Abstract
Serotonin (5-HT) and its specific transporter, SERT play important roles in pregnancy. Using placentas dissected from 18d gestational SERT-knock out (KO), peripheral 5-HT (TPH1)-KO and wild-type (WT) mice, we explored the role of 5-HT and SERT in placental functions in detail. An abnormal thick band of fibrosis and necrosis under the giant cell layer in SERT-KO placentas appeared only moderately in TPH1-KO and minimally present in WT placentas. The majority of the changes were located at the junctional zone of the placentas in SERT. The etiology of these findings was tested with TUNEL assays. The placentas from SERT-KO and TPH1-KO showed 49- and 8-fold increase in TUNEL-positive cells without a concurrent change in the DNA repair or cell proliferation compared to WT placentas. While the proliferation rate in the embryos of TPH1-KO mice was 16-fold lower than the rate in gestational age matched embryos of WT or SERT-KO mice. These findings highlight an important role of continuous 5-HT signaling on trophoblast cell viability. SERT may contribute to protecting trophoblast cells against cell death via terminating the 5-HT signaling which changes cell death ratio in trophoblast as well as proliferation rate in embryos. However, the cell death in SERT-KO placentas is in caspase 3-independent pathway.
Keywords: Serotonin, Serotonin transporter, Placenta
INTRODUCTION
Serotonin (5-HT) is a vasoconstrictor compound that also acts as a developmental signal early in rodent embryogenesis (Lauder et al., 1981). Genetic and pharmacological disruption of 5HT-signaling causes maternal and prenatal morbidity and mortality via mediating high blood pressure and neuroanatomical abnormalities, respectively. The actions of 5HT are mediated by different types of receptors, and terminated by a single 5-HT transporter (SERT). On the plasma membranes of the trophoblast cells SERT regulates extracellular 5-HT levels and prevents the vasoconstriction in the placental vascular bed and thereby secures a stable blood flow to the embryo SERT cDNA’s have been cloned and sequenced from a number of sources, including human placenta (Ramamoorthy et al., 2993), platelets (Lesch et al., 1993), and brain (Lesch et al., 1993a); rat (Blakely et al., 1991; Hoffman et al., 1991) and mouse brain (Gregor et al., 1993). SERT is a well characterized member of Na+/Cl−-dependent solute carrier 6 (SLC6A4) family (Amara and Arriza, 1993; Rudnick and Clark, 1993). In different tissues, SERT is encoded by the same gene (Lesch et al., 1993, 1993a; Blakely et al., 1991; Hoffman et al., 1991; Gregor et al., 1993). However, the accumulating evidences suggest that low affinity, high capacity transport mechanisms may also contribute to the clearance of 5-HT (Matthaeus et al., 2015).
Trophoblast cells express many components of the 5-HT system such as SERT (Viau et al., 2009; Bottalico et al., 2004), monoamine oxidase, MAO (Auda et al., 1998) which catabolizes 5-HT, and receptors, 5-HT1A and 5-HT2A (Viau et al., 2009; Huang et al., 1998). As a mitogen, 5-HT promotes cell division and mitosis and acts as a developmental signal early in embryogenesis (Cote et al., 2007). However, continuous 5-HT signaling is involved in cell death mechanisms via activation of phosphatidylinositol-3 kinase (PI3K)/AKT and extracellular signal-regulated kinase (ERK) 1/2 signaling pathways (Kim et al., 2015; Nebigil et al., 2003). Additionally, 5-HT signaling upregulates the expression of the interleukin (IL)-6, which activates Janus kinases signal transducers and activators of the transcription (JAK-STAT3) signaling pathway in human placenta leading to cell death (Kim et al., 2015; Mercado et al., 2013a). Therefore, the 5-HT level in intervillous space and inside the cells plays different roles in the growth, differentiation and the apoptosis of trophoblast cells and must be tightly regulated. Supporting the importance of the role of SERT in trophoblast, many clinical studies (but not all) report increased rates of congenital prematurity and malformations, including defects in fetal forebrain development, induced by selective serotonin uptake inhibitors (SSRI) during pregnancy (Chambers et al., 2006; Huybrechts et al., 2014, 2015; Wemakor et al., 2015). Reducing 5-HT uptake rates of SERT by SSRI alters the plasma vs platelet 5-HT ratio; as reported using SSRI in the first trimester had approximately a 2-fold increased risk for cardiac and a 1.8-fold increased risk for other congenital malformations compared to the entire national registry population (http://www.ncbi.nlm.nih.gov/pubmed/22052679) (Balsell et al., 2012).
Furthermore, studies with preclinical models report that mice lacking the gene for SERT (SERT-KO) (Uceyler et al., 2010; Chen et al., 2012; Walther et al., 2003; Bengel et al., 1998a) or the gene for TPH1 (TPH1-KO) (Cote et al., 2007; Kim et al., 2010; Paulmann et al., 2009) exhibit insulin resistance and glucose intolerance, and progressively develop obesity and hepatic steatosis (Armitage et al., 2008). These studies clearly throw additional role to 5-HT and emphasize the importance of SERT in trophoblast more than functioning as a transporter, protecting the trophoblast cells against continuous 5-HT signaling.
In order to evaluate the impact of SERT and 5-HT on placenta, E18.d transgenic mice, SERT-KO (Bengel et al., 1998) and TPH1-KO (Walther et al., 2003) were characterized by comparing the plasma levels of 5-HT, glucose and insulin with the gestational age matched wild-type (WT) counterparts.
Using two different approaches, the histopathologic analysis and the TUNEL assays, the placentas from 18d E18.d SERT-KO (Bengel et al., 1998a) and TPH1-KO mice (Walther et al., 2003), and WT counterparts were evaluated. Our findings reveal that in SERT-KO mouse, the plasma 5-HT levels is 2-fold higher, the plasma glucose level is slightly higher, and the insulin level is 4-fold higher than these determinants in the plasma of WT mice. While these findings agreed with previously published studies, we then evaluated the impact of 2-fold higher 5-HT signaling on SERT-KO placentas. In TUNEL and proliferation assays, the cells death rates in SERT-KO placentas were found several-fold elevated at the maternal side as compared to the placentas of TPH1-KO and WT mice. Further analysis of the placentas from SERT-KO mice for Caspase3 staining suggested that the placental damage in the absence of SERT, at elevated 5-HT signaling, occurs in caspase3-independent pathway.
