Abstract
Homeostasis posits that physiological systems compensate setpoint deviations in an attempt to maintain a state of internal constancy. Allostasis, on the other hand, suggests that physiological regulation is more appropriately described by predictive modulatory actions that, by adjusting setpoints, anticipate and react to changes in internal and external demand. In other words, “maintaining stability through change.” The allostatic perspective enabled the rationalization of predictive and reactive homeostasis. While the latter reflects external perturbations, the former refers to systemic adaptation in response to anticipated changes − not necessarily related to unexpected external disturbances. Therefore, the concept of allostasis accounts also for adaptation to circadian variations (seasonal, circannual or other predictive variability) and interprets the system’s adaptation of its setpoints not as reactive/subnormal adjustments, but rather as a proper response. Therefore, systemic entrainment to periodic demands is handled by predicting and implementing setpoint changes. Given the important role of circadian variability and regulation in maintaining health, and the loss of circadian entrainment as a predisposing factor and sequel of stress, we elaborate on an allostasis model which demonstrates the ability of the systems to adapt to circadian demands and quantifies the deteriorative natural wear and tear of a system constantly adapting, i.e. the irreversible damage and its consequences on system function and overall survival. While developing a system of cascaded nature, we demonstrate the importance of phase coordination and the implications of maintaining proper phase relations. The disruption of these relations is a hallmark of circadian disruption, a predisposing factor to increased vulnerability and/or a sequel to chronic stress.
Keywords: Mathematical bioscience, Systems biology
1. Introduction
Homeostasis posits that physiological systems compensate or equilibrate variations from their expected behavior and describes how systems maintain their internal environment [1]. This concept, broadly, presumes the existence of an internal condition whose characteristics are expected to vary within narrow bounds [1]. Even when subject to changing external demand, the sensed discrepancy is equilibrated and the system continues with its static goal of internal constancy [1]. The allostatic approach argues that the convention that systems do not adjust to changing demand should be re-evaluated [2] and describes that parameters held constant by error-correcting feedback (negative feedback) cannot respond effectively to changes in demand [2]. Therefore, homeostasis does not “efficiently match capacities across stages that are functionally coupled [2].” Similar to their inability to adjust their setpoint, homeostatic systems are also not designed to adjust themselves in order to minimize future error [2]. Homeostats attenuate all errors but are not sensitive to those that are acutely catastrophic; acute trauma capable of irreparably damaging physiology is not demonstrated in the classic homeostat model [2].
The concept of allostasis extends conventional homeostasis by describing the implications of adaptable physiology [2, 3, 4, 5, 6, 7]. Physiological systems, it is argued, are not passive and setpoint deviations are often required to adapt to changing demands. Therefore, increasing body temperature to fight a virus, or varying hormone level during the day, is an appropriate course of action. However, this continuous adaptation “taxes” the host, which eventually manifests natural “wear and tear”. Although systems exhibit resilience and can successfully adjust to changing demands [2, 3, 5], over time, irreversible damage, defined as allostatic load (AL), builds up [2, 3, 4, 5]. An example of physiology incurring irreversible damage is observed in the expression of catecholamine intermediates, such as epinephrine or norepinephrine, in response to an entraining circadian transcription mechanism involving adrenergic receptors [8]. This dictates the dilation of target peripheral blood vessels via catecholamine mediated adrenergic receptor binding, thus altering blood pressure [8]. Chronic demand for high blood pressure causes fibrosis in vasculature, also known as atherosclerosis [9]. Thus, irreversible damage is observed in cases of chronic hypertension.
Motivated by investigations into allostatic development of chronic addiction [10, 11, 12] and disease [6, 11, 13, 14, 15] conceptual models were suggested to describe physiological modulation under stress [13, 16]. These investigations discuss the life-prolonging consequence of activating compensatory effectors (biological entities that expend energy and resources to effect change in a system) in physiological context [13, 16]. For example, Goldstein discusses the experimentally observed compensatory activation of the arginine vasopressin and renin-angiotensin-aldosterone systems for barostat maintenance in response to a compromised sympathetic nervous system (SNS) [16]. Blood pressure is maintained despite chemical sympathectomy (knockdown of SNS) due to this compensatory activation [16]. These effectors are tangible physiology that induce change on a system including cells, tissues, organs, organ systems, etc., depending on the definition of the model [13, 16]. In early investigations, [13, 16] classic homeostats are used to describe physiology and all in silico systems are defined by sets of static parameters, parameter sets which are interpreted as representations of the physical state of the system. Systems are heavily taxed for adapting and the concept of allostatic load (AL) is used to express this burden [6, 13, 14]. AL results from compensatory activity and further impairs the ability of the of the system to adapt.
Previous literature has established stress as a causative agent of chronic disease development [17, 18, 19, 20] while numerous studies have established the bi-directional relations between stress and (disruption of) circadian rhythms [17, 19, 20]. These and many other results indicate how disruption of coordination among rhythmic physiological signals induces stress. The catecholamine system discussed above is an example of a circadian entrained system. Relatedly, the three-homeostat format where the entrainer and peripheral systems are each subject to independent circadian reference signals, is exemplified in circadian rhythmicity in cell division, which has been reported in both unicellular and multicellular organisms [21]. Studies have found that a number of critical cell cycle mediators, including wee1, mdm2, and p21 are under the control of the circadian clock. Moreover, evidence suggests that the circadian rhythmicity in cell proliferation in multicellular organisms might also be modulated by systemic gating signals in addition to the endogenous circadian clock [22] thus, providing multiple layers of regulatory control. Motivated by these observations, we present in this paper an allostasis model which demonstrates the dependence of synchronicity between integrated homeostats, is capable of aggregating allostatic load and which demonstrates the consequences of irreversible damage when modulating a homeostat’s physical state. Entraining, intermediate and peripheral homeostats are integrated with hill repressor controllers [23] that incur irreversible damage as a function of integrated absolute error. The state of the system’s ability to maintain itself and interact with linked systems is observed to depend on the phase relationship between the homeostats (synchronicity) as well as the history, frequency and severity of stress challenges. We observe a phase dependence of the survival time (ST) of the peripheral homeostat, revealing the downstream sensitivity to upstream stressors. We demonstrate an allostatic system capturing an entrainer-intermediate-periphery structure. In this way, we can better observe disease development within periodic systems that desynchronize.
