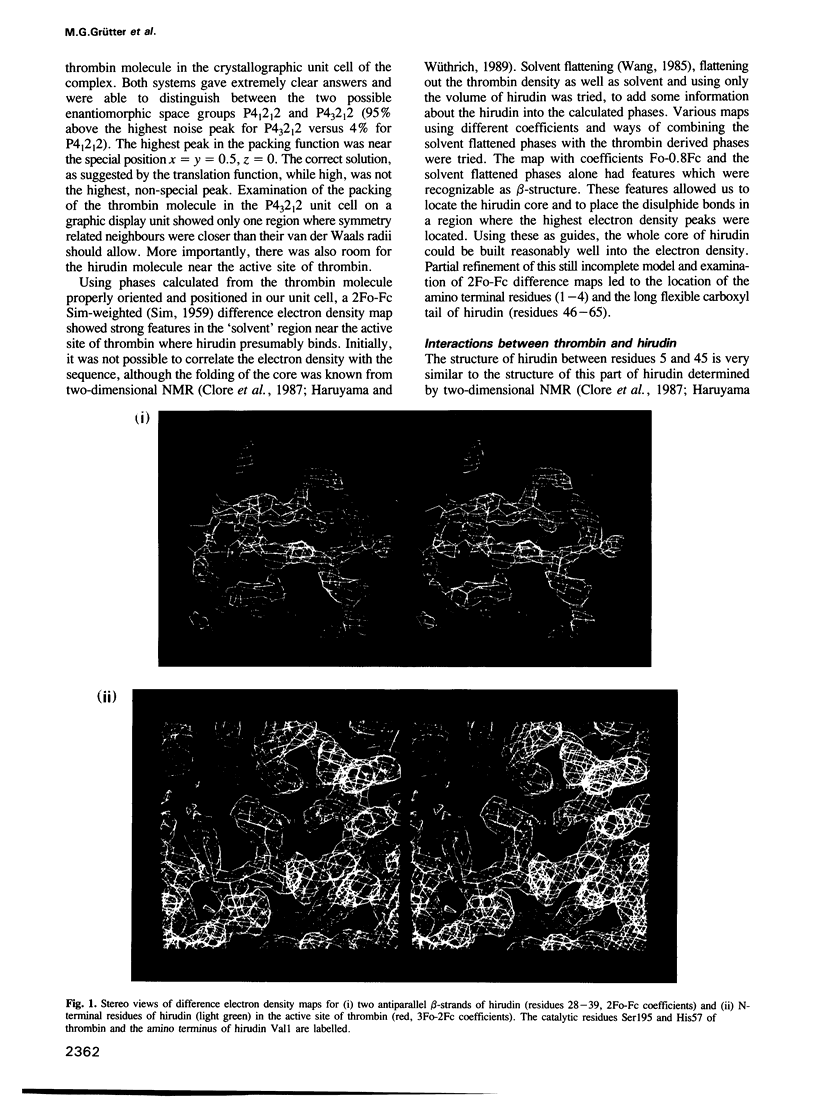
Abstract
Thrombin is a serine protease that plays a central role in blood coagulation. It is inhibited by hirudin, a polypeptide of 65 amino acids, through the formation of a tight, noncovalent complex. Tetragonal crystals of the complex formed between human alpha-thrombin and recombinant hirudin (variant 1) have been grown and the crystal structure of this complex has been determined to a resolution of 2.95 A. This structure shows that hirudin inhibits thrombin by a previously unobserved mechanism. In contrast to other inhibitors of serine proteases, the specificity of hirudin is not due to interaction with the primary specificity pocket of thrombin, but rather through binding at sites both close to and distant from the active site. The carboxyl tail of hirudin (residues 48-65) wraps around thrombin along the putative fibrinogen secondary binding site. This long groove extends from the active site cleft and is flanked by the thrombin loops 35-39 and 70-80. Hirudin makes a number of ionic and hydrophobic interactions with thrombin in this area. Furthermore hirudin binds with its N-terminal three residues Val, Val, Tyr to the thrombin active site cleft. Val1 occupies the position P2 and Tyr3 approximately the position P3 of the synthetic inhibitor D-Phe-Pro-ArgCH2Cl. Thus the hirudin polypeptide chain runs in a direction opposite to that expected for fibrinogen and that observed for the substrate-like inhibitor D-Phe-Pro-ArgCH2Cl.
