Abstract
In Brazil, human and canine visceral leishmaniasis is caused by infection with Leishmania infantum, a Protist parasite transmitted by blood-feeding female Lutzomyia longipalpis sand flies. The objective of this study was to determine if the odour of hamsters, infected with Le. infantum, was more attractive than the odour of the same hamsters, before they were infected. The attractiveness of odour collected from individual hamsters (n = 13), before they were infected, was compared in a longitudinal study, with the attractiveness of the odour of the same hamster in a Y-tube olfactometer bioassay, at a late stage of infection. The odour of six of the golden hamsters was significantly more attractive to 50% of the female sand flies at the end of infection compared to before infection and the odour of four of the golden hamsters was significantly more attractive to 75% of the female sand flies at the end of infection. These results strongly indicate that hamsters infected with Le. infantum become significantly more attractive to a greater proportion of female sand flies as the infection progresses.
Introduction
In South America the sand fly species complex Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae) is responsible for transmission of the Protist, Leishmania infantum (Cunha & Chagas) (Kinetoplastida: Trypanosomatidae), the causative agent of visceral leishmaniasis (VL) a disease that is fatal if left untreated1. The parasites are transmitted from domestic dogs, which act as the reservoir host, to humans by blood-feeding female sand flies.
Blood-feeding insects use host odours to identify and orientate towards potential host animals. These host odours are used in combination with visual, thermal and tactile host cues. The precise contribution of each of these elements to host finding depends on the insect species involved and is modulated by a combination of intrinsic and extrinsic factors2, 3. Within a population of potential host animals, the attractiveness of each individual host to a haematophagous insect is different and can range from very attractive to repellent4, 5. This differential attraction can in part be explained by the balance between the attractive and repellent chemicals that comprise their odour profile6.
Lu. longipalpis females feed on a wide range of host animals including chickens, dogs, horses, cattle and humans7. They are attracted by a range of host cues including host odour7–9, heat and CO2 10. Male Lu. longipalpis produce a sex pheromone which although moderately attractive by itself, is synergized by the presence of host odour, to become powerfully attractive to both females and males to the vicinity of the host animal11.
Some parasites are able to improve their chances of transmission by manipulating the host animal, e.g. by altering its appearance or behaviour12. In arthropod transmitted diseases such as Malaria, the parasite has been shown to alter the behaviour of the mosquito vector by increasing frequency and persistence of probing activity13 and through a heightened response to host odour14.
Few studies have examined the potential of the parasite to alter the odour of a host animal3. Yet, for insect transmitted parasites, the vector response to host odour is a key determinant of feeding success and therefore disease transmission15. It has long been recognised that infection may change host odour, and physicians have used this observation for hundreds of years to help diagnose parasitic infection in their human patients16. However, it is not clear that changes in the host odour, which may be due to altered breath volatiles or epidermal microbial flora17, 18 lead to change in vector response to the host.
In a preliminary olfactometer study, two Golden hamsters, Mesocricetus auratus, infected with a Brazilian strain of Le. infantum (MHOM/BR/74/PP75) were found to be more attractive to female Lu. longipalpis sand flies than uninfected hamsters. In addition, when the volatile odours were collected from these hamsters, and tested in a Y-tube choice experiment, they were found to be significantly more attractive than odours collected from the uninfected hamsters19.
Although these experiments were not longitudinal and therefore an increase in attractiveness of the individual hamsters following infection was not demonstrated, they suggested that the infected hamster’s odour was altered and led to increased attractiveness of the infected animal to the vector.
A parasite manipulation theory would predict that the odour of an animal would increase in attractiveness after infection and that peak attraction would potentially coincide with peak parasite level. Therefore, the objective of this study was to compare the attractiveness of the odour of individual host animals prior to infection with Le. infantum with their attractiveness at a late stage of infection, when the number of transmissible amastigote infective parasites in the circulating periphery blood is likely to be at a maximum.
Results
This study showed that the odour of hamsters infected with the Italian strain of Le. infantum at a late stage of infection was significantly more attractive than odour before infection (Table 1). The odour of 6 out of the 13 hamsters had become significantly attractive compared to the uninfected hamster odour for more than ½ of the sand flies (probability > 0.9) and the odour of 4 of the 13 hamsters had become significantly more attractive for more than ¾ of the sand flies (probability > 0.9) (Table 2 and Supplementary Fig. S1).
Table 1.