2. Methodology
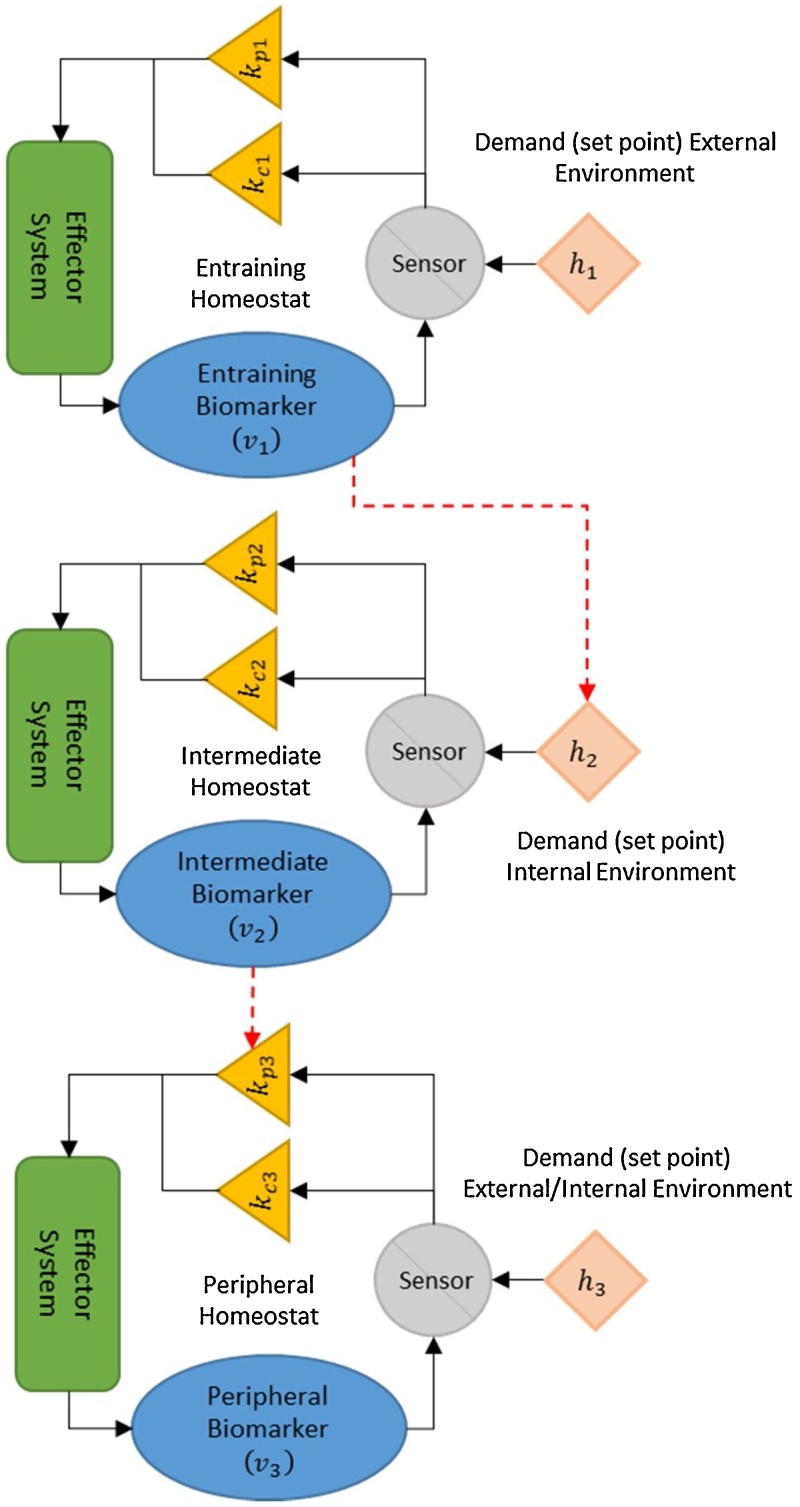
A cascade of inter-connected modified homeostats, designed such that their physiological state and setpoints are adjusted autonomously, is used as the basic structure (Fig. 1). Each homeostat assesses discrepancies between its corresponding regulated substance and, internal or external, demands via appropriate sensors. Detected discrepancies between target and measured values activate appropriate response mechanisms. These effector systems influence the levels of the regulated substance in order to minimize deviation from setpoints [25]. The purpose of the suggested structure is to capture a number of scalable characteristics: 1) the system forms a hierarchical structure of homeostats; 2) we assume that the top and bottom levels need to meet periodic setpoints, i.e., periodic demands that are independently prescribed in order to meet external and/or internal demands; 3) the response mechanism involves effector systems with integral and proportional controllers modified by modulatory gains (Interpreted biologically, gain is a quantity that determines how well a physiological effector can affect change; for example, a measure of the number of muscle fibers capable of being recruited or a measure of fibrosis development); 4) the inter-connectivity among the systems is present at two levels: 4a) the output (regulated substance) of the top level prescribes the periodic setpoint of the middle level homeostat, and 4b) the output (regulated substance) of the middle level homeostat drives the PI effector gain of the bottom level. This system is presented in Equations 2-9, initialized with conditions described in Table 1, and presented as time profiles in Fig. 2. The conditions in Table 1 are easily modified, but have been selected to illustrate effector decay gradually. These values, when changed, illustrate the same consequences on the effectors and variables, except at different rates of decay. The time frame of 0 to 4000 t.u. for the simulations depicted below is selected based on the time required to observe all effectors terminate and all variables fall to zero expression. This simulation timespan can be changed, but will illustrate the same effects, ultimately.
Fig. 1.
