Abstract
Malaria remains a considerable burden on public health. In 2015, the WHO estimates there were 212 million malaria cases causing nearly 429,000 deaths globally. A highly effective malaria vaccine is needed to reduce the burden of this disease. We have developed an experimental vaccine candidate (PyCMP) based on pre-erythrocytic (CSP) and erythrocytic (MSP1) stage antigens derived from the rodent malaria parasite P. yoelii. Our protein-based vaccine construct induces protective antibodies and CD4+ T cell responses. Based on evidence that viral vectors increase CD8+ T cell-mediated immunity, we also have tested heterologous prime-boost immunization regimens that included human adenovirus serotype 5 vector (Ad5), obtaining protective CD8+ T cell responses. While Ad5 is commonly used for vaccine studies, the high prevalence of pre-existing immunity to Ad5 severely compromises its utility. Here, we report the use of the novel simian adenovirus 36 (SAd36) as a candidate for a vectored malaria vaccine since this virus is not known to infect humans, and it is not neutralized by anti-Ad5 antibodies. Our study shows that the recombinant SAd36PyCMP can enhance specific CD8+ T cell response and elicit similar antibody titers when compared to an immunization regimen including the recombinant Ad5PyCMP. The robust immune responses induced by SAd36PyCMP are translated into a lower parasite load following P. yoelii infectious challenge when compared to mice immunized with Ad5PyCMP.
Keywords: Malaria, Simian Adenovirus, Viral Vectored Vaccine, Protein Vaccine, Plasmodium yoelii, Heterologous Prime-Boost Regimens
1. Introduction
Malaria remains a considerable burden on public health. In 2015, there were an estimated 212 million cases of malaria and 429,000 deaths, most of which occurred in children under 5 years of age, being a leading cause of death in this population [1]. A vaccine against malaria is a research priority and an essential tool for control and elimination efforts.
The development of an effective malaria vaccine has been a challenge due to the complex Plasmodium life cycle [2]. The pre-erythrocytic stage is initiated by the injection of sporozoites into the dermis by Anopheles spp mosquitos. The sporozoites then travel via the circulatory system to invade hepatocytes, where the parasite then differentiates and produces tens of thousands of infectious merozoites per infected hepatocyte that burst to release the parasites into the circulatory system where they invade erythrocytes. Within the erythrocyte, the parasite again undergoes a cycle of growth and proliferation, ultimately resulting in the bursting of the erythrocyte and the release of new infectious merozoites to continue the erythrocytic stage of infection, and the clinical symptomatology associated with malaria.
Several key proteins are responsible for the motility and invasion of the infectious forms into their respective target cells. During the pre-erythrocytic stage, the circumsporozoite protein (CSP) and the thrombospondin-related adhesive protein (TRAP) are responsible for the gliding motility and infectivity of the sporozoite [3, 4]. Similarly, the merozoite surface protein 1 (MSP1) is involved in the invasion of the merozoite into the erythrocyte, and antibodies targeting MSP119 have been found to inhibit merozoite invasion of erythrocytes in humans [5].
Many malaria vaccine candidates have therefore aimed to target the pre-erythrocytic antigens to prevent hepatocyte infection and the erythrocytic antigens to prevent clinical manifestations. Radiation-attenuated sporozoites have been used to produce sterilizing immunity, but this method remains impractical for widespread use due to logistical constraints [2]. Furthermore, the most advanced malaria vaccine candidate, RTS,S/AS01, has failed to produce long-lived efficacy [6, 7], likely due to lack of CD8+ T cell responses induced and its design based on a single pre-erythrocytic stage antigen target, CSP [8], as a single sporozoite that evades immune responses induced against CSP can produce tens of thousands of blood stage merozoites [2, 9, 10]. Moreover, preclinical murine studies on a vaccine candidate based on two pre-erythrocytic-stage antigens, CSP and the thrombospondin-related adhesive protein (TRAP), have not shown increased efficacy compared to single antigen vaccines [11]. Therefore, we hypothesize that a malaria vaccine targeting multiple Plasmodium stages is necessary for optimal induction of protective immunity.
We have designed P. yoelii chimeric recombinant protein-based vaccines, constructed by binding of cognate promiscuous T cell epitopes (i.e. capable of binding to 10 or more MHC class II molecules) to well characterized B-cell epitopes, representing CSP and the erythrocytic-stage antigen merozoite surface protein 1 (MSP1) [12, 13]. We also have expressed a hybrid protein by genetic fusion of the chimeric CSP and MSP-1 proteins [14], designated P. yoelii Chimeric Multistage Protein (PyCMP). This vaccine protected mice from experimental challenge through induction of CD4+ T cells and antibodies [14]. However, the lack of induction of protective CD8+ T cells led us to pursue an adenovirus-vectored malaria vaccine, and we reported that an Ad5 prime and two proteins boosts significantly increased the PyCMP protective effect [15].
