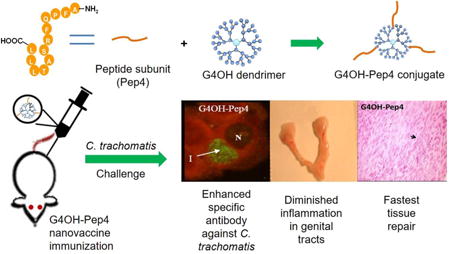
Abstract
Peptide-based vaccines have emerged in recent years as promising candidates in the prevention of infectious diseases. However, there are many challenges to maintaining in vivo peptide stability and enhancement of peptide immunogenicity to generate protective immunity which enhances clearance of infections. Here, a dendrimer-based carrier system is proposed for peptide-based vaccine delivery, and shows its anti-microbial feasibility in a mouse model of Chlamydia trachomatis. Chlamydiae are the most prevalent sexually transmitted bacteria worldwide, and also the causal agent of trachoma, the leading cause of preventable infectious blindness. In spite of the prevalence of this infectious agent and the many previous vaccine-related studies, there is no vaccine commercially available. The carrier system proposed consists of generation 4, hydroxyl-terminated, polyamidoamine (PAMAM) dendrimers (G4OH), to which a peptide mimic of a chlamydial glycolipid antigen—Peptide 4 (Pep4, AFPQFRSATLLL) was conjugated through an ester bond. The ester bond between G4OH and Pep4 is expected to break down mainly in the intracellular environment for antigen presentation. Pep4 conjugated to dendrimer induced Chlamydia-specific serum antibodies after subcutaneous immunizations. Further, this new vaccine formulation significantly protected immunized animals from vaginal challenge with infectious Chlamydia trachomatis, and it reduced infectious loads and tissue (genital tract) damage. Pep4 conjugated to G4OH or only mixed with peptide provided enhanced protection compared to Pep4 and adjuvant (i.e. alum), suggesting a potential adjuvant effect of the PAMAM dendrimer. Combined, these results demonstrate that hydroxyl-terminated PAMAM dendrimer is a promising polymeric nanocarrier platform for the delivery of peptide vaccines and this approach has potential to be expanded to other infectious intracellular bacteria and viruses of public health significance.
Keywords: PAMAM dendrimer, subunit peptide vaccine, Chlamydia trachomatis, nanovaccine, infectious disease
Graphical abstract
1. Introduction
Subunit vaccines are based on synthetic peptides, isolated proteins, polysaccharides, or antigen encoding DNA sequences that elicit specific immune responses.(Gregory et al., 2013) These vaccines are the preferred strategy in the prevention of infectious diseases currently lacking effective vaccines, such as tuberculosis, human immunodeficiency virus (HIV), group A Streptococcus, malaria, hepatitis B, and others.(Heegaard et al., 2010) In addition, vaccines have potential in cancer preventive therapy.(Parmiani et al., 2014) Subunit vaccines can be used as alternatives to attenuated or killed microbes or their toxins in cases where the individual's safety is a concern, such as in the immunocompromised population, the elderly and children.(Gregory et al., 2013) Subunit vaccines can also be employed as an overlap strategy to traditional vaccine formulations that offer low protection levels.(Ivanyi, 2014)
Subunit vaccines may offer new opportunities in the development of a preventive vaccine for Chlamydia infections, a goal that has remained elusive in spite of the significant vaccine development efforts over the past decades.(Yu et al., 2016) Chlamydia trachomatis (C. trachomatis) is an important vaccine target. It is the most prevalent sexually transmitted bacterium worldwide, with the latest statistics showing more than 1.4 million cases annually in the United States alone.(Centers for Disease Control and Prevention) However, there is currently no commercially available vaccine for human chlamydial infection. In an acute infection, C. trachomatis can infect men and women causing urethritis and cervicitis. However, up to 70-75% of infections in women are asymptomatic and, without treatment, might lead to chronic infection and damage to the genital tract.(Hafner et al., 2008) Chlamydia genital infection is directly related to chronic pelvic inflammatory disease (PID), infertility, and adverse pregnancy outcomes, in addition to being a risk factor for other sexually transmitted diseases.(Shaw et al., 2011) Treatment for C. trachomatis infection is currently based on antibiotics, but some studies have shown that C. trachomatis may enter a persistent infectious state before or during treatment, which is also linked to long-term sequelae affecting the reproductive tract as well as joints, in reactive arthritis.(Carter et al., 2010) As for most bacteria, primary chlamydial infection does not prevent reinfection. Protective immunization is, therefore, an essential and widely accepted strategy to control infection by this bacterium.(Yu et al., 2016)
A peptide mimic of the glycolipid exoantigen (GLXA) - a genus-wide chlamydial antigen, has been regarded as a promising antigen candidate.(Stuart and Macdonald, 1989) We previously derived a series of peptides (including Pep4) from a phage display peptide library by selection of peptides bound by an anti-GLXA antibody (mAb1).(Hamzeh-Mivehroud et al., 2013; Petrenko, 2008; Whittum-Hudson and Hudson, 2011) These peptide sequences retain the genus-wide characteristic of GLXA (Stuart and Macdonald, 1989; Vora and Stuart, 2003) and may provide protection against not only the sexually transmitted chlamydial serovars, but against trachoma (eye infection) (Abu El-Asrar et al., 1998) and some human diseases associated with other Chlamydia species such as cardiovascular disease,(Wong and Ward, 1999) chlamydial pneumonia and asthma,(Hahn et al., 1991) some subsets of Alzheimer's disease,(Balin et al., 1998) multiple sclerosis,(Stratton and Sriram, 2003) and reactive arthritis.(Gerard et al., 1998) In addition, the genus-wide nature of Pep4 confers potential for use in veterinary vaccines for cattle, swine and goats, all of which have native chlamydial species that cause spontaneous abortions, infertility and reactive arthritis. Koala bears which are being devastated by chlamydial infections would also benefit from a genus-wide vaccine.(Waugh et al., 2016)
Micro- and nanoparticle vaccine carriers have been proposed to overcome the inherent challenges associated with free peptide-based subunit vaccines.(Dobrovolskaia, 2017; Gregory et al., 2013; Keegan et al., 2003; Moon et al., 2012; Torres-Sangiao et al., 2016) For instance, micro- and nanoparticles as vaccine delivery systems can enhance antigen delivery to the target tissue and cell populations of interest,(Gregory et al., 2013), or act as immunostimulatory adjuvants to activate or augment specific immune responses.(Sahdev et al., 2014) The incorporation of antigenic peptides into the particles are mainly divided into two strategies: encapsulation in carriers and chemical conjugation.(Singh et al., 2007; Zhao et al., 2014) The peptide/protein encapulation in micro- and nanocarriers is a facile and efficient method to prepare nanoscale vaccine formulatoins. Dixit et al. (2014) encapsulated a recombinant peptide of Chlamydia trachomatis major outer membrane protein (MOMP) into a biodegradable poly(lactic acid)–poly (ethylene glycol) (PLA-PEG) nanoparticle. Immunization of mice with encapsulated peptide elicited higher specific T-cell cytokines and potentiated crucial adaptive immunity against Chlamydia.(Dixit et al., 2014) Kelly et al. (2017) designed a vault (ribonucleoprotein) nanoparticle vaccine that loaded recombinant Chlamydia proteins such as the major outer membrane protein (MOMP) or polymorphic membrane protein G-1 (PmpG-1). The vaccines delivered via intranasal delivery induced strong anti-chlamydial immunity at distant genital mucosal sites and significantly attenuated bacterial burden following challenge infection, while avoiding destructive inflammation.(Champion et al., 2009; Jiang et al., 2017) One limitation of these MOMP-loaded nanovaccine formulations is the lack of genus-wide specificity. Whittum-Hudson et al (2001) developed a monoclonal anti-idiotypic antibody (a molecular mimic of GLXA)-encapsualted polylactide microsphere vaccine which demonstrated significant protection against topical vaginal challenge with C. trachomatis after either mucosal (oral or intranasal) or systemic (subcutaneous) immunization.(Whittum-Hudson et al., 2001) However, encapsulated formulations may face the challenge of less spatially/temporally controlled release of peptides/proteins. Chemical conjugation of antigens to nanoparticles is an alterantive strategy to overcome this issue as it enables stronger interaction between nanoparticles and antigenic peptides (i.e. covalent bonding), potentially leading to a stronger protection of peptide from degradation. Additionally, the linkers between the therapeutic molecule and nanoparticles can be designed to allow for the release of the cargo in a controlled fashion, and within specific environments such as the cell cytosol or acidic endolysosomes, thus affording further spatial and temporal control of the release of the therapeutic cargo.(Kurtoglu et al., 2010; Zhong and da Rocha, 2016) However, nanoparticle-conjugated peptide vaccines have not been reported for Chlamydia. Of various nanoparticles, PAMAM dendrimers — a type of synthetic hyperbranched polymer — are of particular of interest as they are highly monodisperse, and their size (ca. 3-10 nm) and molecular weight can be controlled easily.(Esfand and Tomalia, 2001) More importantly, they possess a high density of functionalizable peripheral groups (Svenson and Tomalia, 2005) that can be modified with a variety of ligands,(Gillies and Frechet, 2005) including imaging agents,(Menjoge et al., 2010) targeting moieties,(Kurtoglu et al., 2010) transport modulators,(Heyder et al., 2017; Sadekar and Ghandehari, 2012) and therapeutic molecules.(Mishra et al., 2011; Zhong et al., 2016a) PAMAM dendrimers demonstrates no immunogenicity in in vivo evaluation,(Roberts et al., 1996) although the adjuvancy of cationic PAMAM dendrimers has been reported.(Wright, 1998) NH2-terminated PAMAM dendrimers have been demonstrated as nanocarriers of subunit vaccines in controlled systems using covalent bonds.(Daftarian et al., 2011; Verminnen et al., 2010) Mannose-modified, NH2-terminated PAMAM dendrimers with a measles virus hemagglutinin-derived peptide conjugated were seen to improve cellular uptake by antigen presenting cells (APCs).(Gaertner et al., 2011) Mannose oligomer-modified PAMAM (G4NH2 and G8NH2) also worked as a scaffold against HIV-1 by covalently bonding a carbohydrate-based vaccine candidate.(Kabanova et al., 2010) Sheng et al reported that G4NH2 dendrimers conjugated to ovalbumin (OVA) peptides induced higher T cell responses compared to free peptides both in vitro and in vivo.(Sheng et al., 2008) NH2-terminated dendrimers, however, may have the disadvantage of their limited safety profiles for vaccination.