Material and Methods
Animals
Twelve weeks old adult male C57BL/6J mice WT, or SERT-KO and TPH1-KO mice were engineered on a C57BL6 genetic background. Tryptophan hydroxylase (TPH1) mice (Walther et al., 2003) were donated by Dr. M. Bader (Max-Delbrück-Center for Molecular Medicine). These mice lack the gene for TPH1, which is the rate-limiting enzyme in the synthesis of 5-HT in peripheral tissues. SERT-KO mice were provided by The Jackson Laboratory (Bar Harbor, Maine). Homozygous SERT−/− or TPH1−/− male and female mice (12 weeks old) were bred together and litters were analyzed. These mice were genotyped by PCR amplification of genomic DNA prepared from the tails of SERT−/− and TPH1−/− mice (Mercado et al., 2013b). The WT TPH1 allele produce bands at 570 bp and SERT allele produced bands at 310 bp, respectively indicating the deletion of the SERT or TPH1 genes totally (Walther et al., 2003; Bengel et al., 1998a). Procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Hematoxylin and Eosin (H&E) and Trichromie Histology of Placentas
Placentas from 18d gestation SERT-KO, TPH1-KO, and WT mice were dissected from the uterus, formalin fixed and paraffin embedded. 5 μm slides were prepared for Hematoxylin and Eosin (H&E) staining, TUNEL, and immunohistochemistry (IH). An experienced placental pathologist (DJR) examined the placental histology in H&E-stained sections. Representative placenta sections from each group were stained with trichrome using routine methods.
Slides are loaded into glass slide holders and dewaxed first in 100% xylene (Fisher Scientific) for 5 minutes, then in 100% ethanol (Fisher Scientific) with 10–20 seconds agitation, once in 90% ethanol with 10–20 seconds agitation, once with 70% ethanol with 10–20 seconds agitation and twice in H2O with 10–20 seconds agitation. The rack is transferred into pre-warmed (94°C–96°C) retrieval solution in a glass container in the water bath. Antigen retrieval is 30 minutes in the water bath, 20 minutes on the bench and 5 minutes in running water.
TUNEL and IH
To measure tissue injury (cell death), placental sections were subjected to TUNEL assay using the In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s protocol (Apostolov et al., 2009). Ki67 antibody (Ab) (Abcam, Cambridge, MA) was used on formalin fixed paraffin embedded placental and embryonic tissues and countered with goat anti-rabbit IgG-Alexa Fluor 594 (Invitrogen) to detect the primary Ab (Apostolov et al., 2009). Control staining was performed in the absence of the primary Ab. After staining, sections and cells were counterstained with 4′,6-diamidino-2-phenylindol (DAPI) to visualize cell nuclei, mounted under cover slips with Prolong® Antifade kit (Invitrogen, Carlsbad, CA) and acquired using the Olympus IX-81 inverted microscope (Olympus America, Center Valley, PA) equipped with Hamamatsu ORCA-ER monochrome camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan).
The quantification of the TUNEL and Ki67 data iwas performed by using SlideBook 4.2 software on images captured at 200X magnification which counts the nuclei (DAPI staining) and the TUNEL and Ki67 positive cells and calculates the percentage of positive cells to the total number of cells. For Ki67 assessment, quantification by mean intensity was performed to confirm the results.
Image Analysis
The analysis of fluorescent images was performed using the SlideBook 4.2 software. For quantification, 14 independent fields of view were collected per each mouse placenta section, and mean optical density (MOD) was recorded for Alexa Fluor 594 channel by a person blind to the genotypes. The data were presented as averages of MODx/field of view for each channel. The percentage of TUNEL-positive (dead) cells was quantified by measuring the area of TUNEL-positive nuclei per total area of DAPI+TUNEL; the TUNEL data were presented as the percentage of TUNEL positive cells per total number of cell per field view.
Western blotting (WB) analysis and quantitation of protein expression
Placentas dissected from 18d gestational age mice and the cells were prepared following a previously published method (Li et al., 2014). Cells were lysed and solubilized in PBS containing 0.44% SDS, and protease inhibitor mixture (PIM). Samples were analyzed by 12% SDS-PAGE and transferred to nitrocellulose. Blots were incubated with primary Ab, rinsed, and then with horseradish peroxidase (HRP)-conjugated secondary Abs. The signals were visualized using the enhanced chemiluminescence (ECL) detection system. The polyclonal SERT Ab was generated by Proteintech Group, Inc. (Chicago, IL) on a peptide corresponding to the last 26 amino acids of the C terminus (positions 586–630) of SERT. Anti-SERT (diluted 1:500) gave one major band at 90 kD. For determination of Bcl2, Bax and Caspase-3, membranes were probed with antibodies against Bax or Bcl2 or Caspase-3 (Cell Signaling cat #: 14796, 3498 or 9662, respectively) in the dilutions provided by the manufacturers. Caspase-3 Antibody detects endogenous level of full length caspase-3 (35 kD) and the small fragments of resulting from cleavage (17 kD). Signals were quantitated with a VersaDoc 1000 analysis system.
Statistical Analysis
The placental pathologic parameters were compared and used to evaluate the differences in placental pathology and vasculature between SERT-KO and WT. Continuous variables were summarized using means and standard deviations (SD), while frequency distributions accounting for missing values were used for categorical variables. The Student’s t-test was used when two groups were compared, and the SAS for Windows version 9.1 statistical software package (SAS Institute, Cary, NC) for TUNEL and proliferation assays. A p value less than 0.05 was considered statistically significant.
Results
Plasma concentrations of 5-HT were 2-fold higher in SERT-KO (1.75 ± 0.05 ng/ml of blood) than in WT mice (0.85 ± 0.04 ng/ml of blood). By contrast, deletion of TPH1 gene resulted in undetectable 5-HT levels in plasma (Table 1).
Table 1.
