Abstract
The emergence of herbicide-resistant weeds is a major threat facing modern agriculture. Over 470 weedy-plant populations have developed resistance to herbicides. Traditional evolutionary mechanisms are not always sufficient to explain the rapidity with which certain weed populations adapt in response to herbicide exposure. Stress-induced epigenetic changes, such as alterations in DNA methylation, are potential additional adaptive mechanisms for herbicide resistance. We performed methylC sequencing of Arabidopsis thaliana leaves that developed after either mock treatment or two different sub-lethal doses of the herbicide glyphosate, the most-used herbicide in the history of agriculture. The herbicide injury resulted in 9,205 differentially methylated regions (DMRs) across the genome. In total, 5,914 of these DMRs were induced in a dose-dependent manner, wherein the methylation levels were positively correlated to the severity of the herbicide injury, suggesting that plants can modulate the magnitude of methylation changes based on the severity of the stress. Of the 3,680 genes associated with glyphosate-induced DMRs, only 7% were also implicated in methylation changes following biotic or salinity stress. These results demonstrate that plants respond to herbicide stress through changes in methylation patterns that are, in general, dose-sensitive and, at least partially, stress-specific.
Keywords: Methylkit, Arabidopsis, Glyphosate, Herbicide resistance, Cytosine DNA methylation, MethylC-seq
Introduction
The development of herbicide-resistant weed populations is a major crisis facing modern agriculture (Bonny, 2016). Herbicide resistance has evolved in populations of at least 470 different weeds, including 35 species that have developed resistance to the herbicide glyphosate (Heap, 2016), the most widely used herbicide in the history of agriculture (Duke & Powles, 2008). Mechanisms of herbicide resistance are easily explained in some cases, as when a mutation occurs in the protein target of the herbicide that reduces herbicide binding (González-Torralva et al., 2012) or even the copy number of herbicide target genes (Gaines et al., 2010). Other cases, termed non-target site resistance, are poorly understood, but have been attributed to quantitative accumulation of minor resistance alleles selected from standing genetic variation at multiple gene loci (Busi, Neve & Powles, 2013; Délye, 2013). Non-target site resistance may involve various mechanisms that affect herbicide metabolism or translocation (González-Torralva et al., 2012). Resistance to glyphosate is interesting in that it often appears to involve non-target site mechanisms, which can emerge and dominate a population after as few as three generations of sub-lethal exposure to the herbicide (Busi & Powles, 2009), and can spread through a population faster than predicted by gene flow or propagule dispersal (Escorial et al., 2011; Espeby, Fogelfors & Milberg, 2011; Okada et al., 2013). Given that herbicides induce a strong abiotic stress, it is likely that weeds respond by activating stress-signaling networks that reprogram gene expression (Busi, Neve & Powles, 2013), and we hypothesize that this involves epigenetic regulation of gene function that could contribute to herbicide resistance as implicated in weed adaptation to other stresses (Verhoeven et al., 2010) and in the response of rice to the pesticide atrazine (Lu et al., 2016).
A prominent mechanism of epigenetic regulation of gene expression is through cytosine methylation. Methylated cytosines (mCs) occur in three different sequence contexts in plants: CG methylation, which is the predominant form of methylation in gene-rich regions, and CHH or CHG (H = A, T, or C) methylation predominantly in transposable elements and repetitive sequences (Cokus et al., 2008). CHH and CHG mCs are much more common in plants than animals (Cokus et al., 2008). Multiple RNA-dependent methylation pathways control de novo methylation in the mustard weed Arabidopsis thaliana (Matzke & Mosher, 2014) with methylation at the different sequence contexts altered and maintained through both overlapping and sequence-context-specific mechanisms. Mutations in the genes of these pathways lead to aberrations in the methylome (Stroud et al., 2013). Additionally, wildtype A. thaliana plants accumulate epimutations (i.e., changes in the methylome) over generations of greenhouse propagation (Becker et al., 2011; Schmitz et al., 2011). Stress exposure can lead to substantial methylome reprogramming as observed in A. thaliana following pathogen attack or salicylic acid treatment (Dowen et al., 2012), phosphate starvation (Secco et al., 2015; Yong-Villalobos et al., 2015), and salinity stress (Jiang et al., 2014; Wibowo et al., 2016). The majority of methylation changes following these stresses were considered transient—not transgenerationally stable—but a subset of stress-responsive methylations is stably fixed in populations over several subsequent generations of exposure to stress (Jiang et al., 2014). Thus, transgenerational epigenetic changes in gene expression could lead to enhanced and adaptive stress tolerance. However, the ability of epigenetic changes to be transgenerationally stable and affect the adaptation of plants in their environment remains contentious (Hagmann et al., 2015; Kawakatsu et al., 2016).
Because the specificity of methylome reprogramming following stress may appear inexact [e.g., compare Secco et al. (2015) to Yong-Villalobos et al. (2015)] and the effect of alterations in methylation levels on gene expression may seem inconsistent (Karan et al., 2012; Kawakatsu et al., 2016; Li et al., 2012), we tested whether there was a dosage effect by glyphosate stress on specific alterations of the plant methylome. Glyphosate has been previously shown to alter DNA methylation in wheat (Nardemir et al., 2015) using relatively low resolution techniques. In addition, the herbicide atrazine was recently shown to induce global changes in methylation in rice (Lu et al., 2016). We used bisulfite sequencing to determine the effect of glyphosate on the Arabidopsis methylome at single base pair resolution to identify specific genetic loci that have altered methylation patterns following glyphosate exposure.
Materials and Methods
Plant growth conditions and herbicide treatment
Seeds of A. thaliana ecotype Columbia were sown in Sunshine Number 1 media and stratified for three days in the dark at 4 °C. The flats were then transferred to a Conviron growth chamber with a 12 h light cycle and light intensity of 90 μmol m−2 s−1 and allowed to grow to a fully developed rosette, pre-floral shoot stage. Blocks of four plants were then randomly assigned treatment of glyphosate at 0%, 5%, 10%, or 15% of the label rate (0.9 kg acid equivalency (ae) ha−1 of RoundUp Pro Concentrate). Glyphosate-treated plants were sprayed at 187 L ha−1 in a spray booth. Following glyphosate treatment, plants were transferred to a growth shelf with a 12 h light cycle and light intensity of 90 μmol m−2 s−1 and grown until fully developed siliques were formed (approximately two weeks for the 0% and 5% glyphosate-treated plants and eight weeks for the 10% glyphosate-treated plants). Thus, tissues were harvested at equivalent developmental stages, despite slower growth rate of glyphosate-treated plants.
Genomic DNA isolation and methylC sequencing library preparation
Genomic DNA was isolated from two to three cauline leaves formed following glyphosate exposure from individual plants in quadruplicate for each of the three treatment levels (0%, 5%, and 10%) using the Biosprint-15 plant DNA extraction kit (Qiagen, Hilden, Germany). The 12 samples were sent to Genomics Research Laboratory at Biocomplexity Institute of Virginia Tech (Blacksburg, VA, USA) for library preparation and bisulfite sequencing. 100 ng of intact DNA was bisulfite converted using EZ DNA Methylation-Gold Kit (#D5005; Zymo Research, Irvine, CA, USA), following the manufacturers protocol, except eluting into 9 ul. The entire amount of the purified bisulfite-treated DNA was converted to Illumina DNA libraries using EpiGnome Methyl-Seq kit (Epicentre, Madison, WI, USA). Six samples each were individually barcoded, quantitated by qPCR, and pooled to sequence on the entire Illumina HiSeq Rapid Run flowcell. Libraries were clustered on-board at a concentration of 8.5 pM with 3% phiX, onto a flow cell using Illumina’s HiSeq Rapid Paired End Cluster Kit V2 (PE-402-4002), and sequenced 2× 101 cycles using HiSeq Rapid SBS Kit (200-cycles) (FC-402-4021).