The number of female sand flies responding to late stage infection odour hamsters and before infection odour in a Y-tube olfactometer, nr = no response (the number of female sand flies that did not respond after 3 mins).
| hamster | number of ♀ sand flies responding to the “before infection” and “late stage infection” odours | ||||||
|---|---|---|---|---|---|---|---|
| sex | status | duration of inf (d) | before infection | late stage infection | P | nr | |
| 1 | f | inf | 80 | 2 | 2 | 1.000 | 76 |
| 2 | f | inf | 117 | 9 | 8 | 1.000 | 63 |
| 3 | f | inf | 117 | 29 | 32 | 0.798 | 19 |
| 4 | f | inf | 180 | 17 | 53 | <0.001** | 10 |
| 5 | f | inf | 130 | 3 | 48 | <0.001** | 29 |
| 6 | f | inf | 110 | 17 | 45 | 0.005** | 18 |
| 7 | f | inf | 171 | 15 | 17 | 0.860 | 48 |
| 8 | f | inf | 143 | 29 | 25 | 0.683 | 26 |
| 9 | f | inf | 100 | 28 | 24 | 0.678 | 28 |
| 10 | m | inf | 100 | 8 | 60 | <0.001** | 12 |
| 11 | m | inf | 82 | 6 | 36 | <0.001** | 38 |
| 12 | f | inf | 153 | 9 | 50 | <0.001** | 21 |
| 13 | m | inf | 100 | 20 | 20 | 1.000 | 40 |
| Controls | |||||||
| number of ♀ sand flies responding to the before inoc and 99 days odour | |||||||
| before inoc | 99 days | ||||||
| 14 | f | inoc | 99 | 19 | 13 | 0.377 | 48 |
| 15 | f | inoc | 99 | 29 | 25 | 0.683 | 26 |
| 16 | m | inoc | 99 | 11 | 12 | 1.000 | 57 |
| 17 | m | inoc | 99 | 22 | 19 | 0.755 | 39 |
| 18 | m | not-inoc | 99 | 14 | 17 | 0.720 | 49 |
| 19 | f | not-inoc | 99 | 11 | 13 | 0.149 | 56 |
The numbers responding to each side of the olfactometer were compared for each hamster using a 1-proportion exact test (*P < 0.05, **P < 0.01). The Control section of the table includes female sand fly responses to sham inoculated (inoc) or not inoculated (not-inoc) hamsters. The sex of the hamster (m/f) and the length of time for infection to reach the late stage of infection (duration of inf. days) are denoted.
Table 2.
Relative frequency is the proportion of sand flies found in the test arm (late stage infection) compared to the total number of sand flies in the test and control arm (before infection) combined.
| hamster | Relative frequency | 95% credible interval | Probab | Probab | Rel.Freq >0.75 |
|---|---|---|---|---|---|
| 2.50% | 97.50% | Rel.Freq >0.5 | |||
| 1 | 0.5 | 0.16 | 0.86 | 0.5 | 0.1 |
| 2 | 0.47 | 0.26 | 0.69 | 0.4 | <0.01 |
| 3 | 0.52 | 0.4 | 0.65 | 0.64 | <0.01 |
| 4 | 0.75 | 0.65 | 0.85 | 1 | 0.51 |
| 5 | 0.93 | 0.85 | 0.98 | 1 | 0.99 |
| 6 | 0.72 | 0.61 | 0.82 | 0.99 | 0.29 |
| 7 | 0.53 | 0.37 | 0.69 | 0.64 | <0.01 |
| 8 | 0.46 | 0.34 | 0.6 | 0.29 | 0 |
| 9 | 0.46 | 0.33 | 0.59 | 0.29 | 0 |
| 10 | 0.88 | 0.79 | 0.94 | 1 | 0.99 |
| 11 | 0.85 | 0.73 | 0.94 | 1 | 0.93 |
| 12 | 0.84 | 0.74 | 0.92 | 1 | 0.95 |
| 13 | 0.5 | 0.36 | 0.65 | 0.5 | <0.01 |
| controls | |||||
| 14 | 0.41 | 0.25 | 0.57 | 0.14 | 0 |
| 15 | 0.46 | 0.34 | 0.59 | 0.29 | 0 |
| 16 | 0.52 | 0.33 | 0.71 | 0.58 | <0.01 |
| 17 | 0.46 | 0.32 | 0.61 | 0.32 | <0.01 |
| 18 | 0.55 | 0.38 | 0.71 | 0.7 | <0.01 |
| 19 | 0.54 | 0.35 | 0.72 | 0.65 | 0.01 |
The, larger this frequency the more attractive the hamster odour is to sand flies. Credible interval is calculated on the posterior distributions (Supplementary Fig. S1) of the relative frequency of each hamster as result of accounting for uncertainty in the test parameters. The probability that the relative frequency is larger than 50 and 75% is reported in the last 2 columns.
This is a significant result, since only the infected hamsters showed attractiveness to more than ½ (6 hamsters with probability ranging from 0.99 to 1) and ¾ of sand flies (4 hamsters with probability ranging from 0.93 to 0.99); while control hamsters attractiveness to ½ of the sand flies was never significant (the credible interval was always lower than 0.5).
It could be argued that ½ of the hamsters did not attract more than ½ of infected sand flies, and that ¾ of hamsters did not attract ¾ of sand flies. However, from an epidemiological point of view, the presence of more attractive hosts in a population, at the above proportions, is a determinant for the success of disease transmission (for example the 20/80 rule20).
The time before the appearance of symptoms infection was not significantly associated with the proportion of attracted sand flies (P = 0.58).