Characteristics of the study models
| WT | SERT-KO | TPH1-KO | |
|---|---|---|---|
| Blood plasma 5-HT (ng/ml blood) (Mercado et al., 2013b) |
0.85 ± 0.04 | 1.75 ± 0.05 | u.n.d. |
| Blood glucose levels (mg/dl blood) |
65.22 ± 10.3 | 81.77 ± 4.35 | 108.55 ± 5.92 |
| Blood insulin levels (ng/dl blood) |
0.44 ± 0.04 | 1.82 ± 0.4 | 0.25 ± 0.002 |
| Placental Weight (mg) | 170 ± 43.56 | 150 ± 15.81 | 106 ± 16.73 |
u.n.d. = undetectable.
Location of SERT in the mouse placenta
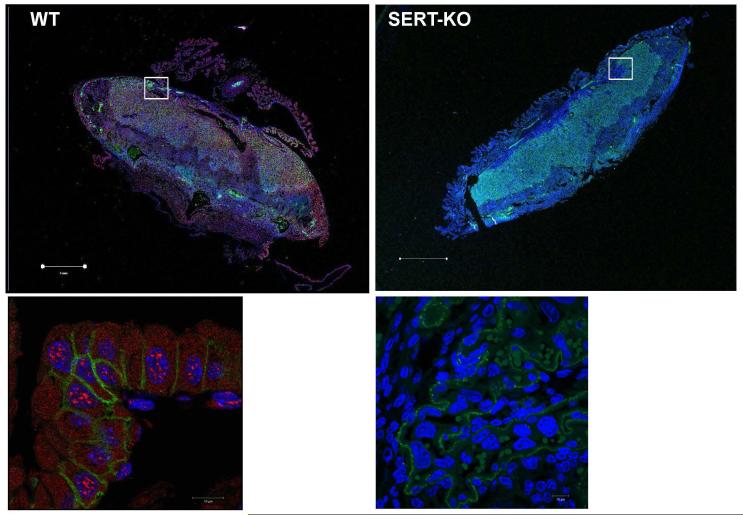
In order to visualize SERT in the placentas from 18d gestational age of SERT-KO and WT mice were processed for IH (Figure 1). The slides were first dewaxed in xylene and then moved for staining with SERT-Antibodies (1:100 dilution; Mercado et al., 2013) or mouse trophoblast marker (1:100 dilution TROP-1; Thermo Fisher Scientific Cat No:PA5-47074) antibodies. The localization of SERT (in red) and TROP-1 (in green) signals were captured in the overlaid images with Alexa Fluor −549 and −488 dye (1:250 dilution, respectively filter sets. The DNA is stained with DAPI in blue.
Figure 1. Expression of SERT in E18 mouse placentas.
The 4 μm sections of parafine embeded placentas dissected from E18 WT and SERT-KO mice were dewaxed, rehydrated and subjected to antigen retrieval. Sections were stained as described in the Methods with polyclonal SERT- and monoclonal TROP-1 antibodies (1:100 dilution) followed by Alexa549-conjugated donkey anti-goat and Alexa488 donkey anti-rabbit dye (1:250 dilution). Nuclei were counterstained with DAPI in blue (1:250 dilution). In placentas dissected from SERT-KO mouse only green TROP-1 and blue DAPI staining are found. In WT placentas red, SERT-staining appeared over TROP-1. Cells were analyzed with a Zeiss LSM510 laser confocal microscope. The lower panels are 10X the magnification of the area framed in white in the upper panels. The scale bars for the upper panels are in 1 μm and for the lower panels 10 μm indicate the magnification of the images. Figures show representative images from at least 2 separate experiments.
If TROP-1 and SERT co-localized then the structures would appear light purple; otherwise, the distinct green and red signals would represent structures containing either one of the two proteins. In enlarged images the TROP-1 mostly appears around the cell plasma and overlaid with red SERT. In SERT-KO placenta only TROP-1 and DAPI staining were determined. Antibodies against SERT localized in both, on the plasma membrane and at the intracellular compartments. Some non-specific staining in the same channel with DAPI was observed but not very distinct. SERT was mostly found in the junction, labyrinth and decidua whereas no detection at all in KO placentas. The morphology of trophoblast in the placentas of SERT-KO mouse was found different than the WT counterparts.
H&E stained sections of placentas from transgenic and WT mice at 18d gestations
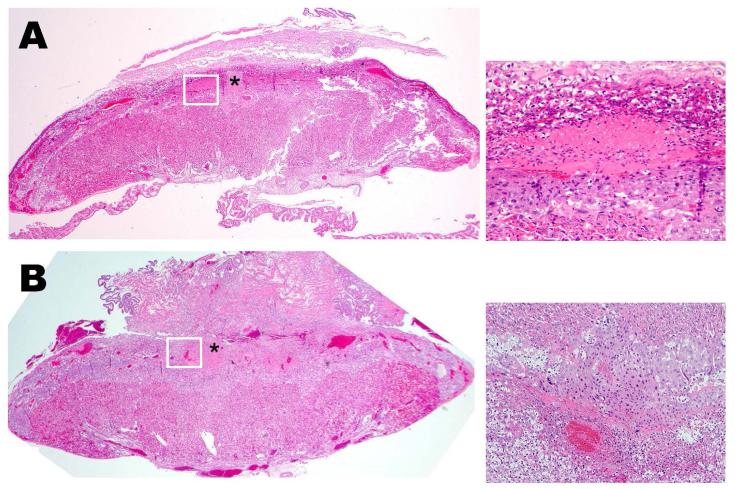
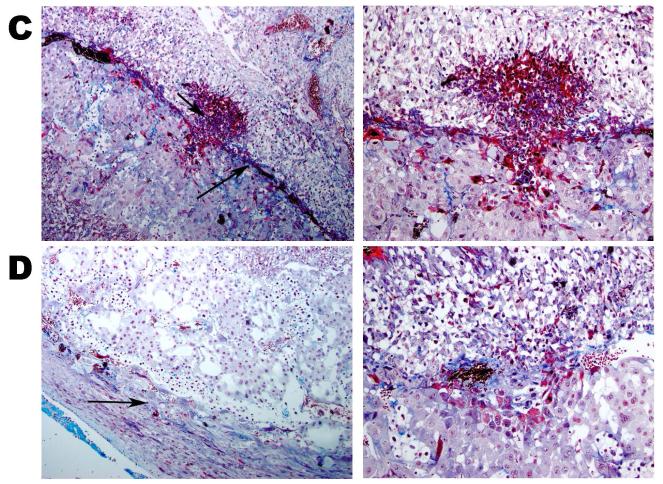
The effect of increasing plasma 5-HT levels was investigated by histological analysis of placentas from SERT-KO and WT mice (Figure 2). Placentas from all three mouse models (10 slides per placenta, 6 placentas from each genotype, n=4 different dams) were harvested, weighed (170 ± 43.56 and 150 ± 15.81 mg, WT and SERT-KO, respectively) and the orientation to the uterus (preserving the implantation site) was maintained for processing. 5 μm sections were stained and analyzed for intravascular thrombosis, vascular wall necrosis, retroplacental thrombus/necrosis, and for placental development. Initially, the histopathologic analysis of the placenta and uterine tissues included careful examination of the junctional zone and maternal decidua. We found no structural changes such as vascular thrombi amongst the SERT-KO and WT placentas. However, the junctional zone of placenta from SERT-KO mice showed significant macroscopic modifications. WT placental junctional zones are typically viable without evidence of necrosis. SERT-KO placentas showed large confluent areas of necrosis with signs of hemorrhage and fibrosis (Figure 2A and B). We confirmed these findings using H&E trichrome staining of placentas (18d gestational age) which demonstrated fibrin deposition at the junctional zone only in the SERT-KO placenta (Figure 2C and D).