MethylC sequencing data analysis
The sequencing reads were subject to pre-processing quality control using FastQC to eliminate adapter sequences and barcodes using Trimmomatic (http://www.usadellab.org/cms/) and FastX Tookit (http://hannonlab.cshl.edu/fastx_toolkit/). Low quality reads (quality score Q < 30) were discarded and only reads passing the quality check were mapped to Col-0 A. thaliana (TAIR 10) reference genome using Bismark aligner (v 0.14.5) under default parameters (−n 1 −l 50) (Krueger & Andrews, 2011). Cytosine methylation information was extracted from aligned reads using Bismark methylation extractor and methylation calls for CG, CHH, and CHG contexts were generated. The conversion efficiency of bisulfite treatment (methylation status of cytosine) was estimated from reads mapped to the chloroplast genome, which is expected to be unmethylated.
Calling DMRs
Methylkit/eDMR
Differentially methylated regions (DMRs) between treated and control plants were identified using the R (v3.0.3) package methylkit/eDMR (Li et al., 2013). Methylkit allows parameter adjustment to identify DmCs based on q-value, percent methylation difference, and types of methylation (hyper or hypo) using statistical tests such as logistic regression and Fisher’s exact test. Pairwise Pearson’s correlation coefficient and Hierarchical clustering (Ward’s method, correlation distance metric) were calculated based on percent methylation values for all 12 samples. Differential methylation between treated (5% and 10%) and control groups were determined using Fisher’s exact test with a minimum 25% difference in methylation ratio between groups and q-value <0.01. DMRs were constructed using weighted optimization algorithm eDMR.
bsseq
Differential methylation between glyphosate-treated and control libraries was also determined in R using bsseq package (bioconductor). The coverage (.cov) files generated by Bismark were used to run methylation smoothing which generates per-CG, CHH, and CHG methylation values based on at least one biological replicate for control and treated samples using mean t-statistics. DMRs were filtered by areaStat which was weighted by the number of methylation sites for each context (Hansen, Langmead & Irizarry, 2012). We identified the overlapping DMR regions for each context between percent of glyphosate exposure using the intersectBed function within the bedtools suite with default parameters (Quinlan & Hall, 2010). The stringency of the parameters to use when calling DMRs was optimized through analyzing the effect of increasing the number of analyzed replicates on the number of identified DMRs by calling DMRs using all possible combinations of two replicates, three replicates, and four replicates from our methylC-seq data. For the less stringent parameters (see Fig. S1 legend) relying on two replicates likely leads to many false positives because of the sharp decline in the number of DMRs when increasing the number of replicates (Fig. S1). When using the more stringent parameters, the overall number of DMR calls is lower and increasing replicates has no effect on raw number of called DMRs suggesting fewer false positives. We, therefore, used the DMRs called using the more stringent parameters for downstream analyses.
Gene ontology enrichment analysis
Annotations from the TAIR10 database were assigned to DMRs using a custom Perl script (script provided in supplementary materials) in which genomic features were associated with DMRs that overlapped within 2Kb in either direction. Arabidopsis thaliana ID lists from associated DMRs were processed and analyzed in VirtualPlant 1.3 software (http://virtualplant.bio.nyu.edu/cgi-bin/vpweb/) using the BioMaps module (p ≤ 0.05) to find significantly over-represented gene ontology (GO) categories. To select significant functional categories, we set a cutoff point in a normalized frequency (relative frequency of input gene list/relative frequency of reference) that was greater than 1.5-fold.
Classification of dose-dependent response of DMRs identified in both 5% and 10% datasets
Statistical hypothesis tests using the Student’s t-test with 5% significance levels were conducted to determine if the mean difference in 5% glyphosate vs. control group (Δ5) is significantly different from the mean difference in 10% glyphosate vs. control group (Δ10) for each DMR. We then fitted one nonlinear curve for sites in which Δ5 is significantly greater than Δ10 and all DMRs below the fitted blue curve in hypermethylation case and above the fitted blue curve in hypomethylation case were classified as inverse dose-dependent (see Fig. S2). Another nonlinear curve for sites in which Δ5 is significantly less than Δ10 was then fitted. All DMRs above the fitted green curve in hypermethylation case and below the fitted green curve in hypomethylation case were classified as positive dose-dependent. Nonlinear curves are appropriate for fitting the data based on both Akaike information criterion (AIC) and Bayesian information criterion (BIC). The nonlinear curve fitting AIC and BIC was smaller compared to linear fitting.
Statistical comparison of overlapping DMR-associated genes across different stresses (glyphosate, phosphate starvation, and biotic)
The number of DMR-associated genes following glyphosate treatment, phosphate starvation and biotic stress are 3,680, 712, and 884, respectively (Dataset S7). Only 13 genes were identified as being differentially methylated following all these stresses (Dataset S7). We considered the possibility that the lack of shared DMR-associated genes among all three stresses is evidence for DMRs being randomly distributed across the genome as opposed to selective in response to stress. The total number of genes in the TAIR10 annotation of the A. thaliana genome is 33,602. We included the total number of A. thaliana genes as the baseline rather than previously published lists of methylated A. thaliana genes because a majority of our genic DMRs (73%) were identified in genes previously characterized as unmethlyated. The glyphosate-induced DMR-associated genes account for 11% of all possible genes. If these 3,680 genes are randomly distributed across the genome, and the 884 biotic stress DMR-associated genes are likewise random, then the expected number of overlapping genes can be calculated by 0.11 × 884 = 97. Using the same formula for the phosphate starvation DMR-associated genes, the expected number of overlaps with the glyphosate DMR-associated genes is 78 if the DMRs are randomly distributed. Under the same assumptions, the expected number of DMR-associated genes linked to all three stress responses is 2 [(97/33602) × 712], much smaller than the observed 13 shared genes. In fact, χ2 test of independence of the three stresses is highly significant (χ2 = 179.39, df = 4, p = 1.01e−37), suggesting that the three stresses indeed induce common epigenetic changes in A. thaliana. Moreover, all pairwise comparison tests of independence show statistical significance (glyphosate and phosphate: χ2 = 62.21, df = 1, p = 3.088e−15; glyphosate and biotic: χ2 = 7.557, df = 1, p = 0.005977; phosphate and biotic: χ2 = 100.08, df = 1, p = 1.463e−23). Taken together, the comparison result shows that DMR-associated genes are not randomly distributed in the Arabidopsis genome and DMR-associated genes in response to one stress are not mutually independent of those involved in response to another stress.