The four hamsters showing the strongest attraction when infected (i.e. hamsters 5, 10, 11 and 12) were significantly different from the rest of the hamsters. The odour of these four infected hamsters showed a 30–40% larger relative frequency of attraction of female Lu. longipalpis than the rest of the hamsters (Supplementary Figs S2, S3, S4 and S5). The biggest difference in the dataset is between hamster number 5 and hamster number 14 (Supplementary Fig. S2, first graph on the left in the third row).
Overall the probability that infected hamsters were more attractive than control hamsters was larger than 0.999. Infected hamsters were more attractive than control hamsters, with an estimated relative frequency of 0.69 ([0.65, 0.72] credible interval) compared to 0.48 ([0.41, 0.55] credible interval) respectively.
To establish if the hamsters that were already attractive before infection improved their attractiveness we repeated the comparison on only those hamsters to which a medium-high number of sand flies were attracted before infection (i.e. ≥11 sand flies). These hamsters still improved their attractiveness, by increasing the relative frequency from 0.42 [credible interval 0.37–0.47] to 0.58 [credible interval 0.53–0.63] (probability of relative frequency larger than 0.5 is 0.999); while the control hamsters in both the “before” and “late stage infection” categories or “not inoculated” do not show any significant change in attractiveness (probability for relative frequency larger than 0.5 after 99 days is 0.311).
Within the control group of six uninfected hamsters, four of which were inoculated with uninfected spleen homogenate and 2 that were sham inoculated, there was no change in the attractiveness of their odour over time ca. 99 days (Table 2).
Analysis of the control experiments i.e. air vs. air, hexane vs. hexane and hexane vs. air indicated that there was no bias in the apparatus and the homogeneity test showed that data from different days could be combined for 1-proportion test analysis.
The results indicate that the change in attractiveness was due to altered volatile odour components and was not age related as there was no difference in the age of the group of hamsters that remained unattractive (mean age = 118.3 ± 11.6d) compared to the age of the group that became attractive (mean age = 125.8 ± 14.8d (T-test = ns).
The response of An. gambiae and male Lu. longipalpis sand flies to infected and not-infected hamster odour was variable (Table 3). Female An. gambiae (79% of responders) were attracted to the uninfected odour compared to infected odour (relative frequency of 94% with credible interval [0.78, 1]). However only 18% of the 160 An. gambiae responded in the olfactometer and the response was significant in one of the two replicates only (Binomial Test, P < 0.005).
Table 3.
Response of control insects, female Anopheles gambiae and male Lutzomyia longipalpis to odour of the before and late stage infected hamsters. Responses were compared by a binomial distribution exact test.
| Insect | Y-tube expt | Number of flies to: | Observed proportion | P value | Relative frequency | Credible interval | |||
|---|---|---|---|---|---|---|---|---|---|
| before infection odour | late stage infection odour | nr | 2.5% | 97.5% | |||||
| Anopheles gambiae females | 1 | 12 | 6 | 62 | 0.67 | 0.071 | 0.66 | 0.45 | 0.85 |
| 2 | 11 | 0 | 69 | 1.000 | 0.005** | 0.94 | 0.78 | 1 | |
| Lutzomyia longipalpis males | 1 | 8 | 17 | 55 | 0.32 | 0.032* | 0.33 | 0.16 | 0.51 |
| 2 | 20 | 11 | 49 | 0.65 | 0.150 | 0.64 | 0.47 | 0.79 | |
Significant (*P < 0.05, **P < 0.01) attraction to late stage infected hamsters was found in one of the two runs but not in the other for each control insect. A Bayesian test of proportion shows that only A. gambiae in the second run has a frequency significantly larger than 0.5.
Overall male Lu. longipalpis did not prefer either uninfected or infected hamster odour (total to uninfected = 17.5%; total to infected = 17.5%). Only 35% of the male Lu. longipalpis responded in these experiments.
Discussion
These results show, for the first time, that hamsters became more attractive over time, because of infection with Le. infantum. In a group of 13 hamsters a significant proportion became attractive after infection compared to the proportion that were attractive before infection. These results can be considered to be robust since we are accounting for uncertainty in the model parameters (see methods) of the statistical test.
A pilot scale study carried out by O’Shea et al.19, showed that hamsters infected with a Brazilian strain of Le. infantum were more attractive than uninfected, sex and age-matched hamsters. Their study was carried out both with live animals and odour extracts made from the headspace volatiles of the infected and uninfected hamsters, compared in a dual-choice wind-tunnel and Y-tube olfactometer respectively. In the whole animal study, a significant female sand fly response to infected hamsters was observed. However, in that study only two hamsters were used for each treatment group (infected and uninfected) and there was a consequent possibility that the difference in attractiveness observed was due to variation in the attractiveness of the individual hamsters4, 21, i.e. the uninfected hamsters selected for the study were already less attractive than the infected hamsters and thus the difference in observed sand fly attraction was because of this variation and not related to infection status. In O’Shea’s study the whole animal wind-tunnel studies were carried out with groups of 15–20 female sand flies (replicated 12 times). This experimental design could allow the potential interaction between the female sand flies in each replicate and also as the numbers of sand flies landing on a target area around the odour source was measured, the data could be skewed by a small number of highly active sand flies thus potentially confounding the results.