Figure 2.
H&E stained E18 SERT-KO (A) and WT (B) placenta at 2X highlighting junctional zone showing necrotic area (indicated with an asterisk) in SERT-KO with minimal serous fluid (arrows) in WT placentas. H&E staining are the representative images of 10 slides per placenta, 6 placentas from each mouse, n=4. The insets at the right of the main figures are the 20X the magnification of the areas framed in white. Trichrome staining of placentas from E.18 d SERT-KO (C) and WT (D) mice. Photomicrographs of the junctional zone stained with trichrome. Royal blue color and arrows highlight connective tissue (collagen) within the necrosis in SERT-KO and WT at 10X magnification.
Placenta tissue injury in SERT-KO mice
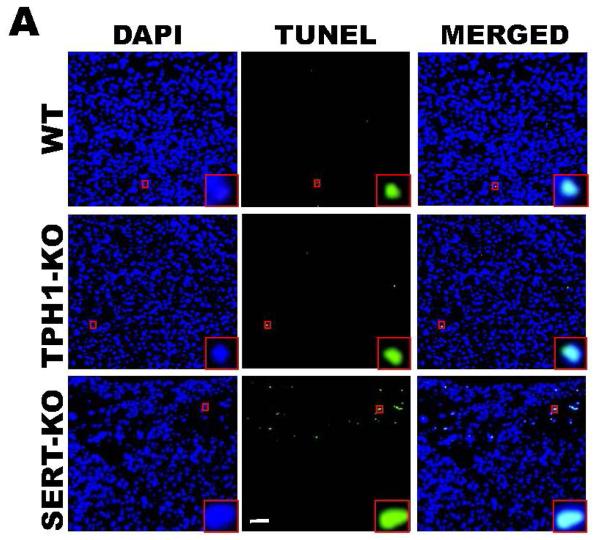
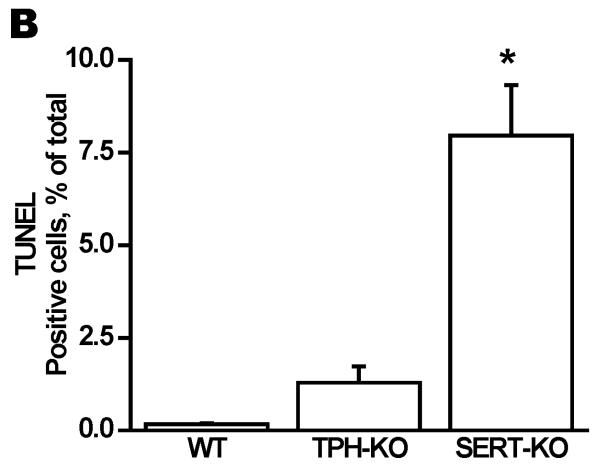
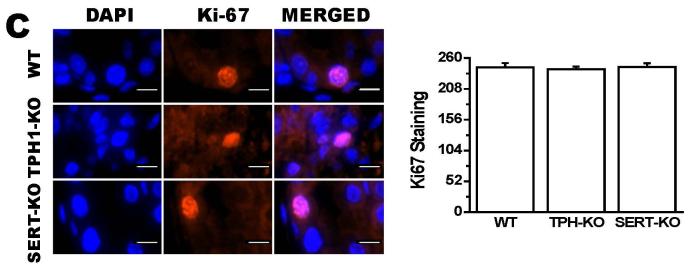
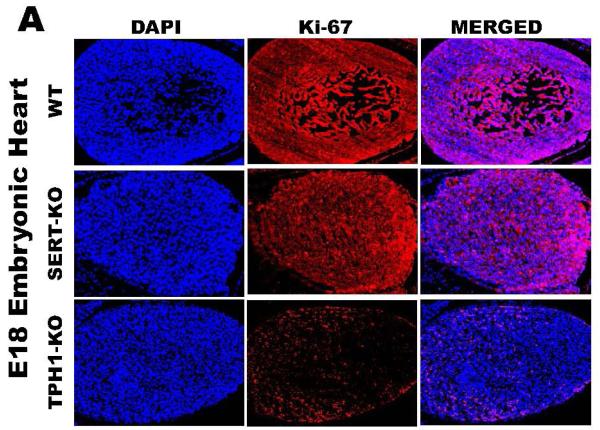
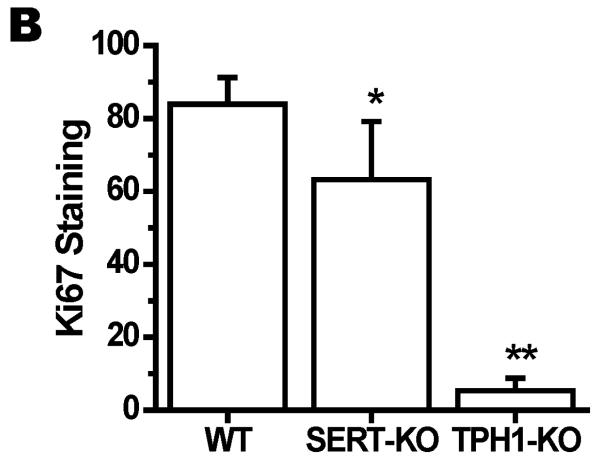
To determine the impact of 5-HT on placental integrity we performed TUNEL assays on placentas of SERT-KO, TPH1-KO and WT mice (n=5/genotype) (Figure 3A). Placentas from SERT-KO mice showed significantly higher percentage of TUNEL-positive cells (49-fold higher) as compared to those from WT mice (Figure 3B). TPH1-KO placental cell death ratio was 8-fold higher than the ratio of the placentas from WT mice. The TUNEL-positive cells were not confluent but rather patchy, potentially indicating that the tissue injury was not equally distributed along the junctional zone (Figure 3A). Since the TUNEL-positivity (free DNA termini), in addition to cell death (pre- and postmortem DNA fragmentation), may be a result of excessive DNA repair (single-stranded DNA gaps and nicks) or elevated scheduled DNA synthesis (Okazaki fragments), we further measured protein expression of Ki67 by quantitative IH. There was no significant difference in the total number of cells in the junctional zone, per specific area of the placentas between the transgenic and WT mouse. There was no difference in the staining of the Ki67 proliferation marker between SERT, TPH1-KO and WT placenta samples (Figure 3C). The percent of TUNEL-positive placental cells in SERT-KO was 65-fold higher than the percent of TUNEL positive cells in placentas from WT and 6-fold higher than TPH1-KO (Figure 4). Ki67 staining of embryonic heart clearly shows a reduced proliferation in with 94% less in TPH1-KO mice, (Figure 5A, B) and 25% less for SERT-KO compared to the embryonic hearts from WT mice.