Results and Discussion
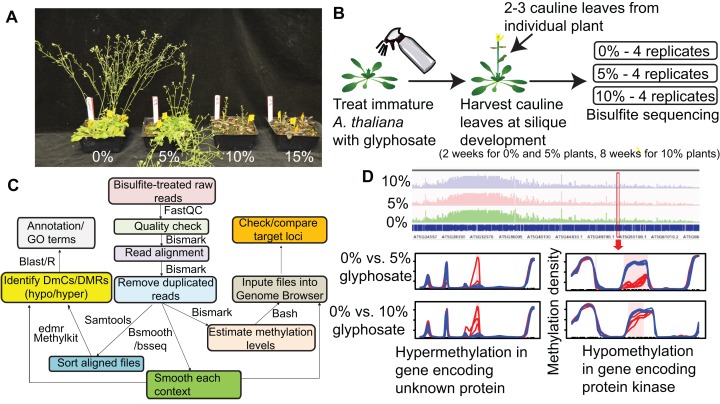
To determine whether glyphosate injury induces changes in plant methylation, we treated A. thaliana with low concentration glyphosate sprays representative of real-world doses a weed on the margin of a treated field could receive. Because a plant must survive herbicide injury and produce viable seed to be a founder of an herbicide resistant population, we first identified the appropriate sub-lethal doses for glyphosate treatment of A. thaliana. Four-week-old A. thaliana rosettes were exposed to 0%, 5%, 10%, or 15% of a typical field rate of 0.9 kg acid equivalency (ae) ha−1 glyphosate, with the 5% and 10% rates causing visible herbicide injury, but allowing for plant survival and reproduction (Fig. 1A). gDNA was collected from newly formed cauline leaves at silique maturation of four individuals from each treatment (Figs. 1B–1D). MethylC-seq of 12 total libraries (four replicates from each treatment) produced a total of 530,042,668 aligned reads resulting in genome coverages from 48× to 76× for each replicate (Table S1).
Figure 1. Overview of experimental pipeline.
(A) Effect of 0%, 5%, 10%, and 15% of a 0.9 kg ae ha−1 field rate of glyphosate on A. thaliana floral shoot development. (B) Schematic of glyphosate treatment and tissue for harvesting gDNA. (C) Pipeline for analyzing methylC-seq data to identify differentially methylated cytosines (DmCs) and differentially methylated regions (DMRs). (D) Example of DMRs identified by bsseq. Blue lines represent methylation levels across the window of individual replicates of control plants and red lines represent methylation levels across the window of individual 5% or 10% glyphosate-treated plants.
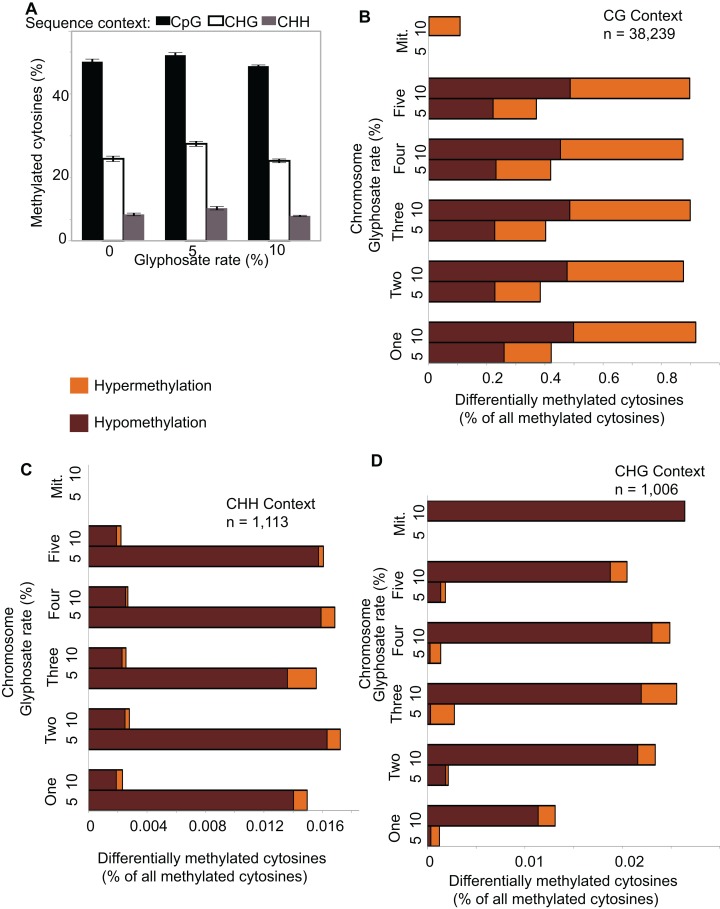
The total amount of methylated cytosines (mCs) ranged from 95 to 143 million for all replicates (Table S1) and were the most abundant in the CG sequence context (Fig. 2A), which is consistent with previous analyses of the A. thaliana methylome (Cokus et al., 2008). Only an average of 0.2% mCs was identified on the chloroplast genome, which is not methylated in A. thaliana (Cokus et al., 2008), demonstrating the fidelity of the methylC-seq protocol and data filtering. mCs were distributed across all five nuclear chromosomes. In addition, a small number of mCs were identified in the mitochondrial genome though these mCs are likely false positives because of the insertion of a region of the mitochondrial genome on chromosome 2 of A. thaliana eco. Columbia (Lin et al., 1999). Neither the abundance of mCs nor the relative frequency of mCs in the three different analyzed sequence contexts (CG, CHG, and CHH) significantly changed due to herbicide injury (Fig. 2A). Clustering the Pearson’s correlation coefficient of the mCs did not result in treatment-specific monophyletic clades, demonstrating the relative similarity of the methylomes across all 12 individuals (Fig. S3). Taken together, these results suggest that glyphosate-induced stress did not lead to changes in overall levels of methylation in any of the three sequence contexts.
Figure 2. Global pattern of methylated cytosines (mCs) and differentially methylated cytosines (DmCs) following methylkit pipeline.
(A) Relative abundance of mCs in the three sequence contexts (CG, CHG, and CHH) following 0, 5%, or 10% of a 0.9 kg ae ha−1 glyphosate treatment to 4-week-old A. thaliana rosettes. N = 4 plants for each treatment. (B–D) Relative abundance of hyper- and hypo-methylated DmCs in the 5% and 10% glyphosate-treated samples compared to the 0% controls across all chromosomes in the CG (B), CHH (C), and CHG (D) sequence contexts. See Dataset S1 for list of all DmCs.
Because glyphosate did not induce global shifts in methylation levels, differences in specific location and context of DNA methylation can be potentially classified as selective responses to herbicide injury. We used Methylkit (Akalin et al., 2012) to compare mCs in the 5% or 10% glyphosate treatments to the control (0% treatment) to identify differentially methylated cytosines (DmCs). This approach enumerated 17,017 DmCs following 5% glyphosate treatment and 23,341 DmCs following 10% glyphosate treatment after normalizing all four replicates and applying a cutoff to only consider the top 25% highest confidence DmCs (Dataset S1). Interestingly, 10% glyphosate treatment led to substantially more DmCs than 5% glyphosate treatment in the two most abundant sequence contexts CG and CHG (Figs. 2B–2D), suggesting that the degree of herbicide injury is correlated with the context and magnitude of methylation changes. However, it is important to note that the 10% glyphosate-treated plants required an additional six weeks to reach silique maturity. Therefore, a subset of the methylation differences could be due to the increased growing time and not the herbicide treatment.