The work reported in this study overcomes the limitations of the previous work as we used a larger sample size of hamsters (n = 13) and the longitudinal study design allowed us to track the change in attractiveness of individual hamsters from before infection to late stage infection, as determined by observation of the hamster. In addition, the use of a Y-tube olfactometer allowed us to treat each individual sand fly as a replicate and thus the outcome based on the observation of a very large number of replicates is more robust than the previous study.
It could be suggested that the change in attractiveness observed in this study was related to aging of the hamsters. However, this is a less likely explanation for the observed change as the group of 6 control hamsters remained unattractive throughout the experiment plus there was no significant difference in the average age of the group of infected hamsters that were attractive compared to the average age of the infected hamsters that were unattractive. Taken together these data suggest that aging by itself is an unimportant determinant of changing attractiveness. A larger group of control hamsters would have removed any possible ambiguity.
Although a comparison between male and female hamsters is not feasible due to the small number of male hamsters sampled, this work also seems to suggest an increase in attraction in both sexes. This will need to be confirmed by additional studies which may test if enhanced attraction is also an effect of the oestrous cycle of the female hamsters which had only been used during previous experiments.
Female An. gambiae mosquitoes were also tested in the Y-tube olfactometer with the same entrained odour samples as the male sand flies. The two separate parts of the bioassay could not be combined because the ratios of the two runs were not homogeneous. However, female An. gambiae were attracted to the before infection odour in both parts of the experiment, suggesting that An. gambiae were more attracted to uninfected Golden hamsters. The response of male Lu. longipalpis was mixed, males were not attracted to the uninfected hamster odour on either day of the bioassay. This would not be predicted from observations of sand fly behaviour in the field, where male sand flies are normally attracted to host animals before females where they establish leks and produce sex pheromone. This combination of host odour and sex pheromone is very attractive to female Lu. longipalpis sand flies. This raises some intriguing possibilities. In an environment with an abundance of potential host animals Lu. longipalpis distribution is heterogenous, it is unclear what drives this heterogeneity and these results may suggest that male sand fly choice of host odour is a key driver of the uneven distribution as they may be responding to different odour components or different concentrations than the females.
In this study not all the infected hamsters became attractive and this may be because of genetic variability between the animals. The mechanism of metabolic translation from genes to odour is currently unknown but likely to be complex22. It is clear from this study that four out of 13 hamsters were very attractive and this may support a “super spreader” hypothesis as they were responsible for attracting more than ¾ of the sand flies (probability > 0.9). In addition, the overall experimental results show a clear difference in attractiveness between “late stage infection” and “before-infection” hamsters, even for those hamsters with relatively medium-high attractiveness before infections. However, individual differences in attractiveness are evident and may be due to intrinsic individual or genetic characteristics of the hamsters or it may be related to the parasite load23. This was not measured in the current study but infectiousness to the sand fly vector has been associated with high parasite numbers in dogs, and skin parasite loads was the best predictor of being infectious23. This study was designed to determine the hamster attractiveness near the end of the infection cycle however, in the future it would be interesting to correlate the change in infection load and skin parasite numbers with changes in attractiveness through the full infection cycle.
In Brazil, Le. infantum is a zoonosis, and the parasite is transmitted primarily among domestic dogs (Canis lupus familiaris) by the sand fly vector, Lu. longipalpis. Although Lu. longipalpis feeds on a variety of mammalian and avian host animals only canids are considered to play a significant role in the epidemiology of the disease and in some parts of Brazil it has been reported that up to 50% of dogs have been exposed to Le. infantum by the time they are 3 years old. It has now been established that dogs infected with Le. infantum, have a different odour profile to uninfected dogs24. It would therefore be interesting to determine the effect of Le. infantum infection on the attractiveness of canid odour to Lu. longipalpis.
A potential relationship between skin parasite loads and an increasingly attractive odour is also an intriguing possibility, offering a potential opportunity to rapidly identify those highly infectious individuals that may be responsible for the majority of transmission23, 25. Furthermore, these results suggest that infection may also add to host selection criteria. Many models have been produced to predict vectorial capacity and disease transmission but none attempt to take into account the variation in host attraction levels26. This work along with the other evidence highlights the necessity for these models to be reviewed and to consider the inclusion of factors such as the level of infection within the population26, 27.
In conclusion, our results indicate that infection with Le. infantum enhances the attraction of hamster host odour to the sand fly vector of this parasite and this could lead to enhanced transmission. Some of the infected hamsters were particularly attractive to the sand flies. Such findings, if they were to be extrapolated to the general epidemiology of the disease, may have profound implications for the use of mathematical models, diagnosis and control of this infection. The implications of this manipulation, whereby infected individuals are more likely to be involved in transmission of disease, might require a revision of the basic epidemiological models28, 29.