We report here the use of a dendrimer-conjugated vaccine platform for the prevention of infectious diseases which we tested in a mouse model of C. trachomatis genital infection. We conjugated the immunogenic peptide Pep4 to hydroxyl-terminated, generation 4 PAMAM dendrimer (G4OH; 64 hydroxyl group on surface) nanocarriers and evaluated its effectiveness in a mouse model of Chlamydia genital infection through the reduction of infectious loads and tissue damage in the genital tract after infectious challenge. G4OH was selected in part due to the favorable biocompatibility of OH-terminated dendrimers (discussed in the 2nd paragraph of the Discussion). By conjugation of Pep4 to G4OH through an esterase-sensitive bond (G4OH-Pep4), we expected that the peptide would be protected from degradation and would provide enhanced sustained release of the peptide(s) at its intracellular target – phagolysosomes, facilitating MHC-II –antigen complex formation (Turley et al., 2000) to thus improve its efficacy compared to the free peptide. To our knowledge, this study is the first use of PAMAM dendrimer conjugates to modulate delivery of a peptide vaccine candidate in an animal model of infectious disease. It is also the first use of OH-terminated PAMAM conjugate for vaccine delivery. The broad relevance of this study resides in the potential that such a PAMAM dendrimer-peptide conjugate can be used as a vaccine platform to elicit immunity to numerous infectious agents or tumors.
2. Materials and methods
2.1. Materials
Generation 4, hydroxyl-terminated PAMAM dendrimer (G4OH) with 64 -OH surface groups was purchased from Dendritech, Inc (Midland, MI, USA). The Pep4 with the sequence AFPQFRSATLLL (MW = 1,363.1) was prepared by RS Synthesis (Louisville, KY, USA). This sequence was obtained by phage display with a monoclonal antibody which is anti-GLXA antibody Ab1 – a mAb produced by a hybridoma cell line deposited in the American Type Culture Collection (ATCC) as accession number HB-11300 (ATCC #HB-11300 (Whittum-Hudson and Hudson, 2011)). Sulfo-N-hydroxysuccinimide (sulfo-NHS) was purchased from ProteoChem, Inc (Cheyenne, WY, USA). Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), 2-(N-morpholino) ethanesulfonic acid (MES), diisopropylethylamine (DIPEA), aluminum hydroxide (Al(OH)3) and 2, 5-dihydroxy benzoic acid (2, 5-DHB) were purchased from Sigma-Aldrich (St Louis, MO, USA). Fluorenylmethyloxycarbonyl-6-amino-hexanoic acid (Fmoc-AHA) and benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) were purchased from VWR International (Radnor, PA, USA). Sodium phosphate (dibasic, anhydrous), sodium phosphate (monobasic, monohydrate), dimethylformamide (DMF, Anhydrous grade), dichloromethane (DCM, ACS grade) and methanol (MeOH, ACS grade) were purchased from EMD Chemicals, Inc (Gibbstown, NJ, USA). Deuterated dimethylsulfoxide (DMSO_d6) was purchased from Cambridge Isotope Laboratories (Andover, MA, USA). Ultrapure deionized water (DI H2O) was obtained from a NANOpure DIamond System (Barnstead, D11911) from Thermo Fisher Scientific (Waltham, MA, USA). Spectra/Por® Float-A-Lyzer® G2 ready-to-use dialysis device (MWCO = 3000-5000Da) was purchased from Spectrum Laboratories, Inc (Rancho Dominguez, CA, USA). Thin layer chromatography (TLC) Silica gel 60 F254 plastic sheet was purchased from Merck KGaA (Darmstadt, Germany). All reagents were of analytical grade and used as received, unless otherwise noted.
2.2. Synthesis and characterization of the Pep4-dendrimer conjugate (G4OH-Pep4)
2.2.1. Synthesis of compound 1
Fmoc-AHA (218.6 mg, 0.619 mmol), PyBOP (389.6 mg, 0.742 mmol) and DIPEA (215.2 μl, 1.238 mmol) were mixed in 10 ml anhydrous DMF and stirred for 30 min (10 min on ice and 20 min at room temperature). G4OH (14374 Da, 220.7 mg, 15.4 μmol) was dissolved in 10 ml anhydrous DMF and then added to the mixture. The reaction was stirred for 24 h at 4°C and another 24 h at room temperature. The product was dialyzed against DMF (ACS grade) until TLC showed no small molecules were present. TLC - eluent: DCM/MeOH = 80/20, v/v; residence factor (Rf): 0.00 (Compound 1 – see Scheme 1), 0.87 (Fmoc-AHA), 0.53 (PyBOP), invisible (DIPEA). The product was subsequently dialyzed against DI H2O overnight. Finally, the purified product (Compound 1) was lyophilized until constant weight and stored in the refrigerator for further use.
Scheme 1.
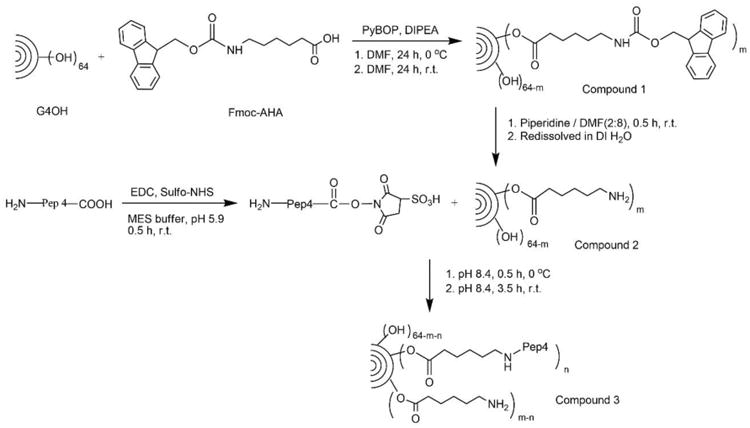
Schematic diagram of the synthesis of the hydroxyl-terminated, generation 4 PAMAM dendrimer (G4OH)-vaccine peptide (Pep4) conjugate (G4OH-Pep4) – compound 3, and intermediates. r.t. = room temperature.
Proton nuclear magnetic resonance spectra (1HNMR) were recorded on a 400 MHz Agilent Mercury spectrometer (Santa Clara, CA, USA) using DMSO_d6. Proton chemical shifts were reported in ppm (δ), and the DMSO_d6 peak at 2.483 ppm was set as the reference peak. Compound 1 (DMSO_d6, ppm): δ 8.089-7.775 (m, 147.72H, NHCO in G4OH and C6H4 in Fmoc), 7.637 (m, 25.92H, C6H4 in Fmoc), 7.418-7.244 (m, 64.89H, C6H4 in Fmoc), 4.371-4.166 (m, 38.88H, CH and CH2 in Fmoc), 3.966 (m, 28.91H, CH2OCO in G4OH), 3.179-3.040 (m, 259.39H, CH2OH in G4OH and CH2 in AHA), 2.623 (m, 257.86H, NCH2 in G4OH and CH2 in AHA), 2.406 ppm (m, 128.47H, CH2N in G4OH), 2.310-2.072 (m, 276.60H, CH2CONH in G4OH and CH2 in AHA), 1.498-1.217 (m, 100.73H, (CH2)3 in AHA).
2.2.2. Removal of Fmoc- protecting group from Compound 1 (Compound 2)
To obtain Compound 2, Compound 1 was first dissolved in 3 ml DMF/piperidine (80/20, v/v) and stirred vigorously for 30 min at room temperature. The organic solvent was completely removed under reduced pressure. The resulting yellowish, oily product was redissolved in 5 ml DMF and then dialyzed against DMF overnight. After removing DMF under reduced pressure, the product was redissolved in DI H2O and then filtered to remove insoluble components. The filtrate was frozen and then lyophilized – Compound 2 – see Scheme 1. 1HNMR of Compound 2 (DMSO_d6, ppm) was developed as described above: δ 8.076-7.789 (m, 126.58H, NHCO in G4OH), 3.980 (m, 11.92H, CH2OCO in G4OH), 3.183-2.970 (m, 245.91H, CH2OH in G4OH and CH2 in AHA), 2.645 (m, 234.54H, NCH2 in G4OH and CH2 in AHA), 2.435 (m, 109.40H, CH2N in G4OH), (2.293-2.094 (m, 259.9H, CH2CONH in G4OH and CH2 in AHA), 1.542-1.277 (m, (CH2)3 in AHA).
2.2.3. Synthesis of Pep4-conjugated G4OH (G4OH-Pep4, Compound 3)
To activate peptide (Pep4), EDC and sulfo-NHS were added to a 5 ml solution of Pep4 (1363.1Da, 16.0 mg, 11.7 μmol) in MES buffer (pH=6.0) with a final concentration of EDC at 4 mM and sulfo-NHS at 10 mM. After the reaction was stirred for 30 min at room temperature, a 3 ml aqueous solution of Compound 2 (15091 Da, 17.7 mg, 1.2 μmol) was added to the EDC-activated Pep4. The pH was immediately raised to 8.4 with 1N NaOH. The reaction was stirred 5 min at 4 °C and another 4 h at room temperature. The resulting product was purified by dialysis (MWCO = 5000Da) against DI H2O for 48 h and lyophilized until constant weight. The resulting white solid - Compound 3 - see Scheme 1 - was stored at 4°C for future use. 1HNMR of Compound 3 (DMSO_d6, ppm): δ 8.046-7.789 (m, 157H, NHCO in G4OH and Pep4), 7.274-7.155 (m, 38.11H, C6H5 in Pep4), 4.711 (m, 40.18H, OH in G4OH), 3.982 (m, 15.98H, CH2OCO in G4OH and CH in Pep4), 3.109-3.066 (m, 224.91H, CH2OH in G4OH and CH2 in AHA), 2.756-2.623 (m, 241.56H, NCH2 in G4OH and CH2 in AHA), 2.408 (m, 98.09H, CH2N in G4OH), 2.309-2.183 (m, 248.00H, in G4OH and CH2 in AHA), 1.508-1.486 (m, 59.54H, (CH2)3 in AHA), - 1.279-1.217 (m, 28.95H, CH2 in AHA and CH3 in Pep4), 1.012 (d, 16.70H, CH3 in Pep4), 0.846-0.793 (m, 40.57H, (CH3)3 in Pep4).
The mass-assisted laser desorption/ionization-time of flight (MALDI-TOF) spectra of G4OH, Compound 2, and Compound 3 was performed on a Bruker Ultraflex spectrometer under positive ion reflector mode. 10 μL of sample (1.0 mg/ml) in DI H2O was mixed with 10 μl methanol containing 2, 5-DHB (10 mg/ml). 2 μl of the sample was spotted on a Bruker Daltonics target plate and dried gently by air flow. The hydrodynamic diameter (HD) and zeta potential (ζ) of G4OH, Pep4, Compounds 2 to 3, and the mixture of G4OH and Pep4 (G4OH+Pep4) were measured using a Malvern Zetasizer NanoCS (Worcestershire, UK). Samples (1.0 mg/ml) were prepared by dissolving the system of interest in phosphate buffer saline (PBS, 10 mM). HD reported in Table 1 represent the average of at least 14 measurements, while ζ is averaged over 100 measurements. Molecular weight s (MW) determined from mass spectra, HD and ζ of the compounds are summarized in Table 1.