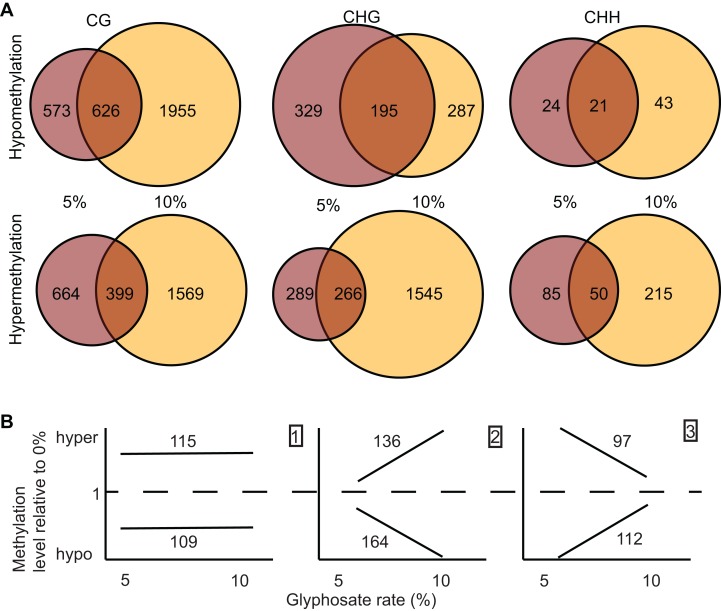
Differentially methylated regions are more strongly associated with regulatory changes in gene expression than DmCs. We called DMRs induced by both 5% and 10% glyphosate treatment using two independent approaches: a bimodal DmC distribution modeling approach using eDMR (Li et al., 2013), and a curve smoothing approach of DmCs using bsseq (Hansen, Langmead & Irizarry, 2012). All DMRs were defined as CG, CHH, or CHG based on the predominance of the sequence context of DmCs comprising the DMR. The eDMR algorithm identified 1,949 hypomethylated and 1,229 hypermethylated non-redundant DMRs following 5% and 10% glyphosate treatments (Dataset S2). More than 95% of the DMRs called by eDMR were defined by the CG sequence context (Fig. S4). Using the bsseq algorithm, 4,053 hypomethylated DMRs and 5,082 hypermethylated DMRs were identified following glyphosate herbicide injury across all three sequence contexts (Dataset S3). We excluded 70 DMRs identified in both the 5% and 10% treatment groups but conflicting in directional change of methylation (i.e., hypo in one treatment and hyper in the other treatment) and therefore unlikely to represent biologically relevant DMRs. In contrast to the eDMR data, only 78% and 51% of hypomethylated and hypermethylated bsseq DMRs, respectively, were categorized as CG (Fig. 3A). The lack of congruence between the bsseq and eDMR datasets suggests that the choice of algorithm remains a primary driver for the specificity of DMRs identified as previously suggested (Yu & Sun, 2016). We consider the 545 DMRs identified by both eDMR and bsseq as our highest confidence DMRs (Dataset S3). We focused on the bsseq DMRs for subsequent analyses because of the relative abundance of non-CG DMRs in this dataset and the observation that non-CG DMRs are more frequent in plants than they are in animals.
Figure 3. Identification and dose-dependency of DMRs.
(A) overlap of DMRs between the 5% and 10% glyphosate treatment groups by sequence context. (B) Number of 5% and 10% overlapping DMRs that exhibit glyphosate dose-independent (panel 1), positive dose-dependent (panel 2), or inverse dose-dependent (panel 3) methylation responses based on non-parametric curve fitting and statistical test for significant difference between the doses at p < 0.05. All DMRs are described in Dataset S3. The list of dosage classification for all overlapping DMRs is available in Dataset S4. Glyphosate percentages based on a 0.9 kg ae ha−1 rate.
We identified 1,964 DMRs (counting both hypo- and hyper-methylated DMRs) unique to the 5% glyphosate-treated samples, and 5,614 DMRs unique to the 10% samples (Fig. 3A), which are consistent with the trend observed for DmCs in which the severity of herbicide injury correlates with the magnitude of methylome shifts. The 5,614 DMRs present only in the 10% glyphosate-treated samples were tentatively classified as positive dose-dependent DMRs (i.e., the magnitude of changes in methylation levels is positively correlated with severity of herbicide injury) and the 1,964 DMRs unique to the 5% glyphosate-treated samples as inverse dose-dependent DMRs (i.e., more severe herbicide injury correlated with decreases in the magnitude of changes in methylation levels). The 1,557 DMRs present in both the 5% and 10% treated samples were further analyzed for dose-dependency resulting in 224, 300, and 209 DMRs classified with 95% confidence as dose-independent, positive dose-dependent, and inverse dose-dependent respectively, across both hypomethylated and hypermethylated DMRs (Fig. 3B; Dataset S4; Fig. S2; see Methods). We, therefore, conclude that for nearly two-thirds (5,914 out of 9,205) of DMRs that are positive dose-dependent, the magnitude of changes in methylation is dependent on the severity of the stress. We propose that these positive dose-dependent DMRs are the best candidates for identifying biologically relevant genomic loci that are modified in response to glyphosate stress. However, it is important to note that the 10% glyphosate-treated plants were harvested several weeks after the 5% glyphosate-treated plants to compensate for the developmental delay induced in the 10% glyphosate-treated plants (see Methods). It is possible that a number of the identified differential DMRs between the two treatment levels are due to maintenance of these DMRs being uncoupled from development and instead linked to the age of the plants.
We tentatively hypothesize for the smaller number of the inverse dose-dependent DMRs that the severity of herbicide injury following 10% glyphosate treatment prevented the plant from responding to the stress through specific alterations in DNA methylation. Interestingly, the CHH context had the most pronounced inverse dose-dependent trend, with more DmCs in the 5% treatment group than the 10% treatment group (Fig. 2), in stark contrast to the CG and CHG contexts. While some molecular mechanisms that control methylation act on all three sequence contexts [e.g., DRM 1/2 (Cao & Jacobsen, 2002b)], other mechanisms have distinct effects across different sequence contexts with the most pronounced differences between asymmetrical and symmetrical Cs (Cao & Jacobsen, 2002a; Matzke & Mosher, 2014). CHH sites are asymmetrical (no C in the antisense strand) in contrast to CG and CHG sites. Additionally, CHH sites were previously identified as responding uniquely to biotic stress in contrast to CG and CHG sites (Dowen et al., 2012). However, it is impossible to conclude that glyphosate herbicide injury alters different molecular methylation mechanisms specifically until the effects of glyphosate on methylation patterns in known methylation mutant plant lines are empirically assessed.
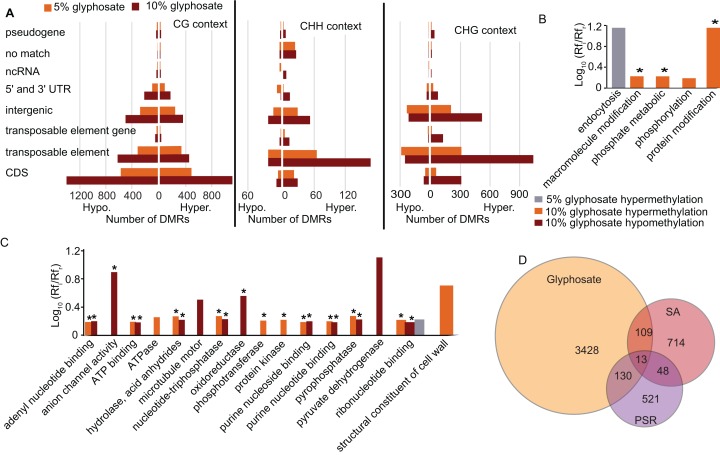
To elucidate which genomic regions are responding to the glyphosate stress through changes in methylation, we annotated all DMRs. A plurality of DMRs were identified in gene coding sequences and in the CG context (Fig. 4A) as expected (Cokus et al., 2008). The DMRs categorized as CHG or CHH were predominantly associated with transposable elements, similar to previous studies in plants (Cokus et al., 2008; Li et al., 2012). In total, 1,818 transposable elements or transposable element (TE) genes were hypermethylated and 936 were hypomethylated (Fig. 4A; Dataset S3). Transposable element-associated DMRs occurred at varying frequency across superfamily groups in response to both the 5% and 10% glyphosate treatments (Fig. S5; Dataset S5). Even though hypermethylated TEs are twice as common as hypomethylated TEs, the 936 hypomethylated TEs may contribute to genome destabilization due to increased transposon mobility associated with reduced transposon body methylation (Chan, Henderson & Jacobsen, 2005; Mirouze & Vitte, 2014). The number of hypomethylated TEs is less than the number of activated TEs in the DNA methylation A. thaliana mutant ddm1 (Zemach et al., 2013), but still greater than the number of TEs activated in A. thaliana following treatment with DNA demethylating agent azacytidine based on expression data (Griffin, Niederhuth & Schmitz, 2016). We, therefore, conclude that glyphosate induces hypomethylation of TEs on a scale comparable to other known treatments capable of destabilizing the A. thaliana genome through TE hypomethylation. The hypomethylated TEs are interesting to explore in terms of their potential for contributing to the phenomenon of gene amplification observed in several glyphosate-resistant weeds (Gaines et al., 2010).