Full text
PDF
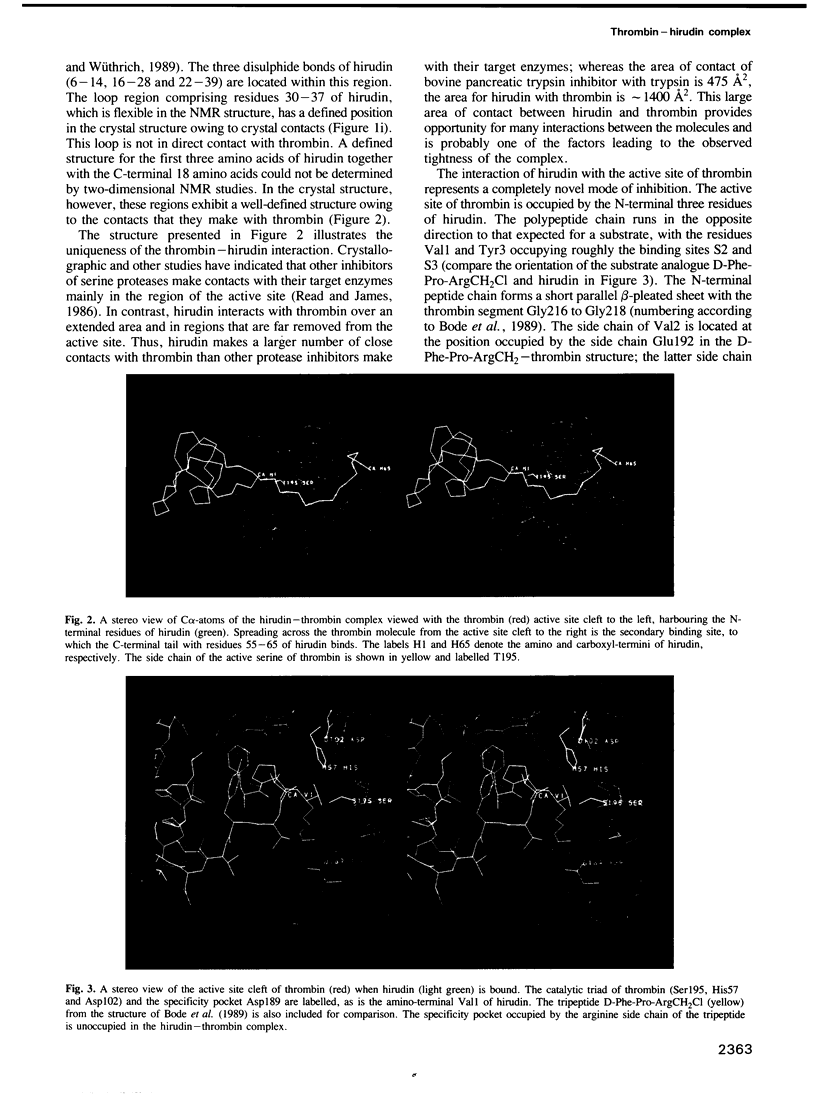
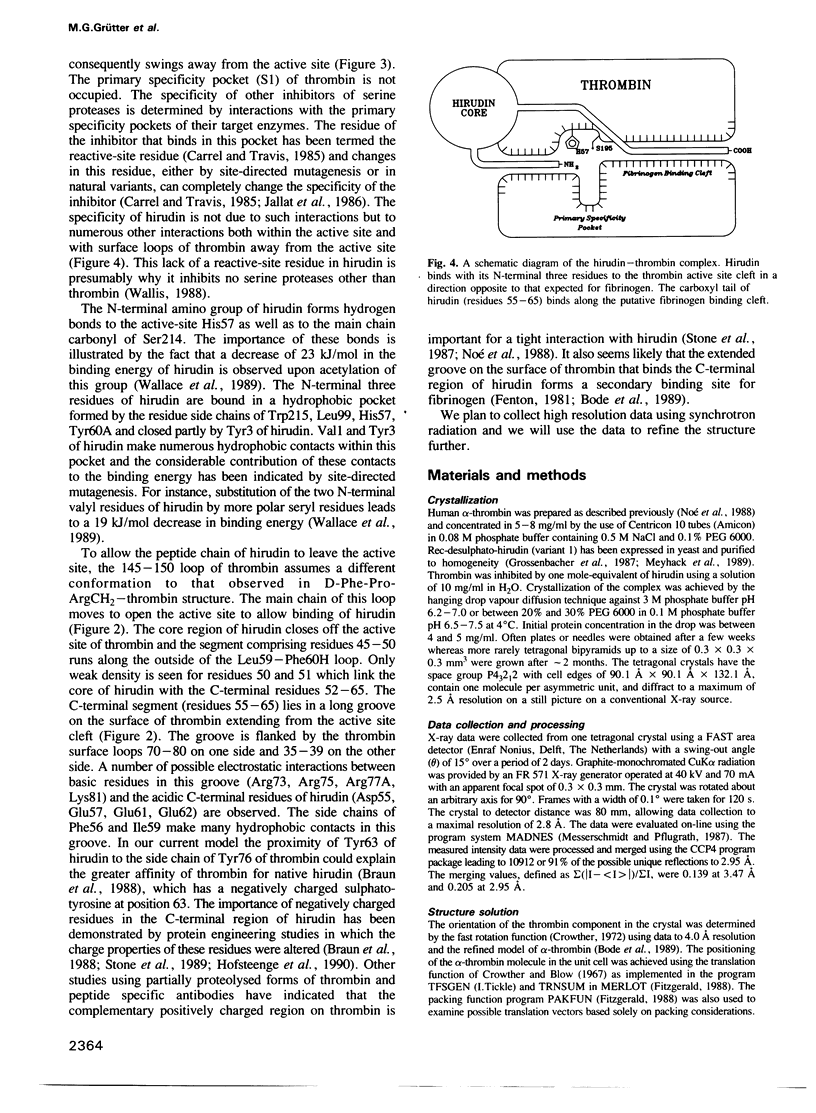

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdy D., Barabas E., Gráf L., Petersen T. E., Magnusson S. Hirudin. Methods Enzymol. 1976;45:669–678. doi: 10.1016/s0076-6879(76)45057-7. [DOI] [PubMed] [Google Scholar]
- Bode W., Mayr I., Baumann U., Huber R., Stone S. R., Hofsteenge J. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun P. J., Dennis S., Hofsteenge J., Stone S. R. Use of site-directed mutagenesis to investigate the basis for the specificity of hirudin. Biochemistry. 1988 Aug 23;27(17):6517–6522. doi: 10.1021/bi00417a048. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. The hirudin-binding site of human alpha-thrombin. Identification of lysyl residues which participate in the combining site of hirudin-thrombin complex. J Biol Chem. 1989 May 5;264(13):7141–7146. [PubMed] [Google Scholar]
- Clore G. M., Sukumaran D. K., Nilges M., Zarbock J., Gronenborn A. M. The conformations of hirudin in solution: a study using nuclear magnetic resonance, distance geometry and restrained molecular dynamics. EMBO J. 1987 Feb;6(2):529–537. doi: 10.1002/j.1460-2075.1987.tb04785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse E., Acker M., Defreyn G., Bernat A., Maffrand J. P., Roitsch C., Courtney M. Point mutations modifying the thrombin inhibition kinetics and antithrombotic activity in vivo of recombinant hirudin. Protein Eng. 1989 Mar;2(6):459–465. doi: 10.1093/protein/2.6.459. [DOI] [PubMed] [Google Scholar]
- Dodt J., Köhler S., Baici A. Interaction of site specific hirudin variants with alpha-thrombin. FEBS Lett. 1988 Feb 29;229(1):87–90. doi: 10.1016/0014-5793(88)80803-2. [DOI] [PubMed] [Google Scholar]