Table 1.
Characterization of dendrimer-based compounds.
| Compounds | y | M | MW (Da) | HD ± s.d. (nm) | PDI | ζ ± s.d. (mV) | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1HNMR | MALDI | 1HNMR | MALDI | |||||
| G4OH | - | - | - | - | 14374 | 4.5 ± 1.9 | 0.121 | -0.1±0.4 |
| G4OH-yNH2 | 5.8 | 6.3 | - | - | 15091 | 4.1 ± 1.6 | 0.107 | +13.2±4.9 |
| Pep4 | - | - | - | 1363 | 9.6 ± 4.2 | 0.225 | +17.4±9.0 | |
| G4OH-mPep4 | 2.7* | 2.9** | 3.1 | 3.4 | 19723 | 6.1 ± 2.5 | 0.163 | +15.7±6.5 |
| G4OH+Pep4 | - | - | - | - | - | 5.4 ± 1.9 | 0.159 | +19.5±10.8 |
Note: Number of peripheral -NH2 groups of G4OH (y), number of Pep4 conjugated to G4OH (m), molecular weight (MW), hydrodynamic diameter (HD) and zeta potential of the dendrimers (G4OH and G4OH-yNH2), Pep4, G4OH-mPep4 conjugate and G4OH+Pep4 mixture as determined by 1HNMR, MALDI-TOF, and dynamic light scattering (DLS).
number of NH2 groups remaining after conjugation of Pep4 as determined by 1HNMR;
number of NH2 groups remaining after conjugation of Pep4 as determined by MALDI-TOF. PDI=polydispersity index.
2.3 In vivo studies
2.3.1. Animals and immunization schedule
Female BALB/c mice (5-7 weeks old) were obtained from Charles River Breeders (Wilmington, Massachusetts). Mice were housed and maintained following the NIH guidelines for Handling of Experimental Laboratory animals under an approved Wayne State University IACUC protocol. Four groups of 7–8 unanesthetized mice were immunized three times, at 2-week intervals. Each treatment group received subcutaneous (SC) injections: group i) 100 μg Pep4 dissolved in a mixture of PBS and an equal volume of Al(OH)3 adjuvant (50/50; v/v), for a total volume of 200 μl/mouse; group ii) G4OH-Pep4, 100 μg of Pep4 conjugated to G4OH dissolved in PBS (456 μg G4OH-Pep4 in 200 μl/mouse); group iii) a mixture of G4OH (356 μg) and 100 μg Pep4 in PBS in 200 μl/mouse); and group iv) the negative control mice received only PBS (200 μl). Prior to beginning immunizations, blood was collected from the tail vein of all mice and sera stored at -20◦C for further analysis. A final pre-challenge blood sample was collected 9 days after the third immunization. Seven days before infectious challenge, all mice received subcutaneous (SC) injections of medroxyprogesterone acetate (2.5 mg per mouse; Greenstone® Peapack, NJ, USA). The schematic diagram of the immunization schedule and other details of the animal work which we have published previously (Keegan et al., 2003; Mishra et al., 2011; Whittum-Hudson et al., 1996; Whittum-Hudson et al., 2001) are shown in Scheme 2.
Scheme 2.
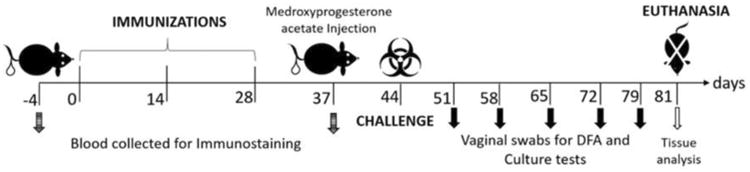
Diagram of immunization and overall in vivo work through the terminal point on day 81.
2.3.2. Preparation of Chlamydia stocks
A human biovar of C. trachomatis (K serovar, Strain UW-31) was used to challenge mice in this study. Stock inocula of chlamydial elementary bodies (EB) were prepared by infecting the human bronchial epithelial cell line HEp-2, using standard methods (33). For immunostaining assays, cells were infected with additional chlamydial species: C. muridarum (a natural mouse pathogen) prepared in McCoy cells, C. pecorum (goat and ovine pathogen) grown in Vero cells or C. abortus (bovine and ovine pathogen) grown in HEp-2 cells, using the same standard methods.
2.3.3. Immunostaining of Chlamydia-infected cells to detect vaccine-induced anti-chlamydial serum antibody
Fixed Chlamydia-infected host cells were incubated with mouse sera to detect the ability of chlamydia-specific Ab induced by immunizations to bind to intracellular chlamydial organisms within membrane-bound chlamydial inclusions. Serum samples tested were obtained prior to exposure to organism (pre-challenge sera). Initially, semi-confluent cells were infected by overlay of EB at a multiplicity of infection equaling 1 EB/cell following standard methods.(Whittum-Hudson et al., 1996) After 48 h infection, cells were harvested with 1X trypsin (Gibco), cytocentrifuged onto microscope slides, fixed with ice-cold absolute methanol for 10 min, and stored at -20°C. Slides remained coded to mask readers to the treatment groups. Our published methods for indirect immunofluorescence staining were followed (Whittum-Hudson and Hudson, 2011; Whittum-Hudson et al., 1996) using sera at 1:40 dilution on replicate slides, followed by 30 min incubation with the secondary Ab (1:100), a FITC-conjugated goat anti-mouse IgG (H+L) (Jackson Immunoresearch Laboratories, West Grove, PA, USA). Slides were mounted with coverslips (#1) using buffered glycerin (Scytek Laboratories, Logan, UT, USA). Cells were viewed at 400× magnification on an epifluorescence microscope (Nikon E600) and images were collected from random fields. Image Pro Plus software version 6.3 (Media Cybernetics, Inc. Rockville, MD, USA) was used to measure pixel intensity of each Chlamydia inclusion detected in the images (green fluorescence). All images were collected under the same camera exposure settings.
2.3.4. Infectious challenge and microbiological evaluation of vaccine-induced clearance of bacteria
Seven days after pre-challenge blood collection and medroxyprogesterone acetate injection, cages were re-coded to mask handlers to prior treatment. Under general anesthesia (Ketamine 100 mg/kg plus Xylazine 8 mg/kg delivered intraperitoneally), mice (5-6 mice/treatment group) were infected by topical vaginal delivery of 1×107 inclusion forming units (IFU) of C. trachomatis in 30 μl of SPG media. Mice were reclined on their backs after challenge to optimize retention of inoculum during the period of anesthesia (ca. 30 min). Infections were confirmed by collection of vaginal swabs at weekly intervals for 5 weeks for microbiological analysis.(Taylor and Velez, 1987; Whittum-Hudson et al., 1996; Whittum-Hudson et al., 1995) Briefly, vaginal swabs containing epithelial cells were spread on microscope slides and cells fixed with cold absolute methanol. Slides were incubated in the dark with 30 μl of FITC-labeled monoclonal anti-chlamydial major outer membrane protein (MOMP) Ab (Bio-Rad Laboratories, Pathfinder™ Chlamydia DFA Kit, #30704). After washes, slides were mounted with coverslips using buffered glycerin. The presence of chlamydial organisms (EB) was graded on a scale of 0 (negative) to 4+ (≥10 EB/400×hpf) using an epifluorescence microscope (Nikon E600).(Taylor and Velez, 1987) In all cases, slides were read in a masked fashion without knowledge of mouse treatment.
For culture confirmation of infectious EB, a second vaginal swab was collected and immersed in ice-cold SPG media. Assay for infectious samples was performed as previously reported.(Whittum-Hudson et al., 1996) After 2 min of vortexing, 100 μl of the sample suspension was added to duplicate flat-bottom microwells containing confluent monolayers of McCoy cells. Plates were centrifuged at 1,203 × g for 1h at 25°C, and incubated for an additional hour at 37°C, after which the mixture was replaced by infection medium as previously described.(Whittum-Hudson et al., 1996) Plates were incubated at 37°C in 5% CO2 for 48h, fixed with absolute methanol for 10 min and stained with mAb specific for the Chlamydia trachomatis LPS within inclusions (Bio-Rad Laboratories, Pathfinder™ Chlamydia Culture Confirmation System #30701).(Whittum-Hudson et al., 1996; Whittum-Hudson et al., 1995) Known positive and negative samples were included in each assay. Inverted plates were read on an epifluorescence microscope at 200× magnification, and mean inclusion counts for duplicate wells calculated for each sample.
2.3.5. Genital tract histopathologic analysis
Thirty-seven days after challenge when the majority of mice had cleared infection, mice were euthanized and genital tracts isolated and photographed ex vivo. Each tissue was scored for inflammatory signs based on tissue color intensity (red/purple, +2, pink/red, +1 and pale pink, 0), dilation intensity of uterine horns (in both; +2; one, +1; or none, 0) and presence of vessels (number and thickness, +2 or +1 and absence of vessels, 0). The excised tissues were photographed and fixed in 10% formalin for 7 days followed by 1 wk in 70% ethanol. Efforts were made to include cervix, right uterine horn, oviducts and ovary. Tissue samples were embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E). Tissues and slides were evaluated in a masked fashion. Pictures (30/genital tract, 150-180/treatment group) were taken from each slide at 400× magnification. The number of inflammatory cells (lymphocytes), fibroblasts, capillary vessels and arterioles were counted to determine any tissue damage or repair process in genital tracts from immunized and challenged animals.
2.4. Statistical Analysis
Immunostaining data, microbiological assays (DFA and culture), genital tract histopathology, and gross pathology data were analyzed by oneway analysis of variance (one-way ANOVA) Dunnett's test using Graphpad Prism 7.0 (San Diego, CA, USA).
3. Results
3.1 Synthesis and characterization of generation 4, hydroxyl-terminated PAMAM dendrimer-Pep4 conjugate (G4OH-Pep4, compound 3)
The G4OH-Pep4 conjugates were synthesized via a two-step reaction shown in Scheme 1: introduction of primary amine into G4OH dendrimer (G4OH-yNH2), and conjugation of activated Pep4 sequence to G4OH-yNH2. The primary amine was introduced by reacting Fmoc-AHA linker with G4OH dendrimer using PyBOP as coupling agent and DIPEA as tertiary base. In the 1HNMR spectrum shown in Fig. 1A, the peak at 3.980 ppm confirmed the formation of ester bond between Fmoc-AHA linker and G4OH.