Schematic of three-homeostat system.
Table 1.
Parameter values for homeostat system.
| Parameter | Description | Value |
|---|---|---|
| Amplitude | 0.5 | |
| Loss rate | −1 | |
| Phase | Varies | |
| Period | 2π | |
| AL upper limit | ||
| AL lower limit | ||
| Modulatory coefficient | ||
| Hill coefficient | 6 | |
| Modulatory coefficient | 1 | |
| Initial values of reference signal sinusoids | 1 |
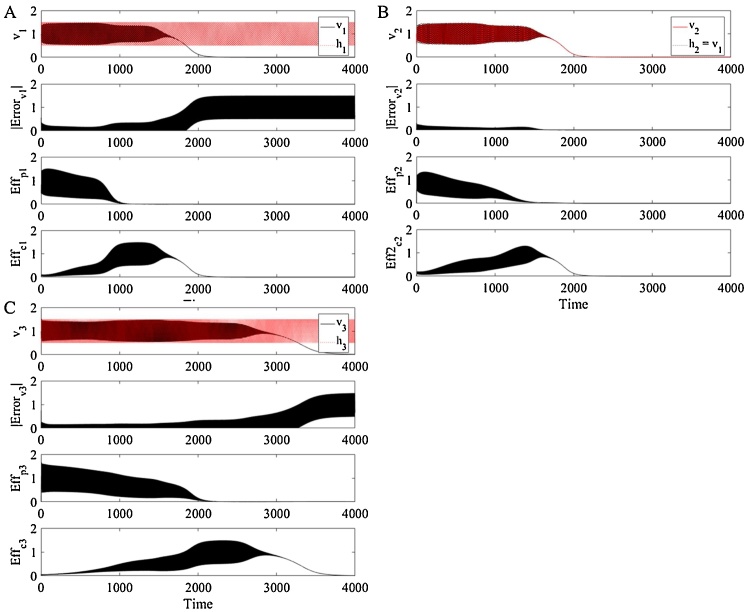
Fig. 2.
Time profiles of components in each homeostat for an unchallenged system. The monitored variable (v1, v2, v3), instantaneous absolute error, as well as the primary and compensatory effectors (kpi, kci) are presented for each homeostat. These profiles were generated after initializing the system with the parameters in Table 1 and letting thus providing the system with the optimal phase difference according to Table 2. Error is denoted e elsewhere in the text. The notation Error and Eff are included here for clarity, in order to distinguish between error generation and effector action.
The Proportional-Integral control allows the system to respond to its instantaneous error (current discrepancy between the reference and the variable) and the history of the error in the system (past discrepancy aggregated and influencing current behavior). The proportional component equips the system with an instantaneous response to deviations from the setpoint, whereas an integral component introduces elements of “memory” to the system, critical for the quantification of natural “wear and tear,” i.e. the consequences of allostatic load. The PI controllers are defined by their ability to respond to error directly (P, proportional control) or to the integral of error (I, integral control). However, in an allostatic system, tissues wear down as their physical limits are tested. This is interpreted in the context of this system to be a reduction in the ability of the controllers to respond to error as more error accumulates. Further described below, the model is equipped with hill repressor components which serve to modify the gain parameters of the controllers as error accumulates [23].
As the system is continuously adapting to meet the periodic demands of the time-varying setpoints, the accumulation of the integral error renders the primary gain kp incapable of maintaining proper function of the homeostats. The integral of error represents cumulative damage − a similar definition for allostatic load was previously described as the aggregation of stress over time [16]. As error accumulates, compensatory effectors with compensatory gain, kc, are activated denoting adaptation of the physical state of the system as it attempts to meet the time-varying demands in the presence of a compromised effector system. Eventually, the compensatory component, followed by the entire system, will fail.
The dynamics of the effectors (Equations (6) and (7)) increase activation as error accumulates, and eventually break down, by means of a Hill-type function (Equation (1)). This function is adapted from [23], and ubiquitously used as a controller model component useful for integrating on/off mechanisms within model types including those describing time-dependent biological events, thermodynamic equilibria, pharmacokinetic-pharmacodynamic non-linear drug dose responses, and gene regulatory response[23, 26, 27].
| (1) |
Equation (1) presents a controller (u) given a setpoint (y*) and detecting error (e), as well as a cooperativity coefficient (n) [23]. This function is adapted for the allostasis model within Equations (6) and (7), parameters defined in Table 1. This Hill repressor conforms to the condition that regardless of the cooperativity coefficient (n) value, the Hill repressor converges to zero (nonlinearly) if the AL , previously described as the aggregation of stress over time in [16]) exceeds a limit γi. Thus, damage aggregates due to inevitable wear and tear of the system [2, 4]. With the Hill repressors, each homeostat exhibits a transient state of resilience, or resistance to damage, to the stress challenge, defined by the AL upper and lower limits, γ and ε, respectively. However, resilience is overwhelmed when AL increases beyond its upper limit. Observed as emergent behavior of the model, repeated use and/or stress challenges alter a homeostat’s ability to meet its own reference signal, weakening the homeostat by over-exertion while working to meet this changing demand.
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
Each homeostat represents the physiological maintenance of a quantifiable biological parameter (regulated substance) via negative feedback [13, 16]. The homeostat system is inter-connected such that while the top-level homeostat follows its reference it dictates the demand (setpoint) for the middle level homeostat . In turn, the middle level homeostat dictates the primary (non-compensatory) control element of the bottom level homeostat (kp3 = v2). Notice that each vi, and by extension its ability to follow its demand, depends on its own internal function as well as interactions (i.e., restrictions or stresses) placed on it via interactions with other homeostats. The proposed structure, albeit simple, is generalizable because it reflects a cascade of information across a system of connected modified homeostats, with functional interactions impacting the demands placed on some homeostats and the ability of other homeostats to respond. Since the primary gain of the bottom level depends on the output of the middle level, clearly its ability to follow the bottom level’s setpoint requires collaboration from the upper levels. Our structure maps a multi-layer framework with connected homeostats. The lower level’s homeostat has a gain determined by the output of the middle level homeostat. As such, the “low” level system needs to activate its compensatory effector to compensate for its primary effector, because the primary effector is limited in that its gain is equal to the output of the second homeostat . This compensatory effort enables v3 to meet its demand (h3).