Figure 4. Location of DMRs in the A. thaliana genome and comparison to DMRs induced by other stresses.
(A) Prevalence of DMRs by annotation context in the A. thaliana genome. See Dataset S3 for annotated list of all DMRs. (B and C) Gene ontology (GO) terms for biological process (B) and molecular function (C) enriched (p < 0.05) in DMR associated genes. Rfi/Rfr represents the ratio of the relative frequency of GO terms in the input (glyphosate DMRs) to the reference (TAIR10 Arabidopsis genome) datasets. *indicates p < 0.01. Redundant GO terms excluded from the figure. See Dataset S6 for full list of GO terms with Rfi/Rfr > 1.5. (D) comparison of DMR-associated genes identified following glyphosate stress compared to DMR-associated genes previously identified as induced by biotic stress mimic (Dowen et al., 2012) or phosphate starvation (Yong-Villalobos et al., 2015). Dataset S8 lists all DMR-associated genes categorized by overlap (or lack thereof) among the three analyzed stresses.
An advantage to studying plant DNA methylation changes in response to glyphosate is that the biochemical mechanism of action of this herbicide has been extensively characterized. Glyphosate stops the flow of carbon through the shikimate pathway by inhibiting activity of the enzyme 5-enolpyruvylshikimate 3-phosphate (EPSP) synthase (Amrhein et al., 1980). Two of the seven genes in the shikimate pathway of Arabidopsis (Tzin & Galili, 2010) were differentially methylated in the CG context in response to glyphosate stress: 3-deoxy-d-arabino-heptulosonate-7-phosphate (DAHP) synthase (At4g33510), which encodes the first enzyme (and major regulatory step for the pathway), and both forms of shikimate kinase (SK1, At2g21940 and SK2, At4g39540) were all hypermethylated in the 10% glyphosate-treated plants. Notably, all three of these genes were previously classified as unmethylated in A. thaliana (Niederhuth et al., 2016). EPSP synthase (At2g45300), which is also considered unmethylated in A. thaliana populations (Niederhuth et al., 2016), was not differentially methylated due to glyphosate injury (Dataset S3). Several biological processes and molecular functions were enriched in the GO terms linked to the DMR-associated genes (genes that contain DMRs in their coding sequence) (Figs. 4B and 4C; Dataset S6). The enrichment of genes associated with phosphate (including phosphorylation, phosphotransferase, protein kinase, and pyrophosphatase) was striking. This could be related to glyphosate induction of microRNAs that regulate phosphate transport pathways observed in maize (Żywicki et al., 2015). The glyphosate molecule is recognized by phosphate transporters (Denis & Delrot, 1993) and some cases of glyphosate resistance have been attributed to alterations in herbicide transport and sequestration (Sammons & Gaines, 2014).
As expected, the majority of the enriched GO terms were associated with genic DMRs in the CG context (Fig. S6; Dataset S6), and these included many of the terms related to phosphate. The GO terms enriched in the CHG and CHH contexts were distinct. In the CHG context, glyphosate herbicide injury was associated with alterations in methylation of cell wall-associated and hydrolase genes. Genes associated with signal reception and transduction were specifically enriched in this analysis for genic DMRs in the CHH context.
Formation and maintenance of DMRs is driven by changes in gene expression (Secco et al., 2015), and DMRs are sometimes associated with downstream changes in gene expression (Li et al., 2012) though often have no effect on gene expression (Kawakatsu et al., 2016). We, therefore, cannot conclude whether glyphosate is directly altering the methylation patterns of the genes associated with phosphate metabolism and the shikimate pathway or if glyphosate is altering transcription of associated genes that in turn modify the methylation states of the underlying genomic loci. Previous work showed that glyphosate caused only minor changes in immediate transcriptional activity in A. thaliana eco. Columbia (Das et al., 2010), but induced major effects on the transcriptome of A. thaliana eco. Lansberg erecta (Faus et al., 2015). Future work including paired transcriptome analyses are required to correlate methylation patterns with gene expression.
Next, we asked whether the DMRs induced by glyphosate treatment are indicative of a general stress response or potentially at least partially glyphosate specific. We compared the 3,680 non-redundant DMR-associated genes in either the 5% or 10% treatment group to DMR-associated genes that were previously identified following salicylic acid (SA) treatment, which mimics biotic stress (Dowen et al., 2012), or phosphate starvation stress (Yong-Villalobos et al., 2015). Even considering the use of different approaches and cutoffs for calling DMRs, we hypothesized that the three stresses would induce overlapping methylome alterations as a result of the plants’ general response to environmental stresses and would therefore share some DMR-associated genes. Only 13 genes were identified as being differentially methylated following all three stress treatments (Fig. 4D; Dataset S7). These 13 DMR-associated genes are top candidates for genes that are epigenetically regulated as part of a general stress response. An additional 109 and 130 glyphosate-induced DMRs overlapped with DMRs associated with biotic stress and phosphate starvation stress, respectively. In total, 3,428 (93%) of the glyphosate-induced DMR-associated genes were unique to glyphosate exposure. While the number of overlapping DMR-associated genes among the three analyzed stresses seems small, they represent significantly more than would occur by random selection from all A. thaliana genes (see Methods). Moreover, χ2 tests of independence of DMR-associated genes in three stresses show that stress responses are not independent of one another (χ2 = 179.39, df = 4, p = 1.01e−37), and there is indeed a higher than expected number of genes involved in response to all three stresses, indicating the existence of a common methylome reprogramming pathway in Arabidopsis regardless of the stressors. Nevertheless, the majority of identified DMRs appear specific to glyphosate injury.
Conclusion
This work identifies a large set of genes and other genomic regions epigenetically regulated in response to glyphosate herbicide injury. Determining the extent to which stresses induce patterned versus random epimutations is critical for understanding the role of DNA methylation in plant adaptation to stresses. We favor the hypothesis that the methylome changes are, at least partially, stress-specific over the hypothesis that methylome alterations are universally random because: (1) A majority of the DMRs exhibit dose-sensitive response patterns; (2) all analyzed glyphosate-induced DMRs were identified in four independent biological replicates; and (3) DMRs were enriched in gene pathways known to be affected by glyphosate exposure. Identification of transgenerationally stable DMRs and confirmation of specific DMRs directly correlated with glyphosate resistance will further clarify the role of methylome reprogramming in the evolution of herbicide resistance.
Supplemental Information
Overview of sequencing results, breadth of coverage, and identification of methylated cytosines (mCs) following bisulfite sequencing of gDNA collected from newly formed cauline leaves at silique maturation of four A. thaliana individuals from each treatment where four-week-old rosettes were exposed to 0, 5, or 10% of a typical field rate of 0.9 kg acid equivalency ha−1 glyphosate.