Fig. 1.
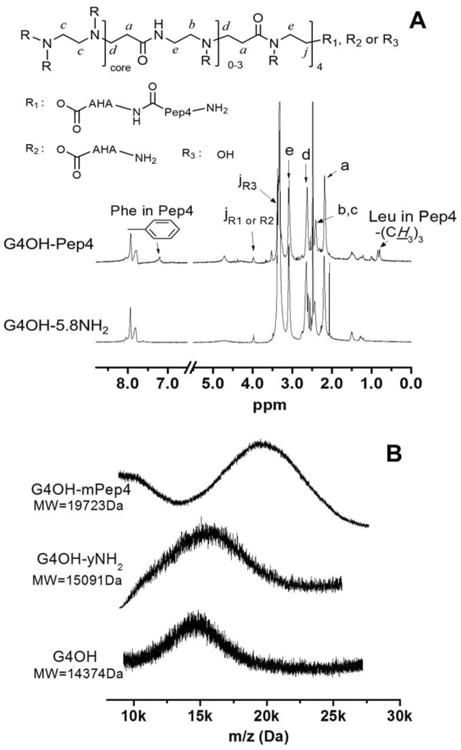
(A) 1HNMR spectra of G4OH-Pep4 conjugate – compound 3. The chemical shifts of all protons are reported in Material and Methods. Inset: chemical structure of the G4OH-Pep4 conjugate. (B) MALDI-TOF spectra of compound 1-3. MW = molecular weight as determined with MALDI-TOF.
Subsequently, the Fmoc protecting group was removed by piperidine/DMF mixture. The disappearance of characteristic peaks of Fmoc group at 7.637, 7.418, 7.294 ppm (aromatic H) and 4.273 ppm (-CH2-) demonstrated the successful modification of G4OH with primary amines. The product was redissolved in DI H2O and the soluble fraction (Compound 2 – G4OH-yNH2) was isolated by centrifugation and lyophilization. We found an average introduction of 5.8 primary amines (yNH2, y = 5.8) to the G4OH dendrimer, by comparing proton integration of methylene (-CH2-) group at 3.980 ppm to methylene proton of G4OH at 2.192 ppm (G4OH-5.8NH2).
Prior to peptide conjugation to G4OH-5.8NH2, Pep4 was activated by EDC and sulfo-NHS in MES buffer (0.1 M, pH 5.9). The active ester of dendrimer based on sulfo-NHS is water soluble and relatively resistant to hydrolysis opposed to that based on NHS.(Anjaneyulu and Staros, 1987) The pH for peptide activation was controlled to 4.5 to 6.0, which is found optimal for carboxyl activation by EDC. The activated Pep4 was then conjugated to G4OH-5.8NH2 at slightly alkaline pH. Such a reaction sequence has been proved to inhibit dipeptide formation effectively due to the protonation of their amino groups at acidic condition.(Anjaneyulu and Staros, 1987; Grabarek and Gergely, 1990) In the 1HNMR spectrum of dendrimer-peptide conjugate (G4OH-Pep4), the aromatic protons of phenylalanine at 7.193 ppm and methyl protons of leucine at 0.85 ppm revealed the successful addition of Pep4 to G4OH-5.8NH2. The payload of Pep4 attached to dendrimer was determined to be 3.1 (G4OH-3Pep4) by 1HNMR integration. The final G4OH-3Pep4 conjugate was readily soluble in aqueous medium.
We also characterized the dendrimer-peptide conjugate with mass spectrometry. The mass spectra and molecular weights (MW) of the compounds were summarized in Fig. 1B and Table 1. The MW shifted from 15091 Da (G4OH-yNH2) to 19723Da (G4OH-Pep4) and only a single peak was detected for G4OH-Pep4 conjugate in entire mass spectra, indicating that the Pep4 sequence is covalently bonded to dendrimer (otherwise, more peaks would be detected) and free Pep4 is completely removed by dialysis. The number of Pep4 per dendrimer was further calculated by comparing the difference in MWs of G4OH-yNH2 and G4OH-Pep4, which resulted in 3.4 Pep4 molecules per dendrimer. Both 1HNMR and MALDI-TOF results revealed similar Pep4 load in dendrimer conjugate.
The hydrodynamic diameter (HD) and zeta potential (ζ) of Pep4, G4OH, G4OH-5.8NH2, G4OH-Pep4 conjugate and G4OH+Pep4 mixture were determined in PBS by DLS (Table 1). The hydrodynamic size of G4OH-Pep4 conjugate (HD: 6.1±2.5 nm) slightly increased compared to the bare G4OH (HD: 4.5±1.9 nm) and G4OH-5.8NH2 (HD: 4.1±1.6 nm), while is smaller than that of Pep4 itself (HD 9.6±4.2 nm). The conjugation of Pep4 to dendrimer and physical mixing of both parties significantly decreased the polydispersity of Pep4 molecules potentially due to disassembly of Pep4 aggregates.
3.2 PAMAM dendrimers increase peptide immunogenicity and have adjuvant effect
Chlamydiae have many pathogenic species that are relevant to human and animal health (poultry, horses, sheep, goats, pigs, cattle and others).(Longbottom, 2003) GLXA antigen is a glycolipid present in most Chlamydia species(Vora and Stuart, 2003) and the peptide vaccine (Pep4) is a vaccine candidate that serves as a molecular mimic of GLXA. The Pep4 sequence was derived by phage display with 12-mer peptides using standard methods.(Hamzeh-Mivehroud et al., 2013; Petrenko, 2008) For our vaccine formulation, Pep4 was conjugated to G4OH PAMAM dendrimer (G4OH-Pep4) and its immunogenic properties were tested through the immunization protocol discussed earlier (see Scheme 2). Four groups of female BALB/c mice were immunized. The Pep4 is a molecular mimic (conformational epitope) of a chlamydial antigen and does not represent a protein epitope. Specific binding of pre-challenge serum antibodies to chlamydial organisms in infected cells demonstrates peptide-induced anti-chlamydial specificity. Many immunogenic chlamydial antigens have failed to protect against infection despite vigorous immune responses so the demonstrated in vivo anti-microbial responses were important. Specific antibody binding to chlamydial organisms in infected cells is a key to specificity induced prior to exposure to Chlamydiae. This is very different from anti-tumor vaccines which are therapeutically delivered after tumors develop. The presence of vaccine-induced Chlamydia-specific serum Abs reactive with organisms of different species of Chlamydiae was determined by immunostaining and the results are summarized in Fig. 2.
Fig. 2.
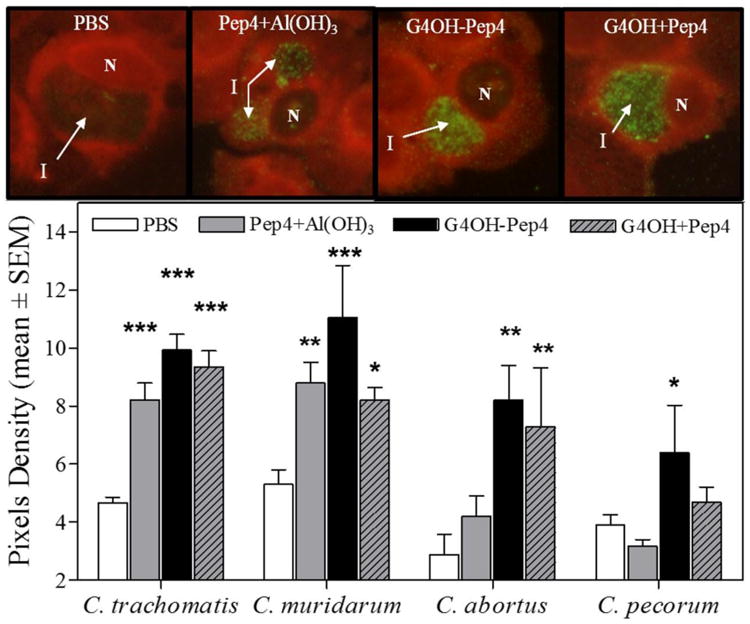
Immunostaining analysis of cells infected in vitro by different Chlamydia species. When Chlamydia-specific antibodies bind to chlamydial organisms and matrix material inside inclusions (I) within infected cells, the secondary anti-IgG antibody – FITC conjugate will show green color. Uninfected cells do not contain inclusions. Sera from mice immunized with: PBS, Pep4+Al(OH)3; G4OH-Pep4, or G4OH+Pep4 were evaluated for the presence of chlamydia-specific IgG Ab. Pictures represent HEp-2 cells infected with C. trachomatis and incubated with immune mouse sera at the same dilutions. Cell nuclei (N) and Chlamydia inclusions (I) are indicated. Animals evaluated per group: C. trachomatis (n=7-8) and C. muridarum, C. abortus and C. pecorum (n=2). 6-9 inclusions were analyzed per slide. Images were photographed under identical conditions. Statistical analyses were performed using one-way ANOVA Dunnett's test with respect to the PBS treatment group.*p<0.05, **p<0.01, and ***p<0.001.
As shown by Ab binding to the chlamydial organisms within chlamydial inclusions (membrane bound vacuoles in which chlamydia develop) in Fig. 2, all groups immunized with Pep4 developed specific Ab against C. trachomatis, independent of the vaccine formulations. As expected, all treatment groups developed significantly more antibody than the group receiving PBS alone, and all prior to exposure to live Chlamydia (p<0.05-0.001). The genus-wide specificity of these vaccine formulations was demonstrated with staining of organism in cells infected with different Chlamydia species important in other Chlamydia-induced infections of humans and animal species. Similar trends were observed for other tested Chlamydia species, except for the Pep4+Al(OH)3 immunized group which did not develop detectable Ab against C. pecorum. Overall, these results demonstrate the genus-wide immune responses induced by the chosen subunit vaccine peptide, Pep4. It can be also observed that the G4OH-Pep4 conjugate produced the highest specific Ab levels against all measured chlamydial species, followed by G4OH+Pep4 and Pep4+Al(OH)3 groups. Furthermore, only the G4OH-Pep4 conjugate induced significantly higher reactivity against all measured chlamydial species compared to the PBS group: specific Ab against C. trachomatis (p<0.001), C. muridarum (p<0.001), C. pecorum (p<0.02), and C. abortus (p<0.01). The G4OH+Pep4 group induced significant Ab against C. trachomatis (p<0.001), C. muridarum (p<0.05), and C. abortus (p<0.01) while the Pep4+Al(OH)3 group induced high reactivity against C. trachomatis (p<0.001) and C. muridarum (p<0.01). Overall, these results suggested that PAMAM dendrimers not only stimulated, but also enhanced, Pep4 immunogenicity when conjugated to or mixed with peptide. It is important to note that formulations with Pep4 and PAMAM did not include any adjuvant, yet they induced better Ab responses compared to Pep4 with Al(OH)3 adjuvant.