The main focus of the proposed analysis is the response of networked homeostats under periodic operation. Allostasis, i.e., achieving stability through change to accommodate for physiological setpoint changes, has been particularly influential in the context of periodic systems. Diurnal (circadian) variations in, practically any, biological function fall under this broad category. Circadian rhythms are believed to have emerged as the attempt of living organisms to maintain a balance of resource distribution throughout the active and inactive periods, most likely in an attempt to regulate energy homeostasis [28]. In fact, the likelihood of metabolic mobilization to optimize energy homeostasis, i.e., the interplay between catabolic and anabolic processes with the ensuing shift in the levels of the associated metabolites and hormones during the course a circadian cycle (day/night), strongly motivated the development of the concept of allostasis [7] and the challenge to the concept of homeostasis. The concept of homeostasis being the idea that physiological and biological mediators need to be confined within narrow ranges in order to maintain “constancy” of the extra-cellular medium [29]. Therefore, we aim to explore the proposed model to assess the following: a) The periodic setpoints of linked physiological sub-systems need to satisfy specific phase relations for optimal response − we quantify optimality with respect to accumulated AL, i.e., natural wear and tear; b) Non-optimized phase relations induce stress which compromise the ST of the overall system, i.e., increases the rate of accumulation of AL leading to faster break time; and c) Intermittent (acute) stress compromises the overall health of the system in a dose dependent manner. These observations shed light on the relations between disruption of periodic signals and the development of chronic disease [17].
The model (Fig. 3), and all simulations to be discussed, were developed in Matlab’s Simulink [24].
Fig. 3.
3. Results and discussion
3.1. Phase dependent allostatic load aggregation
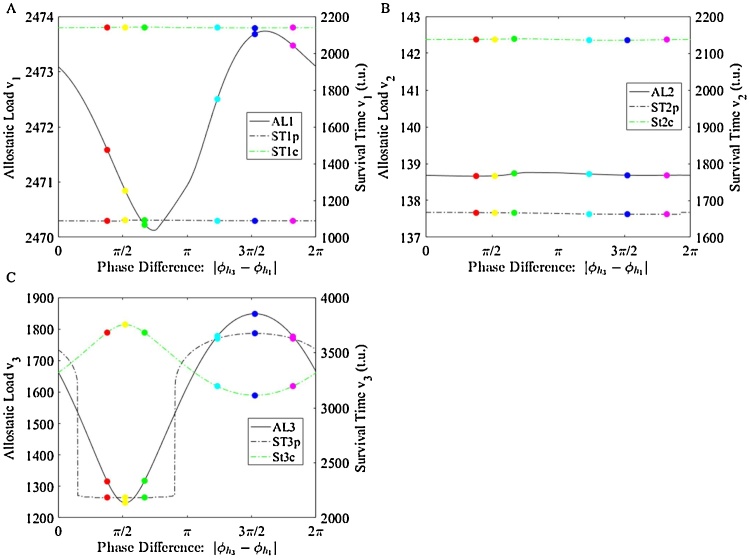
Challenging the three-homeostat system with all phase shifts in hundredths from 0 to 2π reveals a phase dependence depicted in Fig. 4C. AL is defined as the integral of the absolute error , for each phase tested. ST is calculated as the time period during which the compensatory effector is activated above 3%. Phases corresponding to minimum and maximum AL are determined from these results. The AL of our system is minimized for the normalized phase relationship between the homeostat signals of the entrainer and the periphery, . AL is maximized at . The AL’s dependence on phase speaks to the importance of synchronicity in minimizing AL. These results indicate that specific synchronicity between entraining and peripheral systems corresponds to the system’s ability to minimize irreversible damage, AL.
Fig. 4.
Phase modulation response in each homeostat, where the (C) bottom (peripheral) homeostat is of note. As the difference between the phases of h1 and h3 changes, the allostatic load and survival time are presented for each homeostat. Allostatic load and survival time for the bottom homeostat oscillate. Phase dependence reveals min AL/max ST at phase 1.64 and max AL/min ST at phase 4.79. Phase modulation response in all homeostats.
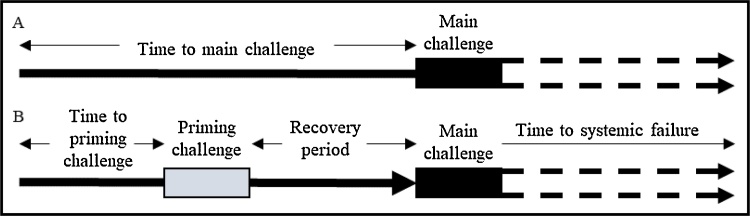
In order to determine the influence of stress on ST and AL, stress is introduced to the system as a reference signal phase shift within the entraining homeostat signal and represents a change in demand, whether acute or chronic. All parameters defining the applied stress pattern are varied independently (Fig. 5) and these include: 1) varying the duration of the stress challenge; 2) presence of a priming challenge; 3) intensity (phase) of the priming and secondary challenges (i.e. the degree of phase shift); and 4) length of rest between challenges. Fig. 6 presents a reference signal modified with stress challenge phase shifts, illustrating how stress is applied to the model: through modification of the reference signal to the entraining homeostat.
Fig. 5.
Construction of stress challenges. Each parameter (time to challenge, severity of challenge, recovery and challenge duration) is modified independently, in order to observe system sensitivity to each parameter. (A) and (B) differentiate challenge patterns applied below.