Effect of increasing the number of replicates in limiting the number of DMRs called by the BSmooth/bsseq R package (Bioconductor) using less stringent qcutoff of 0.1 (left panel) and more stringent qcutoff of 0.01 (right panel) parameters. The more stringent parameters and all four replicates were used to identify the DMRs in Dataset S3. Colored lines indicate 5 and 10% of a 0.9 kg acid ha−1 glyphosate rate.
Categorization of overlapping DMRs identified following treatment at 5 and 10% of a 0.9 kg acid ha−1 glyphosate rate based on dose-dependency of methylation response. Using a 95% confidence interval cutoff, overlapping DMRs were classified as either doseindependent (red points), positive dose-dependent (i.e. larger methylation differences in the 10% glyphosate-treated plants than the 5% glyphosate-treated plants–green points), inverse dose-dependent (i.e. larger methylation differences in the 5% glyphosate-treated plants than the 10% glyphosate treated plants–blue points) or insufficient support for categorization (grey points). See Dataset S4 for list of DMRs categorized by this analysis.
Treatments comprised percentages of a 0.9 kg acid equivalency ha−1 glyphosate rate applied to four-week-old A. thaliana rosettes.
Number of DMRs identified using eDMR across the three sequence contexts and separated by hypomethylation vs hypermethylation and whether or not the DMR was identified in the 5% glyphosate-treated samples only, the 10% glyphosate-treated samples only, or in both treatment groups. See Dataset S2 for detailed list of all DMRs identified using eDMR. Glyphosate percentages based on an application rate of 0.9 kg acid equivalency ha−1.
Frequency of DMRs associated with transposable elements superfamilies, identified from the TAIR10 genome release, across all contexts (CG, CHG, CHH). Panels separate hypomethylation vs hypermethylation and colors differentiate the 5% (orange) and 10% (maroon) glyphosate-treated samples. Methylation events are diverse across superfamily, treatment, and context. See Dataset S5 for a detailed list of all transposable element families and superfamilies associated with the identified DMRs. Glyphosate percentages based on an application rate of 0.9 kg acid equivalency ha−1.
Gene ontology (GO) terms for molecular function enriched (p<0.05) in DMR associated genes for each of the three sequence contexts. Rfi/Rfr represents the ratio of the relative frequency of GO terms in the input (glyphosate DMRs) to the reference (TAIR10 Arabidopsis genome) datasets. * indicates p<0.01. See Dataset S6 for full list of GO terms with Rfi/Rfr >1.5 in each of the three sequence contexts.
All differentially methylated positions (DMPs) identified by methylkit.
All differentially methylated regions (DMRs) identified using eDMR algorithm.
All differentially methylated regions (DMRs) identified using bsseq algorithm.
DMRs categorized by dose dependency.
DMR-associated transposable elements.
GO term enrichment in DMR-associated genes.
All DMR-associated genes following glyphosate stress (this work), biotic stress mimic (1), or phosphate starvation (2).
References:
1. Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, et al. Widespread dynamic DNA methylation in response to biotic stress. Proceedings of the National Academy of Sciences. 2012;109(32):E2183–E91. 2. Yong-Villalobos L, González-Morales SI, Wrobel K, Gutiérrez-Alanis D, Cervantes-Peréz SA, Hayano-Kanashiro C, et al. Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proceedings of the National Academy of Sciences. 2015;112(52):E7293–E302.
Annotation of genomic features with DMRs. This custom Perl script identifies differentially methylated regions (DMRs) and provides genomic features annotation with TAIR10 gff file.
Acknowledgments
The authors thank the Genomics Research Laboratory (GRL) at the Biocomplexity Institute of Virginia Tech for excellent MethylC-seq service.
Funding Statement
Seed funds were provided by the Virginia Tech College of Agriculture and Life Science and Department of Plant Pathology, Physiology and Weed Science, and additional support from the National Institute of Food and Agriculture grants nos. 2015-68004-23492 and 2013-67013-21306 (J.B.), 2015-67012-22821 (C.R.C.) and 135997 (J.H.W.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Gunjune Kim performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, and reviewed drafts of the paper.
Christopher R. Clarke performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, and reviewed drafts of the paper.
Hailey Larose analyzed the data and reviewed drafts of the paper.
Hong T. Tran analyzed the data and reviewed drafts of the paper.
David C. Haak analyzed the data and reviewed drafts of the paper.
Liqing Zhang analyzed the data and reviewed drafts of the paper.
Shawn Askew conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, and reviewed drafts of the paper.
Jacob Barney conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, and reviewed drafts of the paper.
James H. Westwood conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, and reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
All sequencing data and employed scripts can be downloaded from NCBI Sequence Read Archive (SRA) BioProject ID: PRJNA322493.
References
- Akalin et al. (2012).Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, Mason CE. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biology. 2012;13(10):R87. doi: 10.1186/gb-2012-13-10-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrhein et al. (1980).Amrhein N, Deus B, Gehrke P, Steinrücken HC. The site of the inhibition of the shikimate pathway by glyphosate: II. Interference of glyphosate with chorismate formation in vivo and in vitro. Plant Physiology. 1980;66(5):830–834. doi: 10.1104/pp.66.5.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker et al. (2011).Becker C, Hagmann J, Muller J, Koenig D, Stegle O, Borgwardt K, Weigel D. Spontaneous epigenetic variation in the Arabidopsis thaliana methylome. Nature. 2011;480(7376):245–249. doi: 10.1038/nature10555. [DOI] [PubMed] [Google Scholar]
- Bonny (2016).Bonny S. Genetically modified herbicide-tolerant crops, weeds, and herbicides: overview and impact. Environmental Management. 2016;57(1):31–48. doi: 10.1007/s00267-015-0589-7. [DOI] [PubMed] [Google Scholar]
- Busi, Neve & Powles (2013).Busi R, Neve P, Powles S. Evolved polygenic herbicide resistance in Lolium rigidum by low-dose herbicide selection within standing genetic variation. Evolutionary Applications. 2013;6(2):231–242. doi: 10.1111/j.1752-4571.2012.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busi & Powles (2009).Busi R, Powles SB. Evolution of glyphosate resistance in a Lolium rigidum population by glyphosate selection at sublethal doses. Heredity. 2009;103(4):318–325. doi: 10.1038/hdy.2009.64. [DOI] [PubMed] [Google Scholar]
- Cao & Jacobsen (2002a).Cao X, Jacobsen SE. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proceedings of the National Academy of Sciences of the United States of America. 2002a;99(4):16491–16498. doi: 10.1073/pnas.162371599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao & Jacobsen (2002b).Cao X, Jacobsen SE. Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Current Biology. 2002b;12(13):1138–1144. doi: 10.1016/s0960-9822(02)00925-9. [DOI] [PubMed] [Google Scholar]
- Chan, Henderson & Jacobsen (2005).Chan SWL, Henderson IR, Jacobsen SE. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nature Reviews Genetics. 2005;6(5):351–360. doi: 10.1038/nrg1601. [DOI] [PubMed] [Google Scholar]