Methods
Golden hamsters and Leishmania
An Italian strain of Le. infantum, (MHOM/IT/95/LEM3437) supplied by the London School of Hygiene and Tropical Medicine, was used to inoculate the hamsters used in this study. Ten female and three male, 4-week old Golden hamsters were inoculated intra-peritoneally with 0.5 ml of spleen homogenate containing amastigotes obtained from a single, previously infected hamster that displayed symptoms (cachexia and ascites) of late-stage Leishmania infection30. Controls were two female and two male, 4-week old hamsters inoculated with 0.5 ml of spleen homogenate from a single non-infected hamster and an additional female and male hamster not inoculated, but treated in the same way as the infected animals.
All work with hamsters was carried out with the approval of the Keele University School of Life Sciences PhD committee and Keele University Ethics Committee. Research involving the hamsters was carried out in accordance with the guidelines and regulations of the Animals in Science Regulation Unit (ASRU) and in accordance with the terms of a regulated licence (PPL40/2693) in compliance with the UK Home Office, Animals (Scientific Procedures) Act (ASPA) regulations.
Sand flies
The sand flies were originally collected in Jacobina, Bahia State, Brazil (40°31′ W, 11°11′S) and kept in Barraud cages (18 × 18 × 18 cm) at 27 °C, 95% RH, with a photoperiod of 12:12 (L:D)31. Female sand flies were routinely blood-fed on anaesthetized Golden hamsters to maintain the colony.
Host odour entrainment
Hamster odours were collected from the air passed over the animals immediately after they had been inoculated (before infection) with Leishmania amastigotes on 3 consecutive days for 1 hour in a volatile collection apparatus (Fig. 1). The three samples that were obtained were combined to provide the ‘before infection’ extract for each of the hamsters. The odours of the same hamsters were then collected again individually when they were nearing the end of the infection cycle (late stage infection). The late stage of infection was established by visual inspection of the hamster and by weekly monitoring of the weight of the hamster to determine cachexia (loss of weight, muscle atrophy, fatigue, weakness, and significant loss of appetite) and ascites (excess fluid build-up in the abdomen). These symptoms are indicative of late stage of infection30. As the infection progressed at different rates in each hamster, the odour of late stage infected hamsters was collected when they had reached the same stage of infection, (this was not dependant on chronological age of the animal) or at a pre-determined time (99 days) from inoculation (Table 1). The odour of the control animals was entrained immediately after non-infected or sham inoculation and again after 99 days.
Figure 1.
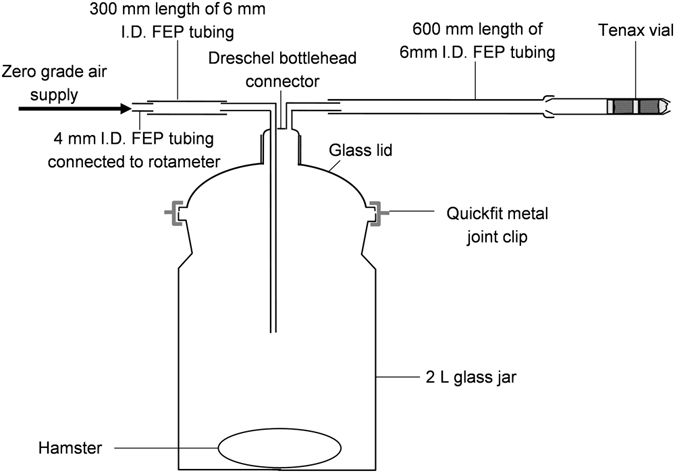
Odour entrainment apparatus. To collect the odours of M. auratus, the animal was placed in a 2 L glass jar; clean, zero grade air was passed over the animal and entrained odours adsorbed on Tenax TA.
Each animal was placed individually into a clean Quickfit culture flask (2 L) fitted with a single-port lid (Fig. 1). A Drechsel bottle head was inserted into the port and cleaned zero-grade air (7 ml s−1) was passed through the flask. The components of the apparatus were connected with fluorinated ethylene propylene (FEP) tubing that had been cleaned by rinsing internally before and after each entrainment with hexane (Pesticide Grade). All glassware was cleaned by first washing thoroughly with a 10% Teepol solution, rinsed with distilled water and then acetone and heated in an oven overnight at 200 °C. Airflow was periodically confirmed using a bubble-meter. The effluent air containing the hamster odour passed through an ORBO 403 filter (Tenax TA (60/80)) and after one hour the airflow was measured again and the tube disconnected and the adsorbed chemicals were eluted in hexane (1.5 ml). Control entrainments without the hamster (i.e. apparatus only) were also carried out. The volume of the entrained odour samples was reduced to 100 µl under air, and the samples stored in heat-sealed glass Pasteur pipette vials at −20 °C prior to bioassays.
Bioassays
Six-day old, mated, sugar-starved, female sand flies were used in the Y-tube bioassays unless otherwise stated. To provide a batch of 40 female sand flies for use in the bioassays, 50 female and 20 male sand flies were collected 1-day post emergence and held together in a Barraud cage within a plastic bag (to maintain humidity), for 6 days without access to sugar. One hour before the experiment started, the sand fly holding cages were moved into the bioassay room (70 ± 5% rh; 24 ± 2 °C; fluorescent lighting), removed from the plastic bag and the sand flies allowed to acclimatise to the bioassay room conditions.