3.3 Vaccination with Pep4 and PAMAM dendrimers reduced infectious loads and tissue damage
An important step in a preclinical vaccine trial is the test for protection after infectious challenge. When an immunized host is exposed to a live pathogen, it is possible to evaluate the level of protective immunity by reduction of infectious loads and tissue pathology. We used the mouse model of C. trachomatis genital infection to evaluate our vaccine formulation. All mice were challenged with infectious Chlamydia EB. Weekly, from 7 to 35 days post infection (dpi), vaginal shedding was evaluated by swab collection (Scheme 2). Direct fluorescent antibody (DFA) staining was performed to detect Chlamydia EBs in the samples collected as previously described.(Whittum-Hudson et al., 1996) Chlamydial viability was tested by the ability of vaginal samples to infect McCoy cells in culture. These results are summarized in Fig. 3A and 3B.
Fig. 3.
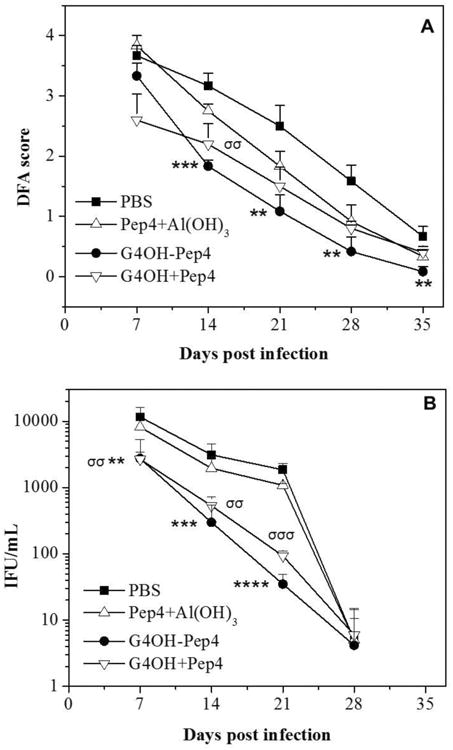
Vaginal swab collections from immunized and control mice were evaluated after C. trachomatis genital infection. (A) Chlamydia elementary bodies were detected in vaginal smears by DFA staining. Smears were scored on a 0-4+ scale for each slide. (B) Inclusions counts were made from cultures exposed to vaginal swab samples, after staining with chlamydia-specific antibody. In each case, results are expressed as the mean of 5-6 animals/treatment group after infectious challenge. Statistical analyses were performed using one-way ANOVA Dunnett's test with */σp<0.05, **/σσp<0.01, ***/σσσp<0.001, and ****p<0.0001. The asterisks (*) indicate comparisons of the G4OH-Pep4 conjugate vs PBS, while sigmas (σ) indicate comparisons of the G4OH+Pep4 mixture vs PBS.
Animals immunized with Pep4 and G4OH (conjugated or mixed) exhibited significant reductions in the DFA scores (Fig. 3A) and culture positivity (Fig. 3B) when compared to the PBS treated group, thus indicating reduced infectious loads in mice receiving the PAMAM vaccine formulations. Additionally, G4OH-immunized groups were significantly different from the Pep4+Al(OH)3 group in both microbiologic assays: culture test (p<0.04-0.001 from 7-21 dpi) and DFA test (p<0.01-0.001 from 14-35 dpi). These results are consistent with the immunostaining data (Fig. 2) in which G4OH induced better responses even when mixed with Pep4. Although statistically significant differences were not shown between G4OH-Pep4 conjugate and G4OH+Pep4 mixture formulations for some of the DFA scores and IFU values, the mean values at most times post-infection were reduced more in recipients of conjugate than in recipients of the mixture formulation.
Effects of the vaccine on tissue damage after infectious challenge were evaluated at termination of the experiment. Genital tracts were scored for gross pathology (Fig. 4) and histopathology of upper and lower tracts (Fig. 5). Genital tracts viewed ex vivo demonstrated that tissues from the G4OH-Pep4 group had fewer signs of inflammation compared to other groups. As expected, no hydrosalpinx (fluid accumulation inside blocked fallopian tubes) was detected because mice infected with C. trachomatis (a human pathogen) do not develop hydrosalpinx.(De Clercq et al., 2013; Lyons et al., 2005) Tissues were scored based on the average of 3 inflammation parameters (tissue color intensity, dilation intensity of uterine horns and presence of vessels). The ex vivo scores for each mouse are shown in Fig. 4 (lower panel). Representative views of genital tracts showing no (score=0) to maximum inflammatory signs (score = 2) are shown in Fig. 4 (upper panel). G4OH-Pep4 was most effective in reduction of inflammation at the macroscopic level: the G4OH-Pep4 group median score was significantly lower than the PBS group (p<0.05). All other treatment groups exhibited persisting inflammatory changes that were not significantly lower than the PBS controls. In order to assess the damage and the repair stage of the tissues, we performed a histopathological analysis on H&E stained genital tract sections from all mice. The results are summarized in Fig. 5.
Fig. 4.
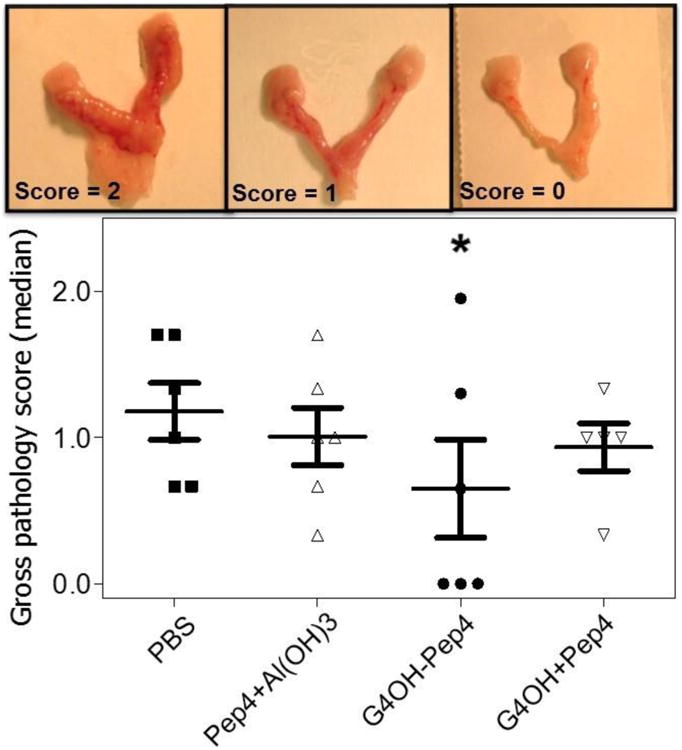
Effect of immunization on the development of genital tract gross pathologies after C. trachomatis infection. Genital tracts were scored 0-2+ according to three inflammation signs. Pictures are representative of the gross pathology scores from a 2+, 1+ and 0 genital tract. The statistical analysis was performed using one-way ANOVA Dunnett's test with *p<0.05 (n=5-6 per group). The asterisk symbols denote comparisons of treatments to the PBS group.
Fig. 5.
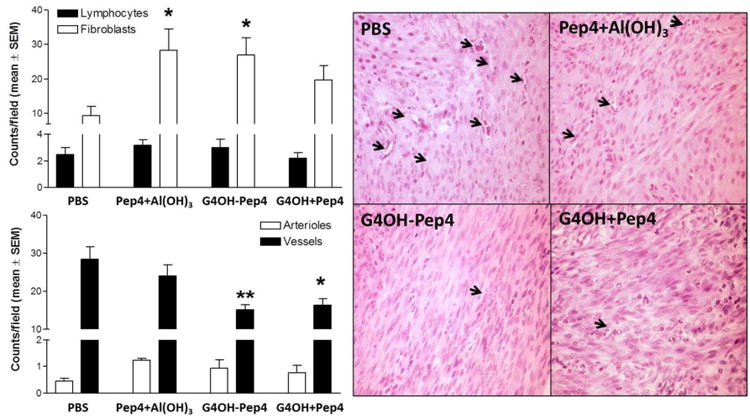
Histopathological analysis of genital tracts from immunized mice 37 days after C. trachomatis infection. Whole sections of H&E stained genital tracts were analyzed for lymphocytes, and fibroblasts (top panel) and arterioles and capillary vessels (bottom panel). Thirty pictures were taken per slide and cells manually counted in a masked fashion at 800× mag. under bright field. Bars represent an average of 5-6 animals. Pictures are representative of each group, and arrows indicate capillary vessels. The statistical analysis was performed using one-way ANOVA Dunnett's test with respect to PBS group with *p<0.05 and **p<0.01.
The number of lymphocytes in all sections was low and not different between groups, indicating none of the experimental groups were in the initial phase of tissue repair. This result was expected as animals were euthanized at 37 dpi after active infections were cleared. The number of fibroblasts was high in tissues for all groups immunized with Pep4 although the number of fibroblasts in groups treated with G4OH-Pep4 and Pep4+Al(OH)3 formulations were statistically higher than seen for the PBS control group (p<0.05). Additionally, the number of capillary vessels was lower in the G4OH groups compared to the PBS group (p<0.01 for G4OH-Pep4 and <0.05 for G4OH+Pep4 mixture). These results together indicate that all groups were in the proliferative phase of tissue repair.(Broughton et al., 2006; Diegelmann and Evans, 2004) The PBS group was the most delayed in the repair process compared to immunized groups, as indicated by higher numbers of vessels and lower numbers of fibroblasts. In contrast, the G4OH-Pep4 treatment group was the most advanced in repair, with a histopathology profile significantly different from that of the PBS group. The Pep4+Al(OH)3 group showed high fibroblast counts and number of vessels, suggesting these mice were in intermediate stages of repair compared to the PBS (early repair) and G4OH-Pep4 (late repair) groups.
4. Discussion
We have developed a Chlamydial vaccine carrier system by covalent conjugation of PAMAM G4 dendrimers to a peptide which is a molecular mimic of a Chlamydial antigen. The dendrimer-peptide conjugate was characterized physicochemically and compared to a mixture of dendrimer and peptide and peptide alone. Microbiologically and immunologically, the dendrimer-peptide conjugate surpassed the other presentations to demonstrate feasibility of the conjugated vaccine platform to reduce infection and pathology in the murine model of Chlamydial genital infection.