Figure 3. Tissue injury in placentas of E.18 d SERT- and TPH1-KO and WT mice placentas.
(A) TUNEL assay performed in placentas of these mice. Note the uneven (patchy) TUNEL staining. The images in each panel are acquired at 200X magnification. (B) Quantification of the TUNEL. The apoptosis in placentas from SERT-KO mice were 65-fold higher than WT, 6-fold higher than the rates of placentas from TPH1-KO mice. (C) Quantification of the proliferation marker Ki67 mean intensity. The images in each panel are acquired at 400X magnification. No difference was seen on the proliferation rates of placentas from transgenic or WT mice. TUNEL assay and Ki67 staining were performed on 10 slides per placenta, 6 placentas from each mouse, n=4. Asterisks indicate statistical difference between WT and SERT-KO (*) mice P<0.05 assessed by one way ANOVA and a Bonferroni post hoc test. The scale bars represent 10 μm.
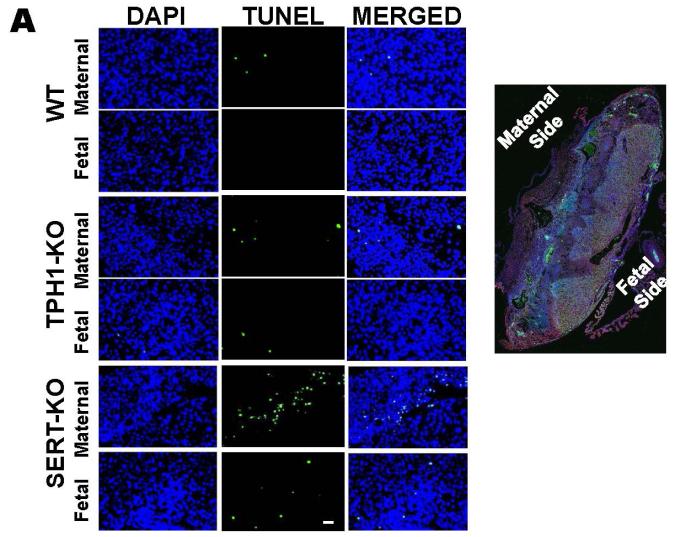
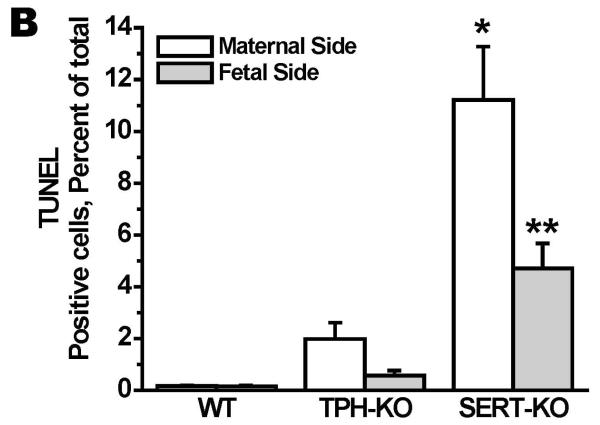
Figure 4. The location of the death cells in placentas of E.18 d SERT- and TPH1-KO and WT mice placentas.
A. TUNEL assays were performed on maternal and fetus sides of the placentas from E.18 d SERT-KO, TPH1-KO and WT mice. The images in each panel are acquired at 200X magnification. The scale bars represent 10 μm. B. Quantification of the TUNEL assays showed that the highest level of the cell death occurred at the maternal side of the SERT-KO placentas was 65-fold higher than the apoptosis of the cells at the maternal side of the WT placentas. TUNEL assays were performed on 10 slides per placenta, 6 placentas from each mouse, n=4. Asterisks indicate statistical difference between WT and SERT-KO maternal (*) and fetal side (**) of placentas P<0.05 and P<0.001 assessed by one way ANOVA and a Bonferroni post hoc test.
Figure 5. Ki67 staining of Embryonic heart from E.18 d WT and transgenic mice.
(A) The embryonic tissues were stained with Ki67, proliferation marker and DAPI to orient the staining in nucleus. Merged image show the level of staining in each tissue. The images in each panel are acquired at 400X magnification. The scale bars represent 10 μm. (B) Quantification of the proliferation marker Ki67 mean intensity. The proliferation rates in embryos of TPH1-KO were 16-fold lower than the rates of WT. This difference was much less pronounced in between the proliferation rates of embryos from SERT-KO and WT which was only 1.3-fold less in SERT-KO embryos. Asterisks indicate statistical difference between WT and SERT-KO or TPH1-KO mice (**) P<0.01 or (***) P<0.001 assessed by one way ANOVA and a Bonferroni post hoc test. All assays were performed on 10 slides per embryo, 6 embryos from each mouse, n=4.