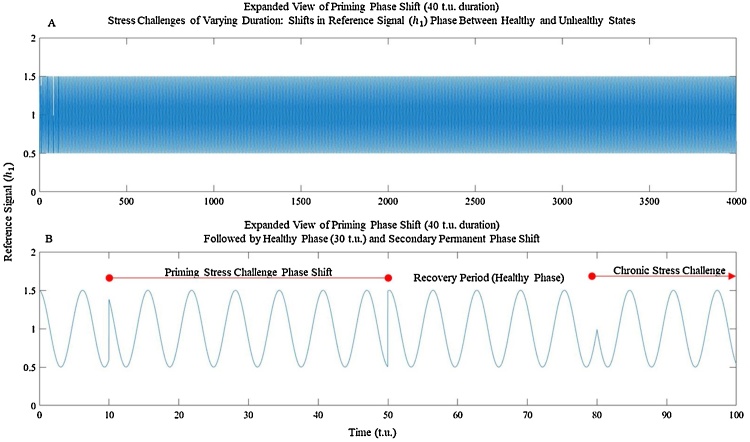
Fig. 6.
Example design of stress challenge illustrating that stress challenges are phase shifts in the reference signal, whose duration is modified to mimic acute or chronic challenges. (A) Entire reference signal of 4000 t.u. duration. (B) Expanded view.
The duration of the stress challenge and the recovery period are varied from 1 to 200 t.u, independently. Each simulation calculates AL and ST for all simulations. The failure of the homeostat is defined when the modulatory effectors converge to zero, representing the time at which the effectors are overwhelmed and permanently compromised.
Shifts in circadian rhythmicity are attributed to altered physiology and health [17]. We mimic these shifts by introducing stress into our system as phase shifts in the reference signal to the entraining homeostat, h1. The longevity of each effector was calculated as the consecutive time period during which kci was greater than or equal to 3% active, as defined previously. Failure of the compensatory controller is defined when this controller’s output converges to zero and was taken as the timepoint when the controller dropped under 3% activation. As is modulated from 0 to 2π, the ST is calculated for each peripheral homeostat’s effector. The entraining and intermediate homeostats exhibit about the same longevity for each phase shift. These results indicate that although stress is induced in the entraining homeostat, the burden of this stress is felt predominantly in the peripheral system (Fig. 4).
3.2. Influence of priming
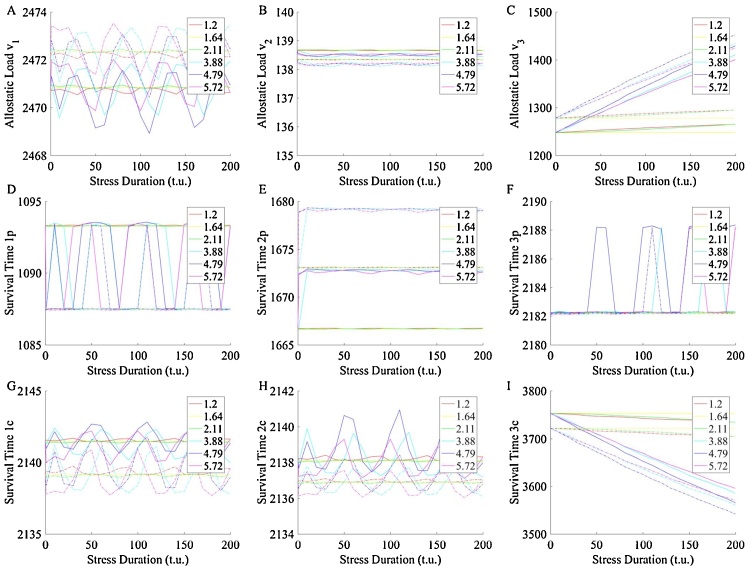
To assess the influence of stress duration, the system was exposed to stress beginning at time 80 t.u., and of increasing duration, referred to as the single challenge illustrated in Fig. 5A. To assess whether an additional early life challenge alters this influence, the double challenge was implemented which prefaces the single challenge with a representative acute early-life challenge, illustrated in Fig. 5B. This double challenge includes subjecting the system to a priming challenge from 10 t.u. to 50 t.u. before subjecting the system to the challenge of changing duration at 80 t.u.
Representative phases (Table 2) were selected from the chronic stress challenges in Fig. 4C and used for the single and double challenges. These phases represent the coordinates of min AL and max ST, max AL and min ST, as well as two pair of intermediate phases that represent behavior near the maxima and minima. Fig. 7 depicts the results of the single and double challenges, overlaid. This juxtaposition reveals the difference in ST and AL between single and double challenges. For example, phases and yield the same AL and ST within the peripheral homeostat, and the data points corresponding to these phases align as expected. These two phases are representative of the behavior of phases near the minimum AL and maximum ST. When comparing the single and double challenges, these points exhibit less difference in AL and ST than do the phase datapoint corresponding to max AL and min ST, i.e. phases and . This indicates that systems with more severe stress (i.e. phase nearer the phase of shortest ST and greatest AL) are more sensitive to stress frequency.
Table 2.
Allostatic load (AL) and survival time (ST) corresponding to phase differences applied as stress challenges.
| AL1 | AL2 | AL3 | ST1p | ST1c | ST2p | ST2c | ST3p | ST3c | Note | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.2 | 2471.6 | 138.7 | 1315.2 | 1087.9 | 2140.6 | 1667.0 | 2137.7 | 2182.3 | 3683.5 | (red) A2 |
| 1.64 | 2470.8 | 138.7 | 1247.5 | 1093.3 | 2141.5 | 1666.7 | 2138.1 | 2182.3 | 3752.9 | (yellow) AL3 min, ST3c max |
| 2.11 | 2470.2 | 138.7 | 1318.7 | 1093.0 | 2141.7 | 1666.1 | 2138.8 | 2182.3 | 3679.6 | (green) A1 |
| 3.88 | 2472.5 | 138.7 | 1777.0 | 1090.4 | 2138.4 | 1663.2 | 2135.6 | 3629.0 | 3194.7 | (light blue/cyan) B1 |
| 4.79 | 2473.7 | 138.7 | 1848.4 | 1089.7 | 2138.1 | 1662.6 | 2135.3 | 3674.0 | 3113.5 | (dark blue) AL3 max, ST3c min |
| 5.72 | 2473.5 | 138.7 | 1776.6 | 1089.3 | 2138.5 | 1662.3 | 2136.8 | 3628.2 | 3195.2 | (purple/magenta) B2 |
Fig. 7.