A Y-tube olfactometer was used to test the attraction of individual female sand flies to the entrained odour samples32. The Y-tube olfactometer was formed from three lengths of glass tubing (10 mm id, ½″ od); it had two 10 cm long arms joined together at an angle of 65° with a 10 cm long stem centrally positioned between the 2 arms in the same plane to form a Y-shape (Fig. 2).
Figure 2.
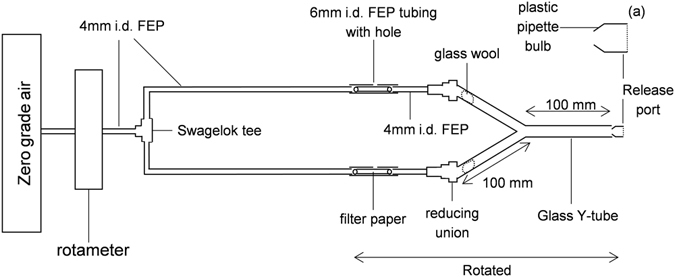
Y-tube olfactometer bioassay apparatus. A sand fly was introduced into the stem of the olfactometer using the release port shown in detail in (2a), and given time to respond to odours which had been introduced through the pierced hole onto the filter paper. Airflow was adjusted to 5 ml sec−1. After every ten replicates within a bioassay the Y-tube was rotated 180° around the horizontal.
Zero-grade air was passed through a rotameter (airflow 5 ml s−1), and a hydrocarbon filter via FEP tubing (¼″ od). The tubing was divided into two with a brass Swagelok T-union. A 150 mm length of FEP tubing (¼″ od) was connected into each side of the T-union and these were pushed into one end of a 30mm long (¼″ id) section of FEP tubing. Each of these short sections had a hole pierced through the wall and a rolled-up, 20 mm diameter Grade 1 filter paper was inserted into it. The other end of each of the short sections of tubing was connected to a longer section of FEP tubing (40 cm long, ¼″ od) and these were connected to the arms of the Y-tube olfactometer with a brass reducing union (½″ to ¼″). Glass wool inserted into the Swagelok connector end of each Y-tube arm prevented flies from escaping. All tubing joints and connections were sealed with PTFE tape. All tubing and glassware was cleaned using the method described for odour entrainment.
To carry out the behavioural experiments the olfactometer was placed horizontally on a solid, vibration-dampened bench. Before each replicate, late stage infection extract (1 µl) was introduced into one arm of the Y-tube apparatus via the hole in the 30 mm long section of FEP tubing; the hole was then sealed with PTFE® tape and before infection extract (1 µl) was introduced into the other arm. A sand fly was then removed from the holding cage and carefully transferred (Fig. 2a) into the open end of the Y-tube stem.
As soon as the sand fly had entered the Y-tube a timer was started and its final location within the Y-tube olfactometer at the end of a 3 min observation period was recorded; either in the test or control arm or, if it remained in the stem, a “no choice” was recorded.
After every 10 replicates, the rolled-up filter paper was removed from the apparatus and replaced and the whole apparatus, from the sections containing the rolled-up filter paper tubing through to and including the Y-tube, was rotated (horizontally) through 180°, so that left and right sides were exchanged. This was done to eliminate any room positional bias. Eighty female sand flies (replicates) were used for each complete experiment.
For the control experiments, one arm of the Y-tube olfactometer was used for odours entrained immediately after the sham inoculation and the other arm was used for odours entrained 99 days after the sham inoculation. Similarly, for non-inoculated hamsters, odours were collected when the hamster was 4 weeks old and then again at 99 days old.
The response of 80 female sand flies to the before infection and late stage infection entrainment samples obtained from each hamster was determined. As the experiments were conducted over 2 days (40 female replicates on each day) the results of each day’s bioassay were tested using a homogeneity test to determine if they could be combined for further analysis.
Control olfactometer bioassays
A series of control experiments i.e. air vs. air, hexane vs. hexane and hexane vs. air were also carried out to check for any bias in the apparatus.
To determine if responses to entrained odour samples were specific to female sand flies alone, the Y-tube olfactometer bioassays were also carried out with female, Anopheles gambiae (5 day-old, un-mated, not blood-fed) and male Lu. longipalpis sand flies. Odour sample used for these control experiments was from hamster 12 that had previously shown enhanced attraction to female sand flies in the Y-tube bioassay. The response of 160 individual female mosquitoes or male sand flies was determined as previously described for female Lu. longipalpis.
Statistical analysis
The data were analysed using both classical statistical methods; chi-squared, 1-proportion and 2-proportions tests and a Bayesian test of proportions in order to obtain probabilistic results and credible intervals from the comparison of the before infection with late stage infection results33, 34. Chi-square tests with exact P values were conducted on the individual olfactometer experiments repeated on different days to test for homogeneity before they were combined and subsequently analysed using a 1-proportion exact test to determine if there was a difference in response to the two odour sources11. Comparison of the change in proportion of the group of mice that were attractive before and after infection used a Fisher’s exact test of 2-proportions, the null hypothesis was that the proportion that were attractive before infection was the same as the proportion that would be attractive at late stage infection. Statistical analyses were carried out using either SPSS v14 or Minitab v17.