The generation and surface charge of PAMAM dendrimers have significant impact on their in vivo toxicity. Generation 5 or higher dendrimers have increased in vivo toxicity such as aggregation of platelets and fibrinogen and red blood cell lysis.(Jain et al., 2010; Roberts et al., 1996) Such a trend is extremely obvious in NH2-terminated PAMAM (cationic dendrimers) Therefore, G4 dendrimer is widely accepted for drug delivery by balancing the requirements of nanoscale size and absence of toxicity. The toxicity of PAMAM dendrimers with different charges follows the sequence of cationic (-NH2) ≫ anionic (-COOH) > neutral (-OH). It is reported that a high maximum tolerance dose is >500 mg/kg for G4OH and no toxicity is observed at 180 mg/kg in murine models, while that of commonly used G4NH2 and G4COOH is ca. 10 mg/kg and 100 mg/kg, respectively.(Sadekar and Ghandehari, 2012) Therefore, OH-dendrimers are extremely suitable to improve the safety profile of dendrimer-based vaccines. Additionally, OH-terminated PAMAM dendrimers permit ease of conjugation of peptide to dendrimers due to significantly reduced electrostatic interactions.(Lee et al., 2003) The antigenic peptide was covalently conjugated to G4OH dendrimer through labile ester bonds which are expected to be broken down primarily by abundant hydrolases at intracellular sites, such as phagolysosomes. The fact that the hydrodynamic size of the conjugate was similar to that of G4OH and obviously smaller than Pep4 molecule (ca. 10 nm), while size distribution narrowed (PDI from 0.225), suggests the formation of a compact structure between the dendrimer and Pep4 upon conjugation. The conjugated Pep4 backbone or side chains may protrude into the dendrimer interior or may adsorb on the surface of dendrimer through van der Waal forces, hydrogen bonding (with surface hydroxyl groups), hydrophobic effects (with hydrophobic dendrimer core), and even electrostatic interaction (with protonated tertiary amine at physiological pH). The similar interaction between dendrimer and conjugated moieties has been discussed in other papers.(Riechers et al., 2015; Yang and da Rocha, 2014) Such a relatively compact structure may effectively protect the integrity of peptides from enzymatic degradation before delivery to immune cells or draining lymph nodes,(Etrych et al., 2011; Kurtoglu et al., 2010) which is one of major challenges for peptide vaccines to elicit/amplify immune responses.(Irvine et al., 2015; Oyewumi et al., 2010) Similar compact structures are observed in partially PEGylated PAMAM dendrimers.(Riechers et al., 2015; Yang and da Rocha, 2014) Mixing Pep4 with dendrimer also seems to disrupt the aggregation of Pep4 (HD=9.6 nm) as indicated by the decrease in hydrodynamic diameter (HD=4.1 nm) and narrower size distribution (PDI changes from 0.225 to 0.159). However, non-covalent bonding in physical mixture is much weaker and susceptible to dissociation caused by physiological fluid.(Cheng and Xu, 2008) It is thus assumed that dendrimer may not provide effective protection for Pep4 from degradation in the mixture system.
The surface charge of dendrimer nanocarriers remained positive and increased slightly upon Pep4 conjugation. This is attributed to the protonation of -NH2 groups in G4OH and amino acids of Pep4. The size and charge of the G4OH+Pep4 were observed to be similar to that of the conjugate, with a size much smaller than that observed for bare Pep4, indicating the formation of dendrimer/Pep4 complexes that serve well as controls to the conjugate. The positive charge of nanocarrier systems is known to enhance or facilitate cellular uptake via nonspecific adsorptive endocytosis and/or receptor-mediated endocytosis,(Albanese et al., 2012; Albertazzi et al., 2010; Kitchens et al., 2008; Nakanishi et al., 1999; Riechers et al., 2015; Win and Feng, 2005) and we thus believe the positive charge may also facilitate internalization of the G4OH-Pep4 nanovaccine and G4OH+Pep4 mixture into antigen-presenting cells (APCs).
Our study demonstrates that G4OH dendrimer-Pep4 conjugates successfully immunized mice so that they developed Chlamydia-specific responses which conferred significant protection from subsequent infectious challenge. The G4OH-Pep4 conjugate or the G4OH+Pep4 mixture sustained the genus-wide immune specificity of Pep4 which is a molecular mimic of the chlamydial antigen, GLXA.(Stuart and Macdonald, 1989; Vora and Stuart, 2003) GLXA, as for other non-protein antigens, has limited potential to elicit immune responses compared to a protein antigen.(Whittum-Hudson and Hudson, 2011; Whittum-Hudson et al., 1996) To overcome this issue, candidate molecular mimic peptides were identified by phage display with GLXA-specific monoclonal antibody (mAb1).(Whittum-Hudson and Hudson, 2011) The peptide sequences on phage which were bound by the Ab1 were deduced by standard methods.(Hamzeh-Mivehroud et al., 2013; Petrenko, 2008; Smith and Petrenko, 1997) Peptide 4 (Pep4) is a 12 amino acid sequence that has been patented along with other vaccine candidate peptides that induced genus-wide chlamydia-specific immune responses in mice. Pep4 was recognized also by serum Ab from patients with documented chlamydial genital infections,(Whittum-Hudson and Hudson, 2011) showing its relevance to human infection. Free peptides are not feasible as vaccine candidates. Since there are few adjuvants available for human use, we chose to test whether the G4OH PAMAM dendrimer for delivery of Pep4 would stimulate protection against C. trachomatis infection. Our results demonstrate that G4OH conferred improved immunogenicity on Pep4 to reduce infectious loads and pathology after C trachomatis challenge compared to an alum type adjuvant. Prior endotoxin assay showed commercial G4OH are endotoxin-free (<0.005 endotoxin units/ml).(Dobrovolskaia et al., 2011) Therefore, our results suggest that G4OH dendrimers have adjuvant properties (For the first time, the adjuvancy of OH-terminated PAMAM dendrimer is reported). Similar adjuvancy of NH2-terminated PAMAM dendrimers has been reported previously and the immunostimulatory properties of dendrimers have been recently reviewed.(Dobrovolskaia, 2017; Heegaard et al., 2010) However, this is the first time to our knowledge that OH-terminated PAMAM dendrimers (actually in all types of OH-terminated dendrimers) demonstrate adjuvant properties for vaccine use. Studies reported in one patent showed that influenza antigens mixed with NH2-terminated PAMAM dendrimers (G3-G8) induced higher serum Ab titers in mice compared to antigens alone.(Wright, 1998) The number of primary amine groups on the PAMAM surface correlated with the Ab titers. PAMAM dendrimers (G5NH2) mixed with ovalbumin (OVA) antigen were shown to induce higher IgM and IgG Abs,(Rajananthanan et al., 1999) although similar results were obtained for OVA mixed with Al(OH)3 in another study.(Rajananthanan et al., 1999) The NH2-terminated PAMAM dendrimers have shown their ability to modulate intracellular cytokines and inflammatory modulators, such as tumor necrosis factor-alpha, interleukin-6, and macrophage inflammatory protein-2.(Naha et al., 2010) In contrast, only one study reported naked and unmodified OH-terminated PAMAM dendrimers had anti-inflammatory properties by interacting with immune cells (e.g. macrophages).(Chauhan et al., 2009) However, the precise mechanisms underlying these PAMAM dendrimers remain unknown. Compared to the mixture of G4OH and Pep4 (G4OH+Pep4), the vaccination with G4OH-Pep4 conjugate induced higher antibody reactivity with a variety of Chlamydia species (Fig. 2), and resulted in significantly lower numbers of shed Chlamydia trachomatis organism (elementary bodies; Fig. 3A) and infectious organisms (Fig. 3B) in vaginal samples of challenged mice. The demonstrated reduced gross genital tract inflammation with histopathological evidence of faster recovery from the infection further support the superiority of the conjugate for clinical application, though dendrimer+Pep4 mixture clearly elicited significant protection. The role of conjugating peptide to dendrimer in the further enhancement of specific immunity and protection remains unclear and requires additional studies for mechanism elucidation. However, logically the conjugate offers superior control of delivery and release kinetics of peptides because the dendrimer conjugate may (1) ensure that the peptide and dendrimer, especially with targeting, will go to the same site/cells where the dendrimer will enhance peptide presentation through protection of peptide cargo from fast enzymatic degradation; (2) target antigenic peptide to regional lymph nodes and increase cellular uptake of peptide by antigen-presenting cells;(Zhong et al., 2016b) (3) retain peptide in circulation longer (due to improved aqueous solubility). A mixture formulation would not facilitate these goals; and most importantly (4) release peptide in a controllable fashion as an enzyme-cleavable ester bond was used to link peptide to dendrimer.
In this study, the observed faster bacterial clearance compared to controls demonstrates the potential of the OH-terminated PAMAM dendrimer for vaccine delivery. These markers of reduced bacterial loads should result in less tissue damage because of reduced ascending infection which is normally associated with late disease sequelae.(Ramsey et al., 1999) A vaccine to prevent such sequelae would have important public health implications. Determination of optimal protective anti-chlamydial effector immune responses induced by G4OH dendrimers will require further study.
In summary, these results indicate that the conjugation of Pep4 to OH-terminated PAMAM dendrimers provide a promising new platform to deliver a peptide vaccine with a potential adjuvant property. Dendrimers offered a very controlled molecular platform for the delivery of peptides, which is expected to lead to reproducible behavior (pharmacokinetics/biodistribution) and also the possibility of further modification for targeting and to modulate the interaction with the physiological environment. Importantly, the latter will also allow tracking of the vaccine carriers to improve our understanding of the mechanism upon which responses are elicited. There is, therefore, a big window of opportunity and many avenues to be followed in subsequent and more detailed studies.
5. Conclusions
There are many infectious diseases worldwide without a preventive vaccine, or which have low efficiency. This lack is in large part due to a need to fine-tune the immune system in specific ways for each infectious disease. This work for the first time developed a dendrimer-conjugated peptide nanovaccine and demonstrated the feasibility and superiority of the formulation over conventional adjuvant such as Al(OH)3 against infectious Chlamydia. Together, our results demonstrate that OH-terminated PAMAM dendrimer with Pep4 conjugated through a pH sensitive linker not only enhanced Pep4 immunogenicity by increasing specific anti-Chlamydia Ab responses in mice, but also sustained the genus-wide property of Pep4. The G4OH-Pep4 conjugate as the vaccine candidate significantly reduced infectious loads and duration of infection in challenged mice. Finally, consistent with reduced microbiological loads, the genital tracts of animals vaccinated with the G4OH-Pep4 conjugates had a healthier gross appearance which indicated faster tissue repair or reduced damage compared to the other vaccine formulations and was supported by histologic examination. In addition to the carrier function, G4OH dendrimers also demonstrated adjuvant effects as shown by G40H+Pep4 mixture, though a dendrimer-peptide mixture is less feasible for an antimicrobial vaccine delivery. The results suggest that PAMAM G4OH dendrimers represent a promising platform for the delivery of peptide-based vaccines candidates for the prevention of infectious diseases. As a new nanoscale platform for vaccine delivery, however, further studies are needed, such as the use of peptide cocktails, optimization of peptide doses and elucidation of underlying immunological mechanisms of dendrimer-conjugated peptide vaccine (e.g. cytokine measurement, immunological functions of dendrimer). This approach could not only be expanded to many other infectious intracellular bacteria and viruses of public health significance such as HIV, herpes simplex virus (HSV), human papillomavirus (HPV), Zika virus, but also provides an efficacious strategy to overcome antibiotic resistance which can develop in the treatment of numerous infectious agents.
