human stromelysin and stromelysin 2: differential expression in rheumatoid synovial fibroblasts. Biochemistry. 1989 Oct 31;28(22):8691–8698. doi: 10.1021/bi00448a004. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Höyhtyä M., Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987 Nov 25;907(3):191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Ueda H., Ono M., Hagino Y., Kuwano M. Isolation of retinoic acid-resistant clones from human breast cancer cell line MCF-7 with altered activity of cellular retinoic acid-binding protein. Cancer Res. 1985 Jul;45(7):3332–3338. [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J. The oncogene jun and nuclear signalling. Trends Biochem Sci. 1989 May;14(5):172–175. doi: 10.1016/0968-0004(89)90268-5. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., LaRosa G. J., Gudas L. J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985 Jan;107(1):75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B. The immunoglobulin heavy-chain B-lymphocyte enhancer efficiently stimulates transcription in non-lymphoid cells. EMBO J. 1986 Mar;5(3):553–560. doi: 10.1002/j.1460-2075.1986.tb04246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Marchio A., Tiollais P., Dejean A. A novel steroid thyroid hormone receptor-related gene inappropriately expressed in human hepatocellular carcinoma. Nature. 1987 Dec 17;330(6149):667–670. doi: 10.1038/330667a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Metzger D. The lack of transcriptional activation of the v-erbA oncogene is in part due to a mutation present in the DNA binding domain of the protein. Nucleic Acids Res. 1990 Aug 11;18(15):4489–4497. doi: 10.1093/nar/18.15.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]