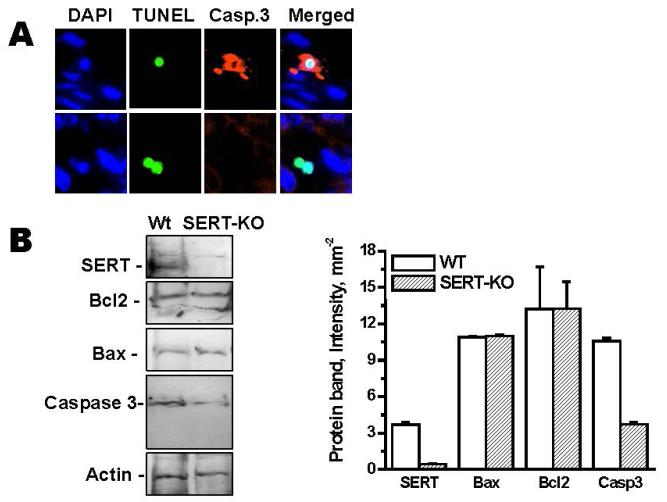
SERT-dependent placental cell death occurs in Caspase3-independent pathway
Placentas from 18d gestation SERT-KO mice were analyzed in TUNEL for Caspase3 staining. The caspase3 staining appeared at a negligible level suggesting that in SERT-KO placenta the cell death may occur in caspase3-independent signaling pathway (Figure 6A). In order to confirm the findings from TUNEL assays, placentas from 18d gestation WT and SERT-KO were harvested, their trophoblast cells were isolated by following previously published method (Li et al., 2014). They were analyzed for the expression levels of Bax, Bcl2 and Caspase-3 in Western blot assays (Figure 6B). The levels of Bcl2 and Bax were found at the same levels in placentas of WT and SERT-KO. However the expression level of Caspase 3 was 65% lower in SERT-KO than WT placentas. The level of SERT and actin were used as control in the same samples. The densitometric determinations indicate the Bcl2 vs Bax ratios are similar in between the placentas from WT and SERT-KO.
Figure 6. Caspase3 staining of the placentas from SERT-KO mice.
(A) E.18d placentas from WT and SERT-KO mice were first analyzed in TUNEL assays and the same field was evaluated for caspase3 staining (n=3). Only one of the three SERT-KO placentas gave a negligible levels of Caspase3 staining suggest that in the absence of SERT the placental damage occurs in caspase3-independent signaling pathway. The images in each panel are acquired at 400X magnification. The scale bars represent 10 μm. (B) Placental cells prepared from WT and SERT-KO (E.18d) (Yi et al., 2014) were analyzed in 5-12% gradient SDS. The WB analysis were performed with antibodies against Bax or Bcl2 or Caspase-3. The results of Western blot analysis are the summaries of combined data from four densitometric scans of the protein bands.
In summary these findings strongly emphasize the importance of SERT in protecting the placenta against cell death in a Caspase3-independent pathway. We did not find evidence of a maternal vascular source of this necrosis (no vascular thrombi or vascular injury) therefore we propose that the protective role of SERT in placenta is through regulating the intervillous/trophoblast 5-HT ratio which influences the cell proliferation in embryos. Furthermore, the placental necrosis is a result of a trophoblastic defect in proliferation or survival.
Discussion
The trophoblast serves as a multi-functional barrier separating the maternal and fetal blood circulation. Defects in trophoblast loose the integrity of trophoblast which causes the formation of necrosis and fibrosis at the junctional zone. In the current studies, we found that by genetically interfering with 5-HT levels there is abnormal hemorrhage (evidenced by fibrin deposition) and necrosis at the junction of the giant cells layer of the placenta. This finding is reminiscent of human placental pathology, such as those complicated by abnormal maternal blood flow (Odibo et al., 2014). We investigated the impact of extracellular 5-HT at elevated level on placenta.
The cellular integrity and the barrier properties of intercellular junctions, which are anchored into cytoskeletal proteins are controlled by a number of factors including 5-HT (Li et al., 2016). The 5-HT concentration in the intervillous space is tightly regulated by SERT expressed on trophoblast (Ramamoorthy et al., 1993). Our current studies with TUNEL assays show that in TPH1-KO mice, with lack of 5-HT, rates of trophoblast cell death were significantly lower than the rates in SERT-KO mice where the plasma 5-HT levels are 2-fold higher than those of WT (Mercado et al., 2013b). Overall, while the SERT-KO placentas showed profound tissue damage, markers for DNA repair or cell proliferation were not elevated.
In exploring the cause of 5-HT-associated placental cell death, our studies underlined a major role for SERT in trophoblast cells. The importance of SERT and the extracellular vs intracellular ratio of 5-HT were demonstrated in different approaches, using preclinical study models. Trophoblast isolated from diabetic placentas where the level of SERT on the cell surface is much less than its level on non-diabetic trophoblast (Li et al., 2014), we found the cell death ratio several fold higher than non-diabetic counterparts. Supporting these findings, the histochemical and biological features of placentas dissected from 18d pregnant mice lacking the gene encoding SERT showed a 49-fold increase in cell death without a concurrent change in the DNA repair or cell proliferation compared to WT counterparts. Interestingly, the necrotic insult was primarily located at the junctional zone of the placenta. These results suggested a relationship between the levels of SERT on cell surface with the rate of cell death and point to an additional role for SERT as protecting placental cells against cell death.
Studies using various selective SERT inhibitors on the placental choriocarcinoma (JAR) cells demonstrated that some, but not all, inhibitors caused DNA fragmentation and apoptosis in these cultured cells (Bengel et al., 1998b). The findings from these previous studies indicate that SERT on the cell surface was required to prevent programmed cell death. The TUNEL assays located the highest rate of cell death at the maternal side of the placentas in both transgenic mice, but predominantly in the SERT-KO model. This finding is similar to the necrosis described in human placentas complicated by maternal hypertension (Odibo et al., 2014). Although we did not observe placental infarct-like histology, we found evidence of placental necrosis by TUNEL assay. Due to the intense DNA fragmentation registered by TUNEL, this tissue damage is likely irreversible at a single-cell level. Judging from the TUNEL intensity that reached 6% on average, and comparing this damage with TUNEL resulting from injuries of other tissues (Apostolov et al., 2009). While the lack of SERT generated placental pathology, the lack of peripheral 5-HT biosynthesis (TPH1-KO) appeared to be associated with only minor placental damage.