- Cokus et al. (2008).Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452(7184):215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das et al. (2010).Das M, Reichman JR, Haberer G, Welzl G, Aceituno FF, Mader MT, Watrud LS, Pfleeger TG, Gutiérrez RA, Schäffner AR, Olszyk DM. A composite transcriptional signature differentiates responses towards closely related herbicides in Arabidopsis thaliana and Brassica napus. Plant Molecular Biology. 2010;72(4–5):545–556. doi: 10.1007/s11103-009-9590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Délye (2013).Délye C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: a major challenge for weed science in the forthcoming decade. Pest Management Science. 2013;69(2):176–187. doi: 10.1002/ps.3318. [DOI] [PubMed] [Google Scholar]
- Denis & Delrot (1993).Denis M-H, Delrot S. Carrier-mediated uptake of glyphosate in broad bean (Vicia faba) via a phosphate transporter. Physiologia Plantarum. 1993;87(4):569–575. doi: 10.1034/j.1399-3054.1993.870418.x. [DOI] [Google Scholar]
- Dowen et al. (2012).Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, Dixon JE, Ecker JR. Widespread dynamic DNA methylation in response to biotic stress. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(32):E2183–E2191. doi: 10.1073/pnas.1209329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke & Powles (2008).Duke SO, Powles SB. Glyphosate: a once-in-a-century herbicide. Pest Management Science. 2008;64(4):319–325. doi: 10.1002/ps.1518. [DOI] [PubMed] [Google Scholar]
- Escorial et al. (2011).Escorial C, Loureiro I, Rodríguez-García E, Chueca C. Population variability in the response of ripgut brome (Bromus diandrus) to sulfosulfuron and glyphosate herbicides. Weed Science. 2011;59(1):107–112. doi: 10.1614/ws-d-10-00033.1. [DOI] [Google Scholar]
- Espeby, Fogelfors & Milberg (2011).Espeby LÅ, Fogelfors H, Milberg P. Susceptibility variation to new and established herbicides: examples of inter-population sensitivity of grass weeds. Crop Protection. 2011;30(4):429–435. doi: 10.1016/j.cropro.2010.12.022. [DOI] [Google Scholar]
- Faus et al. (2015).Faus I, Zabalza A, Santiago J, Nebauer SG, Royuela M, Serrano R, Gadea J. Protein kinase GCN2 mediates responses to glyphosate in Arabidopsis. BMC Plant Biology. 2015;15(1):14. doi: 10.1186/s12870-014-0378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines et al. (2010).Gaines TA, Zhang W, Wang D, Bukun B, Chisholm ST, Shaner DL, Nissen SJ, Patzoldt WL, Tranel PJ, Culpepper AS, Grey TL, Webster TM, Vencill WK, Sammons RD, Jiang J, Preston C, Leach JE, Westra P. Gene amplification confers glyphosate resistance in Amaranthus palmeri. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(3):1029–1034. doi: 10.1073/pnas.0906649107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Torralva et al. (2012).González-Torralva F, Rojano-Delgado AM, Luque de Castro MD, Mülleder N, De Prado R. Two non-target mechanisms are involved in glyphosate-resistant horseweed (Conyza canadensis L. Cronq.) biotypes. Journal of Plant Physiology. 2012;169(17):1673–1679. doi: 10.1016/j.jplph.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Griffin, Niederhuth & Schmitz (2016).Griffin PT, Niederhuth CE, Schmitz RJ. A comparative analysis of 5-azacytidine and zebularine induced DNA demethylation. G3: Genes|Genomes|Genetics. 2016;6(9):2773–2780. doi: 10.1534/g3.116.030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann et al. (2015).Hagmann J, Becker C, Müller J, Stegle O, Meyer RC, Wang G, Schneeberger K, Fitz J, Altmann T, Bergelson J, Borgwardt K, Weigel D. Century-scale methylome stability in a recently diverged Arabidopsis thaliana Lineage. PLoS Genetics. 2015;11(1):e1004920. doi: 10.1371/journal.pgen.1004920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, Langmead & Irizarry (2012).Hansen KD, Langmead B, Irizarry RA. BSmooth: from whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biology. 2012;13(10):R83. doi: 10.1186/gb-2012-13-10-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heap (2016).Heap I. The international survey of herbicide resistant weeds. 2016. [21 March 2016]. www.weedscience.org www.weedscience.org Online. Internet. Monday.
- Jiang et al. (2014).Jiang C, Mithani A, Belfield EJ, Mott R, Hurst LD, Harberd NP. Environmentally responsive genome-wide accumulation of de novo Arabidopsis thaliana mutations and epimutations. Genome Research. 2014;24(11):1821–1829. doi: 10.1101/gr.177659.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan et al. (2012).Karan R, DeLeon T, Biradar H, Subudhi PK. Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PLoS ONE. 2012;7(6):e40203. doi: 10.1371/journal.pone.0040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakatsu et al. (2016).Kawakatsu T, Stuart T, Valdes M, Breakfield N, Schmitz RJ, Nery JR, Urich MA, Han X, Lister R, Benfey PN, Ecker JR. Unique cell-type-specific patterns of DNA methylation in the root meristem. Nature Plants. 2016;2(5):16058. doi: 10.1038/nplants.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger & Andrews (2011).Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27(11):1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2013).Li S, Garrett-Bakelman FE, Akalin A, Zumbo P, Levine R, To BL, Lewis ID, Brown AL, D’Andrea RJ, Melnick A, Mason CE. An optimized algorithm for detecting and annotating regional differential methylation. BMC Bioinformatics. 2013;14(5):1–9. doi: 10.1186/1471-2105-14-s5-s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2012).Li X, Zhu J, Hu F, Ge S, Ye M, Xiang H, Zhang G, Zheng X, Zhang H, Zhang S, Li Q, Luo R, Yu C, Yu J, Sun J, Zou X, Cao X, Xie X, Wang J, Wang W. Single-base resolution maps of cultivated and wild rice methylomes and regulatory roles of DNA methylation in plant gene expression. BMC Genomics. 2012;13(1):300. doi: 10.1186/1471-2164-13-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (1999).Lin X, Kaul S, Rounsley S, Shea TP, Benito M-I, Town CD, Fujii CY, Mason T, Bowman CL, Barnstead M, Feldblyum TV, Buell CR, Ketchum KA, Lee J, Ronning CM, Koo HL, Moffat KS, Cronin LA, Shen M, Pai G, Van Aken S, Umayam L, Tallon LJ, Gill JE, Adams MD, Carrera AJ, Creasy TH, Goodman HM, Somerville CR, Copenhaver GP, Preuss D, Nierman WC, White O, Eisen JA, Salzberg SL, Fraser CM, Venter JC. Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature. 1999;402:761–768. doi: 10.1038/45471. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2016).Lu YC, Feng SJ, Zhang JJ, Luo F, Zhang S, Yang H. Genome-wide identification of DNA methylation provides insights into the association of gene expression in rice exposed to pesticide atrazine. Scientific Reports. 2016;6(1):18985. doi: 10.1038/srep18985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke & Mosher (2014).Matzke MA, Mosher RA. RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nature Reviews Genetics. 2014;15(8):394–408. doi: 10.1038/nrg3794. [DOI] [PubMed] [Google Scholar]
- Mirouze & Vitte (2014).Mirouze M, Vitte C. Transposable elements, a treasure trove to decipher epigenetic variation: insights from Arabidopsis and crop epigenomes. Journal of Experimental Botany. 2014;65(10):2801–2812. doi: 10.1093/jxb/eru120. [DOI] [PubMed] [Google Scholar]
- Nardemir et al. (2015).Nardemir G, Agar G, Arslan E, Aygun Erturk F. Determination of genetic and epigenetic effects of glyphosate on Triticum aestivum with RAPD and CRED-RA techniques. Theoretical and Experimental Plant Physiology. 2015;27(2):131–139. doi: 10.1007/s40626-015-0039-1. [DOI] [Google Scholar]