- Dodt J., Köhler S., Schmitz T., Wilhelm B. Distinct binding sites of Ala48-hirudin1-47 and Ala48-hirudin48-65 on alpha-thrombin. J Biol Chem. 1990 Jan 15;265(2):713–718. [PubMed] [Google Scholar]
- Dodt J., Seemüller U., Maschler R., Fritz H. The complete covalent structure of hirudin. Localization of the disulfide bonds. Biol Chem Hoppe Seyler. 1985 Apr;366(4):379–385. doi: 10.1515/bchm3.1985.366.1.379. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin interactions with hirudin. Semin Thromb Hemost. 1989 Jul;15(3):265–268. doi: 10.1055/s-2007-1002718. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin specificity. Ann N Y Acad Sci. 1981;370:468–495. doi: 10.1111/j.1749-6632.1981.tb29757.x. [DOI] [PubMed] [Google Scholar]
- Folkers P. J., Clore G. M., Driscoll P. C., Dodt J., Köhler S., Gronenborn A. M. Solution structure of recombinant hirudin and the Lys-47----Glu mutant: a nuclear magnetic resonance and hybrid distance geometry-dynamical simulated annealing study. Biochemistry. 1989 Mar 21;28(6):2601–2617. doi: 10.1021/bi00432a038. [DOI] [PubMed] [Google Scholar]
- Haruyama H., Wüthrich K. Conformation of recombinant desulfatohirudin in aqueous solution determined by nuclear magnetic resonance. Biochemistry. 1989 May 16;28(10):4301–4312. doi: 10.1021/bi00436a027. [DOI] [PubMed] [Google Scholar]
- Hofsteenge J., Stone S. R., Donella-Deana A., Pinna L. A. The effect of substituting phosphotyrosine for sulphotyrosine on the activity of hirudin. Eur J Biochem. 1990 Feb 22;188(1):55–59. doi: 10.1111/j.1432-1033.1990.tb15370.x. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Jallat S., Carvallo D., Tessier L. H., Roecklin D., Roitsch C., Ogushi F., Crystal R. G., Courtney M. Altered specificities of genetically engineered alpha 1 antitrypsin variants. Protein Eng. 1986 Oct-Nov;1(1):29–35. doi: 10.1093/protein/1.1.29. [DOI] [PubMed] [Google Scholar]