Response to primed (dashed) and non-primed challenges (solid) of varying severity and duration for each homeostat. (I) Survival time of compensatory controller in third homeostat (denoted 3c) and (C) allostatic load aggregation in the third homeostat. Priming always yields greater AL and shorter ST within the third homeostat and the third homeostat’s compensatory controller, except when the challenges are absent (healthy phase 1.64). AL increases and ST decreases with increasing challenge duration within the third homeostat. Within the other homeostats, variation in stress duration does not have as great an impact on variations in allostatic load and survival time of controllers. Thus, changes in reference signal induce stress on the three-homeostat system which manifests with greatest significance in the periphery (third homeostat). Please note the sawtooth behavior of some of the effectors above is an artifact of how survival time is automatically calculated for discrete data and oscillates about an approximately constant survival time value.
Thus, the priming investigation reveals that systems sick with asynchronicity cannot handle frequent stressors as well as can a synchronous system. Early life stressors compromise the system. Depending on the severity and duration of the priming challenge, the system is not able to respond with the same magnitude of response, as it would have responded given no priming challenge. A primed system is a damaged system and the severity of damage depends on the intensity and duration of early life stress. When presented with the same challenge later in life, a healthy system will meet the demand, a primed system will either meet or fail to meet the demand with variable ability, depending on how it has been damaged in the past.
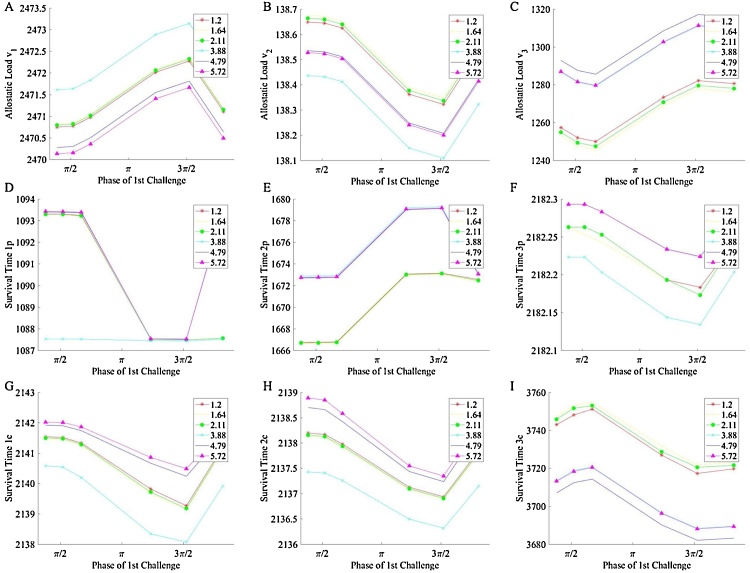
3.3. The influence of priming severity
In order to determine whether priming severity influences each system’s performance and survival, the three-homeostat system was exposed to patterns where the priming and secondary challenges consisted of different phases of stress, i.e. mismatched stresses between the two stress challenges. Both challenges had the same duration. The primer varied by phase only and results are presented in Fig. 8. Homeostats v1 and v2 demonstrate that AL and ST do not vary as drastically as the peripheral homeostat (v3). In the peripheral homeostat, we see the expected phase dependence of AL and ST (Fig. 8C, I). We also observed that the midline of this oscillatory relation changes depending on the phase of the different challenges. For example, in the simulation where the first challenge is , as the phase of the second challenges becomes more severe, the system increases in AL and decreases in ST. These results are observed across all tested phases: as the severity of either stress phase increases, ST decreases and AL increases.
Fig. 8.
AL and ST response for prime and secondary challenges of equal duration (40 t.u.) but different severity. The legend notes the phase of the second challenge. (I) ST of the compensatory controller within the third homeostat and (C) AL of the third homeostat. AL and ST oscillate within each homeostat, but with greatest amplitude in the third homeostat. Within the other first and second homeostats, variation in phase do not have as great an impact on variations in survival time of controllers. Thus, changes in reference signal induce stress on the three-homeostat system which manifests with greatest significance in the periphery (third homeostat).
Thus, we observe that severe priming causes more rapid system failure and greater irreversible damage accrual. The severity of the initial challenge, given identical secondary challenges, controls the eventually response of the system. When the second challenge is fixed, the eventual response of the system depends on the nature of the first challenge itself. Not all the initial challenges are equal in their long-term effect; how the system is stressed is important to its long-term response.
3.4. The influence of recovery period
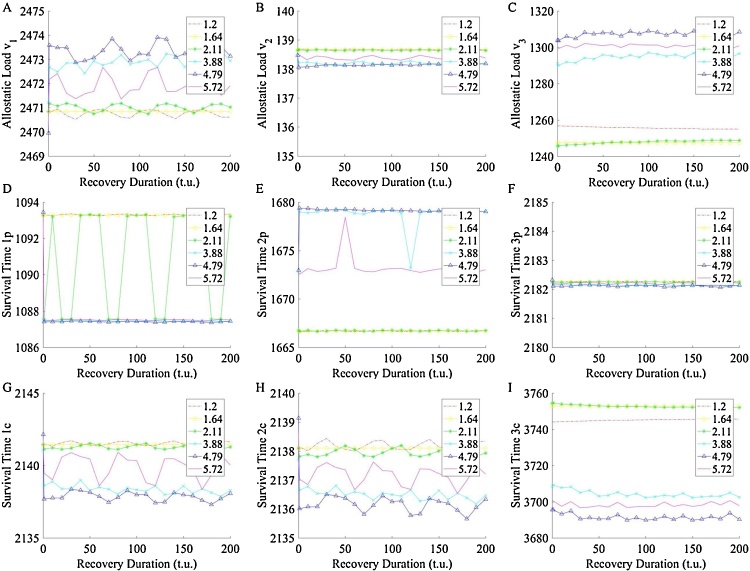
In order to assess the influence of recovery time on each homeostat, the system was again subjected to double stress challenges, of equal severity and duration, but of varying recovery time between stress phases. The results of this challenge are included Fig. 9 where the recovery time is varied from 1 to 200 t.u. It is evident that an extended recovery phase yields marginal changes in AL and ST within the bottom homeostat. These results indicate the lack of influence of in recovery duration on this particular system design and are expected because the system is not designed with a recovery mechanism, discussed further below.