The exact test for proportions is a conservative statistical test as it does not take account of exogenous variability in the female sand fly frequency of response and provides only a P-value. Therefore, a Bayesian test of proportions was also employed. This estimated the relative frequency of sand flies in the test arm (infected odour) (θ) for the 13 infected and 6 control hamsters. The Bayesian model assumed that sand fly movement to one side or the other of the Y-tube is binomially distributed with parameters (θ, n) where θ itself is Beta distributed with parameters (1, 1), which is equivalent to a uniform distribution between 0 and 1 (non-informative prior). The posterior distribution is a Beta distribution with parameters θ + 1 and n − θ + 1 where n is the number of trials (i.e. the sum of the number of sand flies responding to the control and test side of the olfactometer). By employing this method, we were able to provide: the estimated relative frequency of sand flies going to the arm of the Y-tube olfactometer with late stage infection odour and its credible interval; the probability that more than 50% and 75% of sand flies moved to the late stage infection odour arm; the differences in sand fly attraction between hamsters; and the probability that infected hamsters were more attractive to sand flies than control hamsters. The Bayesian statistical analysis was carried out with the BayesianFirstAid package35 within the R statistical software program (R Development Core Team. 2016).
Electronic supplementary material
Acknowledgements
The authors wish to thank Dr Pam Taylor and Dr Shalindra Ranasinghe for their help in maintaining the sand fly colony. Funding was from The Wellcome Trust.
Author Contributions
T.N., R.D.W. and J.G.C.H. designed the research study; T.N. carried out the experimental work. J.G.C.H. and L.S. carried out the statistical analysis. J.G.C.H., L.S. and T.N. drafted the manuscript and read, revised and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
R. D. Ward is deceased.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06313-w
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Conn JE, Mirabello L. The biogeography and population genetics of neotropical vector species. Heredity. 2007;99:245–256. doi: 10.1038/sj.hdy.6801002. [DOI] [PubMed] [Google Scholar]
- 2.Lehane, M. J. The Biology of Blood-Sucking in Insects. 2nd edn, (Cambridge University Press, 2005).
- 3.Hamilton, J. G. C. & Hurd, H. In The Behavioual Ecology of Parasites (ed Lewis, E.E., Campbell, J. F., Sukhdeo, M. V. K.) Ch. 13, 384 (CAB International, 2002).
- 4.Hamilton JGC, Ramsoondar TMC. Attraction of Lutzomyia longipalpis to human skin odours. Medical and Veterinary Entomology. 1994;8:375–380. doi: 10.1111/j.1365-2915.1994.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 5.Jensen KMV, et al. Variation in the load of the horn fly, Haematobia irritans, in cattle herds is determined by the presence or absence of individual heifers. Medical and Veterinary Entomology. 2004;18:275–280. doi: 10.1111/j.0269-283X.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 6.Logan JG, et al. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. Journal of Chemical Ecology. 2008;34:308–322. doi: 10.1007/s10886-008-9436-0. [DOI] [PubMed] [Google Scholar]
- 7.Deane, L., Brazil & Serviço Nacional de Educação, S. Leishmaniose visceral no Brasil; estudos sôbre reservatórios e transmissores realizados no Estado do Ceará. (Serviço Nacional de Educação Sanitária, 1956).
- 8.Dougherty MJ, Guerin PM, Ward RD, Hamilton JGC. Behavioural and electrophysiological responses of the phlebotomine sandfly Lutzomyia longipalpis (Diptera: Psychodidae) when exposed to canid host odour kairomones. Physiological Entomology. 1999;24:251–262. doi: 10.1046/j.1365-3032.1999.00139.x. [DOI] [Google Scholar]
- 9.Rebollar-Tellez EA, Hamilton JGC, Ward RD. Response of female Lutzomyia longipalpis to host odour kairomones from human skin. Physiological Entomology. 1999;24:220–226. doi: 10.1046/j.1365-3032.1999.00133.x. [DOI] [Google Scholar]
- 10.Nigam Y, Ward RD. The effect of male sandfly pheromone and host factors as attractants for female Lutzomyia longipalpis (Diptera: Psychodidae) Physiological Entomology. 1991;16:305–312. doi: 10.1111/j.1365-3032.1991.tb00569.x. [DOI] [Google Scholar]
- 11.Bray DP, Hamilton JGC. Host odor synergizes attraction of virgin female Lutzomyia longipalpis (Diptera: Psychodidae) Journal of Medical Entomology. 2007;44:779–787. doi: 10.1093/jmedent/44.5.779. [DOI] [PubMed] [Google Scholar]
- 12.Poulin R. “Adaptive” changes in the behaviour of parasitized animals: A critical review. International Journal for Parasitology. 1995;25:1371–1383. doi: 10.1016/0020-7519(95)00100-X. [DOI] [PubMed] [Google Scholar]
- 13.Rogers, M. E., Chance, M. L. & Bates, P. A. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology124, doi:10.1017/s0031182002001439 (2002). [DOI] [PubMed]