To investigate the involvement of 5-HT and 5-HT signaling in pregnancy, the fasting blood glucose and insulin levels in transgenic mice, SERT-KO and TPH1-KO were measured. The plasma 5-HT level is twofold higher in SERT-KO than WT mice. During pregnancy 5-HT upregulates the insulin production in beta cells of pancreas (Schraenen et al., 2010) and their secretion (Uceyler et al., 2010). However, 5-HT also regulates glucose-stimulated insulin secretion as a paracrine/autocrine agent via acting on 5-HT3 receptor, lowering the 2 cell threshold for glucose (Ohara-Imaizumi et al., 2013). Agreeing with these studies, in SERT-KO plasma insulin level was fourfold higher and the glucose level was slightly higher than in WT mice. In contrast, in the absence of peripheral 5-HT, in TPH1-KO mice plasma resulting in insulin levels twofold lower and glucose levels 1.5fold higher than in WT mice. Therefore, the elevated plasma 5-HT in SERT-KO mice is the likely cause of their resistance to elevated plasma insulin.
Interestingly, in an earlier study with microarray analysis of megakaryocytes, we made a link between 5-HT signaling and apoptosis by identifying several proteins as apoptosis effectors which were found as up-regulated and their natural inhibitors were listed as down-regulated (Mercado et al., 2013a). Herein we connected these findings with, signaling pathways in cell death of SERT-KO placenta and we investigated if Caspase-3 is activated at Bcl2/Bax ratios in SERT-KO placenta.
In summary our data show an increased in trophoblastic TUNEL-positive cells at the junctional zone in placentas from SERT-KO mice which is coupled with histologic evidence of necrosis and increased fibrin deposition. These findings were mildly present in the TPH1-KO mice and focally present in WT mice. The cell death ratio in maternal versus fetal sides of placentas showed differences between transgenic mice such as in SERT-KO this ratio was 2.4 in TPH1-KO it was 3.5 suggesting more damage on the fetal side of placentas from TPH1-KO. This was almost equal in the placentas of WT mice (1.07). This impact of damaged cells at the fetal side of placentas was observed in the embryonic heart tissues of TPH1-KO which was 94% less than it is in WT embryonic tissues. The findings from the TUNEL assay were supported by IH and WB analysis of caspase3 in the trophoblast cells isolated from SERT-KO and TPH-KO mice. However, the low level of caspase3 positive cells and the ratio of Bcl2 to Bax in SERT-KO cells suggest the SERT-related cell death as independent of caspase3, such as in a “necrosis-like program cell death” (Broker et al., 2005; Cande et al., 2004).
Overall, findings make a link between the abnormal thick band of fibrosis and necrosis under the giant cell layer found in SERT-KO placentas with the high cell death ratio. Furthermore, it is reported that abnormal implantation with chronic abruption and decidual necrosis often present by retroplacental thrombus and necrosis and congenital heart defects (Cote et al., 2007). Indeed, the proliferation assays on embryonic heart showed a significant effect of lack of 5-HT on heart tissue. The cardiac defects observed in earlier studies were atrial or ventricular septal defects in which the wall between the right-left sides of the heart was not completely developed. SERT-KO mice develop obesity and cardiovascular complications and their embryos show various developmental defects (Cote et al., 2007).
Acknowledgement
We would like to thank Dr. J. Cross of University of Calgary for his critical comments on effective display of the images.
The authors also thank the MGH Histology laboratory for their excellent work producing the murine placental H&E and trichrome slides.
We gratefully acknowledge the UAMS Flow Cytometry and the DNA Damage and Toxicology Core Facilities.
This work was supported by the NIH Heart Lung and Blood Institute HL091196, Eunice Kennedy Shriver NICHHD 058697 and 053477; American Heart Association [13GRNT17240014] and the Minnie Merrill Sturgis Diabetes Research Fund, and the Sturgis Charitable Trust to FK.
References
- Amara SG, Arriza JL. Neurotransmitter transporters: three distinct gene families. Curr Opin Neurobiol. 1993;3:337–344. doi: 10.1016/0959-4388(93)90126-j. [DOI] [PubMed] [Google Scholar]
- Apostolov EO, Soultanova I, Savenka A, Bagandov OO, Yin X, Stewart AG, Walker RB, Basnakian AG. Deoxyribonuclease I is essential for DNA fragmentation induced by gamma radiation in mice. Radiat Res. 2009;172:481–492. doi: 10.1667/RR1647.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- Auda GR, Kirk SH, Billett MA, Billett EE. Localization of monoamine oxidase mRNA in human placenta. J Histochem Cytochem. 1998;46:1393–1400. doi: 10.1177/002215549804601208. [DOI] [PubMed] [Google Scholar]
- Balsells M, García-Patterson A, Gich I, Corcoy R. Major congenital malformations in women with gestational diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28(3):252–7. doi: 10.1002/dmrr.1304. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine ("Ecstasy") in serotonin transporter-deficient mice. Mol Pharmacol. 1998a;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bengel D, Isaacs KR, Heils A, Lesch KP, Murphy DL. The appetite suppressant d-fenfluramine induces apoptosis in human serotonergic cells. Neuroreport. 1998b;9:2989–2993. doi: 10.1097/00001756-199809140-00013. [DOI] [PubMed] [Google Scholar]
- Blakely R, Berson H, Fremeau R, Caron M, Peek M, Prince H, Bradely C. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslén B, Marsál K, Hansson SR. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004;25:518–529. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Bröker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–62. doi: 10.1158/1078-0432.CCR-04-2223. Review. [DOI] [PubMed] [Google Scholar]