Acknowledgments
The authors would like to thank Dr. Ronald Thomas (Wayne State University School of Medicine) for help with the statistical analyses; Mr. Christopher McPharlin and Mr. Tayson Lin for assistance with the image analyses, Miss Mariene R. Amorim and Miss Izabel N. Carvalho for assistance with the histopathological analysis and Dr. Alan Hudson for critical reading of the manuscript. We thank Dr. Brian Bao of the Antibody Laboratories of the Michigan University Research Corridor for performing the phage display procedures to identify relevant peptides. The project was supported in part by the Brazilian Federal Agency for Support and Evaluation of Graduate Education (Capes - process # 1284/91-2, scholarship for ISG), the Office of the Vice President for Research at Wayne State University (for JWH and SRPR), the Technology Commercialization Office (for JWH), National Institutes of Health (Grant # AI080928, for JWH), and the National Science Foundation (CBET # 0933144 and DMR # 1508363 for SRPR).
Abbreviations
- PAMAM
poly(amidoamine)
- G4OH
Generation 4 hydroxyl-terminated PAMAM dendrimer
- Pep4
a peptide with the sequence AFPQFRSATLLL
- PID
pelvic inflammatory disease
- C. trachomatis
Chlamydia trachomatis
- GLXA
glycolipid exoantigen
- MHC-II
class II major histocompatibility complex
- MW
molecular weight
- sulfo-NHS
sulfo-N-hydroxysuccinimide
- EDC
ethyl-3-(3-dimethylaminopropyl) carbodiimide
- MES
2-(N-morpholino) ethanesulfonic acid
- DIPEA
diisopropylethylamine
- Al(OH)3
aluminum hydroxide
- 2,5-DHB
2, 5-dihydroxy benzoic acid
- Fmoc-AHA
fluorenylmethyloxycarbonyl-6-amino-hexanoic acid
- Fmoc
fluorenylmethyloxycarbonyl group
- AHA
6-amino-hexanoic acid
- PyBOP
benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate
- MWCO
molecular weight cut off
- DI water
deionized water
- G4OH-yNH2
G4OH dendrimer modified with y amine groups on surface
- G4OH-Pep4
dendrimer-Pep4 conjugate
- G4OH+Pep4
physical mixture of G4OH dendrimer and Pep4
- G4NH2
Generation 4 amine-terminated PAMAM dendrimer
- G4COOH
Generation 4 carboxyl-terminated PAMAM dendrimer
- DMF
dimethylformamide
- DCM
dichloromethane
- MeOH
methanol
- TLC
thin layer chromatography
- 1HNMR
proton nuclear magnetic resonance
- MALDI-TOF
matrix-assisted laser desorption/ionization-time of flight
- DLS
dynamic light scattering
- HD
hydrodynamic diameter
- PBS
phosphate buffer saline
- SC
subcutaneous injection
- EB
elementary bodies
- mAb
monoclonal antibody
- FITC
fluorescein isothiocyanate
- Ab
antibody
- H&E
hematoxylin and eosin
- DFA
direct fluorescent antibody
- IFU
inclusion forming units
- OVA
ovalbumin
- s.d
standard deviation
- mAb
monoclonal antibody
- HIV
human immunodeficient virus
- HSV
herpes simplex virus
- HPV
human papillomavirus virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu El-Asrar AM, Geboes K, Tabbara KF, Al-Kharashi SA, Missotten L, Desmet V. Immunopathogenesis of conjunctival scarring in trachoma. Eye. 1998;12:453–460. doi: 10.1038/eye.1998.104. [DOI] [PubMed] [Google Scholar]
- Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- Albertazzi L, Serresi M, Albanese A, Beltram F. Dendrimer internalization and intracellular trafficking in living cells. Mol pharmaceutics. 2010;7:680–688. doi: 10.1021/mp9002464. [DOI] [PubMed] [Google Scholar]
- Anjaneyulu PS, Staros JV. Reactions of N-hydroxysulfosuccinimide active esters. Int J Pept Protein Res. 1987;30:117–124. doi: 10.1111/j.1399-3011.1987.tb03319.x. [DOI] [PubMed] [Google Scholar]
- Balin BJ, Gerard HC, Arking EJ, Appelt DM, Branigan PJ, Abrams JT, Whittum-Hudson JA, Hudson AP. Identification and localization of Chlamydia pneumoniae in the Alzheimer's brain. Med Microbiol Immunol. 1998;187:23–42. doi: 10.1007/s004300050071. [DOI] [PubMed] [Google Scholar]
- Broughton G, 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117:1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- Carter J, Espinoza L, Inman R, Sneed K, Ricca L, Vasey F, Valeriano J, Stanich J, Oszust C, Gerard H. Combination antibiotics as a treatment for chronic Chlamydia - induced reactive arthritis: a double - blind, placebo - controlled, prospective trial. Arthritis Rheum. 2010;62:1298–1307. doi: 10.1002/art.27394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2014. Atlanta: U.S. Department of Health and Human Services; 2015. [Google Scholar]; Centers for Disease Control and Prevention (CDC) Atlanta, GA, USA: CDC; 2014. 2012 Sexually Transmitted Diseases Surveillance. Online. Available from URL: http://www.cdc.gov/std/stats1412/chlamydia.htm. [Google Scholar]
- Champion CI, Kickhoefer VA, Liu G, Moniz RJ, Freed AS, Bergmann LL, Vaccari D, Raval-Fernandes S, Chan AM, Rome LH. A vault nanoparticle vaccine induces protective mucosal immunity. PloS one. 2009;4:e5409. doi: 10.1371/journal.pone.0005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan AS, Diwan PV, Jain NK, Tomalia DA. Unexpected in vivo anti-inflammatory activity observed for simple, surface functionalized poly(amidoamine) dendrimers. Biomacromolecules. 2009;10:1195–1202. doi: 10.1021/bm9000298. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Xu T. The effect of dendrimers on the pharmacodynamic and pharmacokinetic behaviors of non-covalently or covalently attached drugs. Eur J Med Chem. 2008;43:2291–2297. doi: 10.1016/j.ejmech.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Daftarian P, Kaifer AE, Li W, Blomberg BB, Frasca D, Roth F, Chowdhury R, Berg EA, Fishman JB, Al Sayegh HA, Blackwelder P, Inverardi L, Perez VL, Lemmon V, Serafini P. Peptide-conjugated PAMAM dendrimer as a universal DNA vaccine platform to target antigen-presenting cells. Cancer Res. 2011;71:7452–7462. doi: 10.1158/0008-5472.CAN-11-1766. [DOI] [PubMed] [Google Scholar]
- De Clercq E, Kalmar I, Vanrompay D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infect Immun. 2013;81:3060–3067. doi: 10.1128/IAI.00357-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- Dixit S, Singh SR, Yilma AN, Agee RD, Taha M, Dennis VA. Poly (lactic acid)–poly (ethylene glycol) nanoparticles provide sustained delivery of a Chlamydia trachomatis recombinant MOMP peptide and potentiate systemic adaptive immune responses in mice. Nanomed: Naotech Biol Med. 2014;10:1311–1321. doi: 10.1016/j.nano.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia MA. Dendrimers Effects on the Immune System: Insights into Toxicity and Therapeutic Utility. Curr Pharm Des. 2017;23:1–8. doi: 10.2174/1381612823666170309151958. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Patri AK, Simak J, Hall JB, Semberova J, De Paoli Lacerda SH, McNeil SE. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol Pharmaceutics. 2011;9:382–393. doi: 10.1021/mp200463e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discovery Today. 2001;6:427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- Etrych T, Kovar L, Strohalm J, Chytil P, Rihova B, Ulbrich K. Biodegradable star HPMA polymer-drug conjugates: Biodegradability, distribution and anti-tumor efficacy. J Controlled Release. 2011;154:241–248. doi: 10.1016/j.jconrel.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Gaertner HF, Cerini F, Kamath A, Rochat AFo, Siegrist CA, Menin L, Hartley O. Efficient orthogonal bioconjugation of dendrimers for synthesis of bioactive nanoparticles. Bioconjug Chem. 2011;22:1103–1114. doi: 10.1021/bc1005653. [DOI] [PubMed] [Google Scholar]
- Gerard H, Branigan P, Schumacher H, Jr, Hudson A. Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiter's syndrome are viable but show aberrant gene expression. J Rheumatol. 1998;25:734–742. [PubMed] [Google Scholar]
- Gillies ER, Frechet JM. Dendrimers and dendritic polymers in drug delivery. Drug Discovery Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- Grabarek Z, Gergely J. Zero-length crosslinking procedure with the use of active esters. Anal Biochem. 1990;185:131–135. doi: 10.1016/0003-2697(90)90267-d. [DOI] [PubMed] [Google Scholar]
- Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. 2013;3:13. doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner L, Beagley K, Timms P. Chlamydia trachomatis infection: host immune responses and potential vaccines. Mucosal Immunol. 2008;1:116–130. doi: 10.1038/mi.2007.19. [DOI] [PubMed] [Google Scholar]
- Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA, J Am Med Assoc. 1991;266:225–230. [PubMed] [Google Scholar]
- Hamzeh-Mivehroud M, Alizadeh AA, Morris MB, Church WB, Dastmalchi S. Phage display as a technology delivering on the promise of peptide drug discovery. Drug Discovery Today. 2013;18:1144–1157. doi: 10.1016/j.drudis.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Heegaard PM, Boas U, Sorensen NS. Dendrimers for vaccine and immunostimulatory uses. A review Bioconjug Chem. 2010;21:405–418. doi: 10.1021/bc900290d. [DOI] [PubMed] [Google Scholar]
- Heyder RS, Zhong Q, Bazito RC, da Rocha SR. Cellular internalization and transport of biodegradable polyester dendrimers on a model of the pulmonary epithelium and their formulation in pressurized metered-dose inhalers. Int J Pharm. 2017;520:181–194. doi: 10.1016/j.ijpharm.2017.01.057. [DOI] [PubMed] [Google Scholar]
- Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J. Function and potentials of M. tuberculosis epitopes. Front Immunol. 2014;5:107. doi: 10.3389/fimmu.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain K, Kesharwani P, Gupta U, Jain NK. Dendrimer toxicity: Let's meet the challenge. Int J Pharm. 2010;394:122–142. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Jiang J, Liu G, Kickhoefer VA, Rome LH, Li LX, McSorley SJ, Kelly KA. A Protective Vaccine against Chlamydia Genital Infection Using Vault Nanoparticles without an Added Adjuvant. Vaccines. 2017;5:3. doi: 10.3390/vaccines5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanova A, Adamo R, Proietti D, Berti F, Tontini M, Rappuoli R, Costantino P. Preparation, characterization and immunogenicity of HIV-1 related high-mannose oligosaccharides-CRM197 glycoconjugates. Glycoconj J. 2010;27:501–513. doi: 10.1007/s10719-010-9295-0. [DOI] [PubMed] [Google Scholar]
- Keegan ME, Whittum-Hudson JA, Mark Saltzman W. Biomimetic design in microparticulate vaccines. Biomaterials. 2003;24:4435–4443. doi: 10.1016/s0142-9612(03)00351-x. [DOI] [PubMed] [Google Scholar]
- Kitchens KM, Kolhatkar RB, Swaan PW, Ghandehari H. Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol pharmaceutics. 2008;5:364–369. doi: 10.1021/mp700089s. [DOI] [PubMed] [Google Scholar]
- Kurtoglu YE, Mishra MK, Kannan S, Kannan RM. Drug release characteristics of PAMAM dendrimer-drug conjugates with different linkers. Int J Pharm. 2010;384:189–194. doi: 10.1016/j.ijpharm.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lim YB, Choi JS, Lee Y, Kim TI, Kim HJ, Yoon JK, Kim K, Park JS. Polyplexes assembled with internally quaternized PAMAM-OH dendrimer and plasmid DNA have a neutral surface and gene delivery potency. Bioconjug Chem. 2003;14:1214–1221. doi: 10.1021/bc034095g. [DOI] [PubMed] [Google Scholar]
- Longbottom D. Chlamydial vaccine development. J Med Microbiol. 2003;52:537–540. doi: 10.1099/jmm.0.05093-0. [DOI] [PubMed] [Google Scholar]
- Lyons JM, Morré SA, Airo-Brown LP, Peña AS, Ito JI. Comparison of multiple genital tract infections with Chlamydia trachomatis in different strains of female mice. J Microbiol, Immunol Infect. 2005;38:383. [PubMed] [Google Scholar]
- Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discovery Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Mishra MK, Kotta K, Hali M, Wykes S, Gerard HC, Hudson AP, Whittum-Hudson JA, Kannan RM. PAMAM dendrimer-azithromycin conjugate nanodevices for the treatment of Chlamydia trachomatis infections. Nanomedicine. 2011;7:935–944. doi: 10.1016/j.nano.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Adv Mater. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naha PC, Davoren M, Lyng FM, Byrne HJ. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol Appl Pharmacol. 2010;246:91–99. doi: 10.1016/j.taap.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Nakanishi M, Tanaka K, Mayumi T. Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J Controlled Release. 1999;61:233–240. doi: 10.1016/s0168-3659(99)00097-8. [DOI] [PubMed] [Google Scholar]
- Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev Vaccines. 2010;9:1095–1107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmiani G, Russo V, Maccalli C, Parolini D, Rizzo N, Maio M. Peptide-based vaccines for cancer therapy. Hum Vaccin Immunother. 2014;10:3175–3178. doi: 10.4161/hv.29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko V. Evolution of phage display: from bioactive peptides to bioselective nanomaterials. Expert Opin Drug Deliv. 2008;5:825–836. doi: 10.1517/17425247.5.8.825. [DOI] [PubMed] [Google Scholar]
- Rajananthanan P, Attard GS, Sheikh NA, Morrow WJ. Evaluation of novel aggregate structures as adjuvants: composition, toxicity studies and humoral responses. Vaccine. 1999;17:715–730. doi: 10.1016/s0264-410x(98)00256-4. [DOI] [PubMed] [Google Scholar]
- Ramsey KH, Cotter TW, Salyer RD, Miranpuri GS, Yanez MA, Poulsen CE, DeWolfe JL, Byrne GI. Prior genital tract infection with a murine or human biovar of Chlamydia trachomatis protects mice against heterotypic challenge infection. Infect Immun. 1999;67:3019–3025. doi: 10.1128/iai.67.6.3019-3025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechers S, Zhong Q, Yin NN, Karsai A, da Rocha SR, Liu Gy. High-Resolution Imaging of Polyethylene Glycol Coated Dendrimers via Combined Atomic Force and Scanning Tunneling Microscopy. J Drug Delivery. 2015:2015. doi: 10.1155/2015/535683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst™ dendrimers. J Biomed Mater Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Sadekar S, Ghandehari H. Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv Drug Deliv Rev. 2012;64:571–588. doi: 10.1016/j.addr.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Phar Res. 2014;33:2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K, Coleman D, O'Sullivan M, Stephens N. Public health policies and management strategies for genital Chlamydia trachomatis infection. Risk Manag Healthc Policy. 2011;4:57–65. doi: 10.2147/RMHP.S12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng KC, Kalkanidis M, Pouniotis DS, Esparon S, Tang CK, Apostolopoulos V, Pietersz GA. Delivery of antigen using a novel mannosylated dendrimer potentiates immunogenicity in vitro and in vivo. Eur J Immunol. 2008;38:424–436. doi: 10.1002/eji.200737578. [DOI] [PubMed] [Google Scholar]
- Singh M, Chakrapani A, O'Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines. 2007;6:797–808. doi: 10.1586/14760584.6.5.797. [DOI] [PubMed] [Google Scholar]
- Smith GP, Petrenko VA. Phage display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- Stratton CW, Sriram S. Association of Chlamydia pneumoniae with central nervous system disease. Microbes Infect. 2003;5:1249–1253. doi: 10.1016/j.micinf.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Stuart ES, Macdonald AB. Some characteristics of a secreted chlamydial antigen recognized by IgG from C. trachomatis patient sera. Immunology. 1989;68:469–473. [PMC free article] [PubMed] [Google Scholar]
- Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Taylor HR, Velez VL. Clearance of chlamydial elementary bodies from the conjunctival sac. Invest Ophthalmol Vis Sci. 1987;28:1199–1201. [PubMed] [Google Scholar]
- Torres-Sangiao E, Holban AM, Gestal MC. Advanced nanobiomaterials: vaccines, diagnosis and treatment of infectious diseases. Molecules. 2016;21:867. doi: 10.3390/molecules21070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- Verminnen K, Beeckman DS, Sanders NN, De Smedt S, Vanrompay DC. Vaccination of turkeys against Chlamydophila psittaci through optimised DNA formulation and administration. Vaccine. 2010;28:3095–3105. doi: 10.1016/j.vaccine.2010.02.064. [DOI] [PubMed] [Google Scholar]
- Vora GJ, Stuart ES. A role for the glycolipid exoantigen (GLXA) in chlamydial infectivity. Curr Microbiol. 2003;46:0217–0223. doi: 10.1007/s00284-002-3843-1. [DOI] [PubMed] [Google Scholar]
- Waugh C, Khan SA, Carver S, Hanger J, Loader J, Polkinghorne A, Beagley K, Timms P. A prototype recombinant-protein based Chlamydia pecorum vaccine results in reduced chlamydial burden and less clinical disease in free-ranging koalas (Phascolarctos cinereus) PloS one. 2016;11:e0146934. doi: 10.1371/journal.pone.0146934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittum-Hudson J, Hudson AP. US Patent No. 20110236484 Genus-wide chlamydial peptide vaccine antigens. 2011
- Whittum-Hudson JA, An LL, Saltzman WM, Prendergast RA, MacDonald AB. Oral immunization with an anti-idiotypic antibody to the exoglycolipid antigen protects against experimental Chlamydia trachomatis infection. Nat Med. 1996;2:1116–1121. doi: 10.1038/nm1096-1116. [DOI] [PubMed] [Google Scholar]
- Whittum-Hudson JA, O'Brien TP, Prendergast RA. Murine model of ocular infection by a human biovar of Chlamydia trachomatis. Invest Ophthalmol Vis Sci. 1995;36:1976–1987. [PubMed] [Google Scholar]
- Whittum-Hudson JA, Rudy D, Gerard H, Vora G, Davis E, Haller PK, Prattis SM, Hudson AP, Saltzman WM, Stuart ES. The anti-idiotypic antibody to chlamydial glycolipid exoantigen (GLXA) protects mice against genital infection with a human biovar of Chlamydia trachomatis. Vaccine. 2001;19:4061–4071. doi: 10.1016/s0264-410x(01)00117-7. [DOI] [PubMed] [Google Scholar]
- Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- Wong Y, Ward ME. Chlamydia pneumoniae and atherosclerosis. J Clin Pathol. 1999;52:398–399. doi: 10.1136/jcp.52.5.398b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DC. Adjuvant properties of poly (amidoamine) dendrimers. U.S Patent No. 5,795,582 1998
- Yang L, da Rocha SR. PEGylated, NH2-terminated PAMAM dendrimers: a microscopic view from atomistic computer simulations. Molecular pharmaceutics. 2014;11:1459–1470. doi: 10.1021/mp400630z. [DOI] [PubMed] [Google Scholar]
- Yu H, Karunakaran KP, Jiang X, Brunham RC. Subunit vaccines for the prevention of mucosal infection with Chlamydia trachomatis. Expert Rev Vaccines. 2016 doi: 10.1586/14760584.2016.1161510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Bielski ER, Rodrigues LS, Brown MR, Reineke JJ, da Rocha SRP. Conjugation to Poly(amidoamine) Dendrimers and Pulmonary Delivery Reduce Cardiac Accumulation and Enhance Antitumor Activity of Doxorubicin in Lung Metastasis. Mol pharmaceutics. 2016a;13:2363–2375. doi: 10.1021/acs.molpharmaceut.6b00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, da Rocha SR. Poly (amidoamine) dendrimer-doxorubicin conjugates: in vitro characteristics and pseudo-solution formulation in pressurized metered-dose inhalers. Mol pharmaceutics. 2016;13:1058–1072. doi: 10.1021/acs.molpharmaceut.5b00876. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Merkel OM, Reineke JJ, da Rocha SR. Effect of the Route of Administration and PEGylation of Poly (amidoamine) Dendrimers on Their Systemic and Lung Cellular Biodistribution. Mol pharmaceutics. 2016b;13:1866–1878. doi: 10.1021/acs.molpharmaceut.6b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]