- 14.Smallegange RC, et al. Malaria infected mosquitoes express enhanced attraction to human odor. PLoS ONE. 2013;8:e63602. doi: 10.1371/journal.pone.0063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cator LJ, Lynch PA, Thomas MB, Read AF. Alterations in mosquito behaviour by malaria parasites: potential impact on force of infection. Malaria Journal. 2014;13:164. doi: 10.1186/1475-2875-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penn D, Potts WK. Chemical signals and parasite mediated sexual selection. Trends in Ecology and Evolution. 1998;13:5. doi: 10.1016/S0169-5347(98)01473-6. [DOI] [PubMed] [Google Scholar]
- 17.Smith D, et al. The selected ion flow tube method for workplace analyses of trace gases in air and breath: its scope, validation, and applications. Applied Occupational and Environmental Hygiene. 1998;13:817–823. doi: 10.1080/1047322X.1998.10389161. [DOI] [Google Scholar]
- 18.Braks MAH, Scholte EJ, Takken W, Dekker T. Microbial growth enhances the attractiveness of human sweat for the malaria mosquito, Anopheles gambiae sensu stricto (Diptera: Culicidae) Chemoecology. 2000;10:129–134. doi: 10.1007/PL00001814. [DOI] [Google Scholar]
- 19.O’Shea B, et al. Enhanced sandfly attraction to Leishmania-infected hosts. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:117–118. doi: 10.1016/S0035-9203(02)90273-7. [DOI] [PubMed] [Google Scholar]
- 20.Stein RA. Super-spreaders in infectious diseases. Int J Infect Dis. 2011;15:E510–E513. doi: 10.1016/j.ijid.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan JG, Birkett MA. Semiochemicals for biting fly control: their identification and exploitation. Pest Management Science. 2007;63:647–657. doi: 10.1002/ps.1408. [DOI] [PubMed] [Google Scholar]
- 22.Heth G, Todrank J, Busquet N, Baudoin C. Odour-genes covariance and differential investigation of individual odours in the Mus species complex. Biological Journal of the Linnean Society. 2001;73:213–220. doi: 10.1111/j.1095-8312.2001.tb01358.x. [DOI] [Google Scholar]
- 23.Courtenay O, Carson C, Calvo-Bado L, Garcez LM, Quinnell RJ. Heterogeneities in Leishmania infantum Infection: Using Skin Parasite Burdens to Identify Highly Infectious Dogs. PLoS Neglected Tropical Diseases. 2014;8:e2583. doi: 10.1371/journal.pntd.0002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magalhães-Junior JT, et al. Identification of biomarkers in the hair of dogs: new diagnostic possibilities in the study and control of visceral leishmaniasis. Analytical and Bioanalytical Chemistry. 2014;406:6691–6700. doi: 10.1007/s00216-014-8103-2. [DOI] [PubMed] [Google Scholar]
- 25.Woolhouse MEJ, et al. Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proceedings of the National Academy of Sciences. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkot TR. Non-random host selection by anopheline mosquitoes. Parasitology Today. 1988;4:156–162. doi: 10.1016/0169-4758(88)90151-2. [DOI] [PubMed] [Google Scholar]
- 27.Agrela I, Sanchez E, Gomez B, Feliciangeli MD. Feeding Behavior of Lutzomyia pseudolongipalpis (Diptera: Psychodidae), a Putative Vector of Visceral Leishmaniasis in Venezuela. Journal of Medical Entomology. 2002;39:440–445. doi: 10.1603/0022-2585-39.3.440. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald G. The analysis of equilibrium in malaria. Tropical Disease Bulletin. 1952;49:16. [PubMed] [Google Scholar]
- 29.Garrett-Jones C. The human blood index of Malaria vectors in relation to epidemiological assessment. Bulletin of the World Health Organization. 1964;30:20. [PMC free article] [PubMed] [Google Scholar]
- 30.Melby PC, Chandrasekar B, Zhao W, Coe JE. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. The Journal of Immunology. 2001;166:1912–1920. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
- 31.Modi GB, Tesh RB. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. Journal of Medical Entomology. 1983;20:568–569. doi: 10.1093/jmedent/20.5.568. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton, J. G. C., Ibbotson, H. C., Hooper, A. M. & Pickett, J. A. 9-Methylgermacrene-B is confirmed as the sex pheromone of the sandfly Lutzomyia longipalpis from Lapinha, Brazil, and the absolute stereochemistry defined as S. Chemical Communications, 2335–2336, doi:10.1039/a907910f (1999).
- 33.Nuzzo R. Scientific method: Statistical errors. Nature. 2014;506:150–152. doi: 10.1038/506150a. [DOI] [PubMed] [Google Scholar]
- 34.Wasserstein RL, Lazar NA. The ASA’s statement on P-values: context, process, and purpose. The American Statistician. 2016;70:129–133. doi: 10.1080/00031305.2016.1154108. [DOI] [Google Scholar]
- 35.Bayesian First aid: a package that implements Bayesian alternatives to the classical*. Test functions in R. (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