- Johnson P. H., Sze P., Winant R., Payne P. W., Lazar J. B. Biochemistry and genetic engineering of hirudin. Semin Thromb Hemost. 1989 Jul;15(3):302–315. doi: 10.1055/s-2007-1002723. [DOI] [PubMed] [Google Scholar]
- Noé G., Hofsteenge J., Rovelli G., Stone S. R. The use of sequence-specific antibodies to identify a secondary binding site in thrombin. J Biol Chem. 1988 Aug 25;263(24):11729–11735. [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Braun P. J., Hofsteenge J. Identification of regions of alpha-thrombin involved in its interaction with hirudin. Biochemistry. 1987 Jul 28;26(15):4617–4624. doi: 10.1021/bi00389a004. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Dennis S., Hofsteenge J. Quantitative evaluation of the contribution of ionic interactions to the formation of the thrombin-hirudin complex. Biochemistry. 1989 Aug 22;28(17):6857–6863. doi: 10.1021/bi00443a012. [DOI] [PubMed] [Google Scholar]
- Wallace A., Dennis S., Hofsteenge J., Stone S. R. Contribution of the N-terminal region of hirudin to its interaction with thrombin. Biochemistry. 1989 Dec 26;28(26):10079–10084. doi: 10.1021/bi00452a030. [DOI] [PubMed] [Google Scholar]
- Wallis R. B. Hirudins and the role of thrombin: lessons from leeches. Trends Pharmacol Sci. 1988 Dec;9(12):425–427. doi: 10.1016/0165-6147(88)90124-1. [DOI] [PubMed] [Google Scholar]
- Walsmann P., Markwardt F. Biochemische und pharmakologische Aspekte des Thrombininhibitors Hirudin. Pharmazie. 1981 Oct;36(10):653–660. [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]