- Candé C, Vahsen N, Garrido C, Kroemer G. Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ. 2004;11:591–595. doi: 10.1038/sj.cdd.4401400. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354:579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- Chen X, Margolis KJ, Gershon MD, Schwartz GJ, Sze JY. Reduced serotonin reuptake transporter (SERT) function causes insulin resistance and hepatic steatosis independent of food intake. PLoS One. 2012;7:e32511. doi: 10.1371/journal.pone.0032511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor P, Patel A, Shimada S, Lin CL, Rochelle JM, Kitayama S, Seldin MF, Uhl GR. Mamm. Genome. 1993;4:283–284. doi: 10.1007/BF00417438. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Mezey E, Brownstein MJ. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- Huang WQ, Zhang CL, Di XY, Zhang RQ. Studies on the localization of 5-hydroxytryptamine and its receptors in human placenta. Placenta. 1998;19:655–661. doi: 10.1016/s0143-4004(98)90027-3. [DOI] [PubMed] [Google Scholar]
- Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, Mogun H, Levin R, Kowal M, Setoguchi S, Hernandez-Diaz S. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397–2407. doi: 10.1056/NEJMoa1312828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts KF, Bateman BT, Palmsten K, Desai RJ, Patorno E, Gopalakrishnan C, Levin R, Mogun H, Hernandez-Diaz S. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142–2151. doi: 10.1001/jama.2015.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, Van Der Hart M, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Lee WS, Jeong J, Kim SJ, Jin W. Induction of metastatic potential by TrkB via activation of IL6/JAK2/STAT3 and PI3K/AKT signaling in breast cancer. Oncotarget. 2015;6(37):40158–71. doi: 10.18632/oncotarget.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Wallace JA, Krebs H. Roles for serotonin in neuroembryogenesis. Adv Exp Med Biol. 1981;133:477–506. doi: 10.1007/978-1-4684-3860-4_28. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Wolozin BL, Murphy DL, Riderer P. J. Neurochem. 1993;60:2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Wolozin BL, Estler HC, Murphy DL, Riederer P. J. Neural Transm. 1993a;91:67–72. doi: 10.1007/BF01244919. [DOI] [PubMed] [Google Scholar]
- Li Y, Hadden C, Singh P, Mercado C, Murphy P, Dajani NK, Lowery CL, Roberts DJ, Maroteaux L, Kilic F. GDM-associated insulin deficiency hinders the dissociation of SERT from ERp44 and down-regulates placental 5-HT uptake. Proc. Natl. Acad. Sci. USA. 2014;111:52. doi: 10.1073/pnas.1416675112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hadden C, Cooper A, Ahmed A, Wu H, Lupashin VV, Mayeux PR, Kilic F. Sepsis-induced elevation in plasma serotonin facilitates endothelial hyperpermeability. Sci Rep. 2016;6:22747. doi: 10.1038/srep22747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matthaeus F, Schloss P, Lau T. Differential Uptake Mechanisms of Fluorescent Substrates into Stem-Cell-Derived Serotonergic Neurons. ACS Chem Neurosci. 2015;6:1906–1912. doi: 10.1021/acschemneuro.5b00219. [DOI] [PubMed] [Google Scholar]
- Mercado CP, Byrum S, Beggs ML, Ziu E, Raj VR, Haun RS, Kilic F. Impact of Elevated Plasma Serotonin on Global Gene Expression of Murine Megakaryocytes. PLoS ONE. 2013a;8(8):e72580. doi: 10.1371/journal.pone.0072580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado CP, Quintero MV, Li Y, Singh P, Byrd AK, Talabnin K, Ishihara M, Azadi P, Rusch NJ, Kuberan B, Maroteaux L, Kilic F. A serotonin-induced N-glycan switch regulates platelet aggregation. Sci Rep. 2013b;3:2795. doi: 10.1038/srep02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebigil CG, Etienne N, Messaddeq N, Maroteaux L. Serotonin is a novel survival factor of cardiomyocytes: mitochondria as a target of 5-HT2B receptor signaling. FASEB J. 2003;17(10):1373–5. doi: 10.1096/fj.02-1122fje. [DOI] [PubMed] [Google Scholar]
- Odibo AO, Patel KR, Spitalnik A, Odibo L, Huettner P. Placental pathology, first-trimester biomarkers and adverse pregnancy outcomes. J Perinatol. 2014;34:186–191. doi: 10.1038/jp.2013.176. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Kim H, Yoshida M, Fujiwara T, Aoyagi K, Toyofuku Y, Nakamichi Y, Nishiwaki C, Okamura T, Uchida T, Fujitani Y, Akagawa K, Kakei M, Watada H, German MS, Nagamatsu S. Serotonin regulates glucose-stimulated insulin secretion from pancreatic 2 cells during pregnancy. Proc Natl Acad Sci U S A. 2013;110:19420–19425. doi: 10.1073/pnas.1310953110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann N, Grohmann M, Voigt J-P, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, Walther DJ. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman A, Moore K, Han H, Yang-Feng T, Chang A, Ganapathy V, Blakely R. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Schraenen A, Lemaire K, de Faudeur G, Hendrickx N, Granvik M, Van Lommel L, Mallet J, Vodjdani G, Gilon P, Binart N, in"t Veld P, Schuit F. Placental lactogens induce serotonin biosynthesis in subset of mouse beta cells during pregnancy. Diabetologia. 2010;53:2589–2599. doi: 10.1007/s00125-010-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uceyler N, Schutt M, Palm F, Vogel C, Meier M, Schmitt A, Lesch KP, Mossner R, Sommer C. Lack of the serotonin transporter in mice reduces locomotor activity and leads to gender-dependent late onset obesity. Int J Obes (Lond) 2010;34:701–711. doi: 10.1038/ijo.2009.289. [DOI] [PubMed] [Google Scholar]
- Viau M, Lafond J, Vaillancourt C. Expression of placental serotonin transporter and 5-HT 2A receptor in normal and gestational diabetes mellitus pregnancies. Reproductive BioMedicine Online. 2009;19:207–215. doi: 10.1016/s1472-6483(10)60074-0. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- Wemakor A, Casson K, Garne E, Bakker M, Addor MC, Arriola L, Gatt M, Khoshnood B, Klungsoyr K, Nelen V, O"Mahoney M, Pierini A, Rissmann A, Tucker D, Boyle B, de Jong-van den Berg L, Dolk H. Selective serotonin reuptake inhibitor antidepressant use in first trimester pregnancy and risk of specific congenital anomalies: a European register-based study. Eur J Epidemiol. 2015;30:1187–1198. doi: 10.1007/s10654-015-0065-y. [DOI] [PubMed] [Google Scholar]