- Niederhuth et al. (2016).Niederhuth CE, Bewick AJ, Ji L, Alabady MS, Kim KD, Li Q, Rohr NA, Rambani A, Burke JM, Udall JA, Egesi C, Schmutz J, Grimwood J, Jackson SA, Springer NM, Schmitz RJ. Widespread natural variation of DNA methylation within angiosperms. Genome Biology. 2016;17(1):194. doi: 10.1186/s13059-016-1059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada et al. (2013).Okada M, Hanson BD, Hembree KJ, Peng Y, Shrestha A, Stewart CN, Wright SD, Jasieniuk M. Evolution and spread of glyphosate resistance in Conyza canadensis in California. Evolutionary Applications. 2013;6:761–777. doi: 10.1111/eva.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan & Hall (2010).Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons & Gaines (2014).Sammons RD, Gaines TA. Glyphosate resistance: state of knowledge. Pest Management Science. 2014;70(9):1367–1377. doi: 10.1002/ps.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz et al. (2011).Schmitz RJ, Schultz MD, Lewsey MG, O’Malley RC, Urich MA, Libiger O, Schork NJ, Ecker JR. Transgenerational epigenetic instability is a source of novel methylation variants. Science. 2011;334(6054):369–373. doi: 10.1126/science.1212959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secco et al. (2015).Secco D, Wang C, Shou H, Schultz MD, Chiarenza S, Nussaume L, Ecker JR, Whelan J, Lister R. Stress induced gene expression drives transient DNA methylation changes at adjacent repetitive elements. eLife. 2015;4:e09343. doi: 10.7554/eLife.09343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud et al. (2013).Stroud H, Greenberg Maxim VC, Feng S, Bernatavichute Yana V, Jacobsen Steven E. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis Methylome. Cell. 2013;152(1–2):352–364. doi: 10.1016/j.cell.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzin & Galili (2010).Tzin V, Galili G. The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. The Arabidopsis Book. 2010;8:e0132. doi: 10.1199/tab.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven et al. (2010).Verhoeven KJF, Jansen JJ, van Dijk PJ, Biere A. Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytologist. 2010;185(4):1108–1118. doi: 10.1111/j.1469-8137.2009.03121.x. [DOI] [PubMed] [Google Scholar]
- Wibowo et al. (2016).Wibowo A, Becker C, Marconi G, Durr J, Price J, Hagmann J, Papareddy R, Putra H, Kageyama J, Becker J, Weigel D, Gutierrez-Marcos J. Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. eLife. 2016;5:e13546. doi: 10.7554/elife.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong-Villalobos et al. (2015).Yong-Villalobos L, González-Morales SI, Wrobel K, Gutiérrez-Alanis D, Cervantes-Peréz SA, Hayano-Kanashiro C, Oropeza-Aburto A, Cruz-Ramírez A, Martínez O, Herrera-Estrella L. Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(52):E7293–E7302. doi: 10.1073/pnas.1522301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu & Sun (2016).Yu X, Sun S. Comparing five statistical methods of differential methylation identification using bisulfite sequencing data. Statistical Applications in Genetics and Molecular Biology. 2016;15(2):173–191. doi: 10.1515/sagmb-2015-0078. [DOI] [PubMed] [Google Scholar]
- Zemach et al. (2013).Zemach A, Kim MY, Hsieh P-H, Coleman-Derr D, Eshed-Williams L, Thao K, Harmer Stacey L, Zilberman D. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell. 2013;153(1):193–205. doi: 10.1016/j.cell.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żywicki et al. (2015).Żywicki M, Gracz J, Karłowski W, Twardowski T, Tyczewska A. Expression of miRNAs involved in phosphate homeostasis and senescence is altered in glyphosate-treated maize. Acta Physiologiae Plantarum. 2015;37(12):S160–S161. doi: 10.1016/j.nbt.2016.06.1276. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of sequencing results, breadth of coverage, and identification of methylated cytosines (mCs) following bisulfite sequencing of gDNA collected from newly formed cauline leaves at silique maturation of four A. thaliana individuals from each treatment where four-week-old rosettes were exposed to 0, 5, or 10% of a typical field rate of 0.9 kg acid equivalency ha−1 glyphosate.
Effect of increasing the number of replicates in limiting the number of DMRs called by the BSmooth/bsseq R package (Bioconductor) using less stringent qcutoff of 0.1 (left panel) and more stringent qcutoff of 0.01 (right panel) parameters. The more stringent parameters and all four replicates were used to identify the DMRs in Dataset S3. Colored lines indicate 5 and 10% of a 0.9 kg acid ha−1 glyphosate rate.
Categorization of overlapping DMRs identified following treatment at 5 and 10% of a 0.9 kg acid ha−1 glyphosate rate based on dose-dependency of methylation response. Using a 95% confidence interval cutoff, overlapping DMRs were classified as either doseindependent (red points), positive dose-dependent (i.e. larger methylation differences in the 10% glyphosate-treated plants than the 5% glyphosate-treated plants–green points), inverse dose-dependent (i.e. larger methylation differences in the 5% glyphosate-treated plants than the 10% glyphosate treated plants–blue points) or insufficient support for categorization (grey points). See Dataset S4 for list of DMRs categorized by this analysis.
Treatments comprised percentages of a 0.9 kg acid equivalency ha−1 glyphosate rate applied to four-week-old A. thaliana rosettes.
Number of DMRs identified using eDMR across the three sequence contexts and separated by hypomethylation vs hypermethylation and whether or not the DMR was identified in the 5% glyphosate-treated samples only, the 10% glyphosate-treated samples only, or in both treatment groups. See Dataset S2 for detailed list of all DMRs identified using eDMR. Glyphosate percentages based on an application rate of 0.9 kg acid equivalency ha−1.
Frequency of DMRs associated with transposable elements superfamilies, identified from the TAIR10 genome release, across all contexts (CG, CHG, CHH). Panels separate hypomethylation vs hypermethylation and colors differentiate the 5% (orange) and 10% (maroon) glyphosate-treated samples. Methylation events are diverse across superfamily, treatment, and context. See Dataset S5 for a detailed list of all transposable element families and superfamilies associated with the identified DMRs. Glyphosate percentages based on an application rate of 0.9 kg acid equivalency ha−1.
Gene ontology (GO) terms for molecular function enriched (p<0.05) in DMR associated genes for each of the three sequence contexts. Rfi/Rfr represents the ratio of the relative frequency of GO terms in the input (glyphosate DMRs) to the reference (TAIR10 Arabidopsis genome) datasets. * indicates p<0.01. See Dataset S6 for full list of GO terms with Rfi/Rfr >1.5 in each of the three sequence contexts.
All differentially methylated positions (DMPs) identified by methylkit.
All differentially methylated regions (DMRs) identified using eDMR algorithm.
All differentially methylated regions (DMRs) identified using bsseq algorithm.
DMRs categorized by dose dependency.
DMR-associated transposable elements.
GO term enrichment in DMR-associated genes.
All DMR-associated genes following glyphosate stress (this work), biotic stress mimic (1), or phosphate starvation (2).
References:
1. Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, et al. Widespread dynamic DNA methylation in response to biotic stress. Proceedings of the National Academy of Sciences. 2012;109(32):E2183–E91. 2. Yong-Villalobos L, González-Morales SI, Wrobel K, Gutiérrez-Alanis D, Cervantes-Peréz SA, Hayano-Kanashiro C, et al. Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proceedings of the National Academy of Sciences. 2015;112(52):E7293–E302.
Annotation of genomic features with DMRs. This custom Perl script identifies differentially methylated regions (DMRs) and provides genomic features annotation with TAIR10 gff file.
Data Availability Statement
The following information was supplied regarding data availability:
All sequencing data and employed scripts can be downloaded from NCBI Sequence Read Archive (SRA) BioProject ID: PRJNA322493.