Fig. 9.
AL and ST response given prime and secondary challenges of equal in duration (40 t.u.) and severity, but with increasing rest period. (I) ST of the compensatory controller within the third homeostat and (C) AL of the third homeostat. Increased rest period does not improve survival time of controllers in any homeostat. Rest recovery is not integrated into the model, so this effect is expected.
4. Conclusion
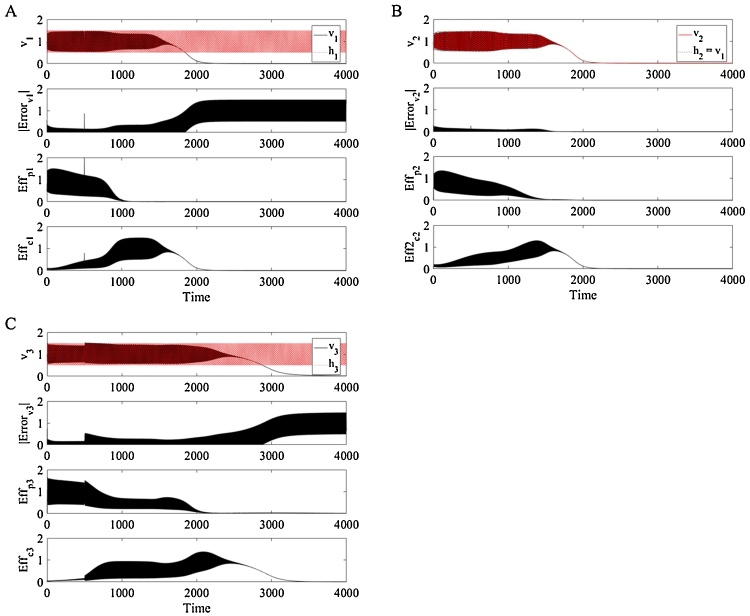
Physiological systems experience general wear over the course of their life, exacerbated by stressors that cause, and are further generated and exacerbated by, disease. To improve upon conventional homeostatic representation of physiology, we present an allostatic system that makes use of homeostat subcomponents, but which are modified such that their physiological state is adjusted autonomously (without any user input) based on the stress experienced, irreversible damage aggregated, and changing internal and external demands; we present an allostatic system which demonstrates allostasis via autonomous growth and development. The system alters itself based on its own experiences. Stress challenges cascade from entrainer to an intermediate system to the periphery, aggregating AL in all systems but most significantly in the periphery. An example of the system’s behavior when subjected to chronic stress is presented in Fig. 10.
Fig. 10.
Example response of the three-homeostat system in response to a challenge is initiated at 500 t.u in the reference signal (h1) of the entraining homeostat (v1). As the primary effectors fail, the compensatory effectors activate to compensate. When both fail, the error becomes large causing v1 and v3 to fail to follow their reference signals. The second homeostat (v2) does not necessarily fail to follow its reference signal, but, by design, follows a reference signal from v1 that has flatlined due to its own effector failure.
Fig. 10 demonstrates how the three homeostats respond to a generic, but chronic, stress challenge introduced as a phase shift at 500 t.u. in h1. Each variable attempts to follow its reference signal but fails with a certain degree of error. When the systems are synchronous, meaning when the systems have a phase relationship that is healthy, error accumulation is minimized and systems survive optimally. This is observed in the error output before t = 500 t.u. Once the stress is applied at t = 500 t.u., the error dramatically increases in each system, eventually overwhelming all systems. Fig. 10 depicts how the peripheral system is overwhelmed. A compensatory effector activates to assist v3 in following its reference signal and temporarily reduces the error in v3, as is observed from about 500 t.u. to 2000 t.u. This effector cannot remain active without consequence. Reflecting actual physiology, tissues wear down when overworked; AL accrues and the compensatory effector fails at about 3500 t.u. As the effectors fail, v3can no longer respond to its exacerbated error or follow its reference signal because its effectors are overwhelmed. Effectors are overwhelmed in each homeostat, resulting in the observed failure of v1 and v3 to oscillate. The intermediate variable, v2, is guided by v1 as its reference homeostat signal. It ceases to oscillate due to both the failure in its reference signal v1 to oscillate as well as the wearing down of the effectors of homeostat 2.
Each stress pattern applied to the three-homeostat system in this investigation demonstrates this breakdown. Considering the relationships between the homeostats, it is evident that the signal cascading from the entrainer communicates with v3via its effector, kc3. It is the synchronicity between v3and its effector which determines the ST and AL of this peripheral system.
The results of the rest duration investigation (Fig. 9) indicate that no measure of rest period improves system response as the system is currently designed. In actual physiology, we expect rest to allow systems to heal and regenerate, not necessarily reverse the irreversible damage that is AL, but allow a compensatory effector component to recover, for example. Our current design has no such recovery component. It is for this reason we do not observe an influence of recovery period on the system’s response. Future investigations may require improvements to this system with an installation of recovery components. Additionally, response variation due to noise is not considered here and noise would influence the rate of failure of the system, likely increasing it as the system is currently designed. Future investigations should include an analysis of system response due to noise in addition to a recovery component.
The three-homeostat model demonstrates, via its reaction to changing stress patterns and severity, the importance of optimal synchronicity between interrelated homeostats. There exists an optimal synchronicity between these homeostats that maximizes survival and minimizes AL. With this understanding, symptom manifestation and disease source can potentially be mapped to actual physiology. As observed, disease manifestation in v3 is the most significant, but applying treatment locally to v3 may not curtail the source of the disease in v1, unless the compensatory effector is therapeutically replaceable or accessible. With this allostatic model, we can observe the development of physiology fitting the entrainer-intermediate-periphery skeleton in order to better understand how these systems adjust to changing demand and manifest dysregulation.
Declarations
Author contribution statement
Alison Acevedo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ioannis Androulakis: Conceived and designed the experiments; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by NIH Biotechnology Training Program (Award T32 GM008339) and NIH GM24211.
Additional information
No additional information is available for this paper.
References
- 1.Vodovotz Y., An G., Androulakis I.P. A systems engineering perspective on homeostasis and disease. Front. Bioeng. Biotechnol. 2013;1:6. doi: 10.3389/fbioe.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sterling P. Allostasis: a model of predictive regulation. Physiol. Behav. 2012;106(1):5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Schulkin J. Cambridge University Press; New York: 2004. Allostasis Homeostasis and the Costs of Physiological Adaptation. [Google Scholar]
- 4.Goldstein D.S., McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002;5(1):55–58. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]
- 5.McEwen B.S. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol. Aging. 2002;23(September–October (5)):921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 6.Romero L.M., Dickens M.J., Cyr N.E. The Reactive Scope Model – a new model integrating homeostasis, allostasis, and stress. Horm. Behav. 2009;55(3):375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Sterling P., Fisher S., Reason J., editors. Handbook of Life Stress, Cogniton and Health. John Wiley & Sons; New York: 1988. Allostasis: a new paradigm to explain arousal pathology. p. 629. [Google Scholar]
- 8.Paschos G.K., FitzGerald G.A. Circadian clocks and vascular function. Circ. Res. 2010;106(5):833–841. doi: 10.1161/CIRCRESAHA.109.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan J.G., Barksdale D.J. Allostasis and allostatic load: expanding the discourse on stress and cardiovascular disease. J. Clin. Nurs. 2008;17B(April):201–208. doi: 10.1111/j.1365-2702.2008.02347.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed S.H., Koob G.F. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl.) 2005;180(3):473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S.H., Graupner M., Gutkin B. Computational approaches to the neurobiology of drug addiction. Pharmacopsychiatry. 2009;42(May (Suppl. 1)):S144–S152. doi: 10.1055/s-0029-1216345. [DOI] [PubMed] [Google Scholar]
- 12.Levy Y.Z., Levy D.J., Barto A.G., Meyer J.S. A computational hypothesis for allostasis: delineation of substance dependence, conventional therapies, and alternative treatments. Front. Psychiatry. 2013;4:167. doi: 10.3389/fpsyt.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein D.S. Computer models of stress, allostasis, and acute and chronic diseases. Ann. N. Y. Acad. Sci. 2008;1148(December):223–231. doi: 10.1196/annals.1410.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galen Buckwalter J., Castellani B., Mcewen B., Karlamangla A.S., Rizzo A.A., John B., O'donnell K., Seeman T. Allostatic load as a complex clinical construct: a case‐based computational modeling approach. Complexity. 2015 doi: 10.1002/cplx.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X., Liu R., Zhao X.M., Chen L. Detecting early-warning signals of type 1 diabetes and its leading biomolecular networks by dynamical network biomarkers. BMC Med. Genom. 2013;6(Suppl. 2):S8. doi: 10.1186/1755-8794-6-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein D.S. Concepts of scientific integrative medicine applied to the physiology and pathophysiology of catecholamine systems. Compr. Physiol. 2013;3(October (4)):1569–1610. doi: 10.1002/cphy.c130006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamashiro K.L., Sakai R.R., Shively C.A., Karatsoreos I.N., Reagan L.P. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14(September (5)):468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- 18.Karatsoreos I.N., Bhagat S., Bloss E.B., Morrison J.H., McEwen B.S. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc. Natl. Acad. Sci. U. S. A. 2011;108(January (4)):1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martino T.A., Tata N., Belsham D.D., Chalmers J., Straume M., Lee P., Pribiag H., Khaper N., Liu P.P., Dawood F., Backx P.H., Ralph M.R., Sole M.J. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension. 2007;49(May (5)):1104–1113. doi: 10.1161/HYPERTENSIONAHA.106.083568. [DOI] [PubMed] [Google Scholar]
- 20.Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U. S. A. 2009;106(March (11)):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson C.H. Circadian clocks and cell division: what's the pacemaker? Cell Cycle. 2010;9(October (19)):3864–3873. doi: 10.4161/cc.9.19.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickmeis T., Foulkes N.S. Glucocorticoids and circadian clock control of cell proliferation: at the interface between three dynamic systems. Mol. Cell. Endocrinol. 2011;331(January (1)):11–22. doi: 10.1016/j.mce.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Correa J.R., Lefranc G., Fernandez-Fernandez M. A new application of the hill repressor function: automatic control of a conic tank level and local stability analysis. Math. Probl. Eng. 2015 271216. [Google Scholar]
- 24.MATLAB and Statistics Toolbox Release, 2016, The MathWorks, Inc., Natick, MA, USA.
- 25.Buchman T.G. The community of the self. Nature. 2002;420(July (6912)):246–251. doi: 10.1038/nature01260. [DOI] [PubMed] [Google Scholar]
- 26.DiStefano J., III . Academic Press; 2015. Dynamic systems biology modeling and simulation. [Google Scholar]
- 27.Goutelle M., Maurin F., Rougier X., Barbaut L., Ducher M., Maire P. The Hill equation: a review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 2008;22(December (6)):633–648. doi: 10.1111/j.1472-8206.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 28.Straub R.H., Cutolo M., Buttgereit F., Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J. Intern. Med. 2010;267(June (6)):543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein D.S., Kopin I.J. Evolution of concepts of stress. Stress. 2007;10(June (2)):109–120. doi: 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]