Despite the relevance of the Ad5-based vector as promising vaccine platform, adult populations exhibit a high prevalence of pre-existing anti-Ad5 neutralizing antibodies, limiting the effectiveness [16, 17]. Simian adenoviruses provide a promising alternative, as they maintain the same safety profile as Ad5 [16, 18] and the level of anti-vector neutralizing activity of human sera has been found to be low [19]. In addition, the use of simian adenoviruses in Ebola Virus [20, 21], HIV [18], HCV [22], and malaria [23–26] vaccine candidates provides further support for the safety and utility of these vectors.
Here we evaluated the immunogenicity and protective efficacy of a heterologous Ad prime – protein boost vaccination regimen, testing three different doses of the simian adenovirus 36 (SAd36), a vector resistant to neutralizing anti-Ad5 antibodies [19]. This vector was engineered to express the synthetic PyCMP gene. We show that immunization regimens including SAd36PyCMP improves immunogenicity and efficacy in comparison to Ad5 vectored PyCMP, making SAd36 a promising vector for the development of an effective malaria vaccine.
2. Materials and Methods
2.1 Viral Vectors
The replication incompetent Ad5PyCMP vector was constructed using the E1-deleted Ad5 backbone as we previously described [15, 27]. To construct the genome of simian adenovirus 36 SAdV-36 from species E containing the PyCMP transgene cassette in place of the deleted E1A/B genes we used the strategy originally described by Roy et al. [19]. We employed the E1-deleted molecular clone pC36.000.CMV.PI.EGFP.BGH (p1411) of an SAd36 vector expressing eGFP and a pShuttle plasmid that were kindly provided by Dr. James M. Wilson (Penn Vector Core – Gene Therapy Program, University of Pennsylvania). The PyCMP-coding sequence was cloned into the pShuttle plasmid between the CMV promoter and BGH polyadenylation signal. The expression cassette was excised with I-CeuI and PI-SceI restriction enzymes and ligated to plasmid DNA containing the SAd36 genome, which was linearized using unique I-CeuI and PI-SceI restriction sites introduced in place of E1 region. The ligated DNA was transformed into E. coli strain, XL10-Gold (Stratagene), to select the plasmid containing viral genome carrying the CMV-driven PyCMP transgene. The constructed genome was released from plasmid DNA by digestion with PacI and transfected into HEK293 cells to rescue the replication incompetent SAd36PyCMP vector. Both SAd36PyCMP and SAd36-GFP vectors were upscaled in HEK293 cells and then purified using double cesium chloride gradient centrifugation as described [28]. The purified vector preparations were dialyzed against PBS containing 10% glycerol, and viral particle (vp) titers were determined based on absorbance at 260 nm as described by Maizel et al. [29].
2.2 Chimeric Vaccine, Immunization Regimens, and Parasites
The PyCMP gene is a 1242bp gene encoding a chimeric antigen based on the P. yoelii CSP genetically linked to a chimeric P. yoelii MSP1. The transgene expression and purification have been previously described [14, 30].
Female CB6F1/J (H-2d/b) mice, 6 to 8 weeks of age, were purchased from Jackson Laboratory. This strain was selected based on the response of syngeneic mice to chimeric antigens [31] and to characterize T cells response via the H-2Kd/SYVPSAEQI/APC tetramer (Tetramer Core Facility, Emory University) which includes the CTL epitope of the P. yoelii CSP included in PyCMP. Mice (n= 10 per group) were primed intramuscularly at day 0 with recombinant SAd36 at a dose of 106 (Low dose), 107 (Intermediate dose), or 1010 v.p. (High dose), or the previously protective recombinant Ad5 107 v.p. dose [15, 30]. All mice received two boosting immunizations with 20 μg of PyCMP emulsified in Montanide ISA 51 (Seppic, Fairfield, NJ) at days 30 and 60. Naïve mice (n=10) were used as a control (Table 1). Mice were bled 20 days after each immunization for assessment of antibody titers via ELISA. PBMCs were obtained from mouse whole blood samples at days 10, 20, 40, 50, and 70 post-priming and were processed for flow cytometry.
Table 1. Immunization Groups.
Mice were immunized intramuscularly at day 0 with adenovirus in PBS at the dose corresponding to their group. Mice were then boosted subcutaneously at days 30 and 60 with 20 μg of the P. yoelii chimeric multistage protein (PyCMP) emulsified in Montanide ISA 51 VG in a 1:1 volume ratio. Control group mice received no immunizations. (n = 10 per group). Mice were bled for analysis of antibody titers via ELISA 20 days after each immunization. PBMCs were obtained from mouse whole blood samples at days 10, 20, 40, 50, and 70 post priming and were processed for flow cytometry.
| Immunization Group | Priming, Day 0 | Protein Boost 1, Day 30 | Protein Boost 2, Day 60 | |
|---|---|---|---|---|
| Ad-transgene | Dose | |||
| SAd36 106 | SAd36-PyCMP | 106 v.p. | PyCMP | PyCMP |
|
| ||||
| SAd36 107 | SAd36-PyCMP | 107 v.p. | PyCMP | PyCMP |
|
| ||||
| SAd36 1010 | SAd36-PyCMP | 1010 v.p. | PyCMP | PyCMP |
|
| ||||
| Ad5 107 | Ad5-PyCMP | 107 v.p. | PyCMP | PyCMP |
|
| ||||
| Control | No immunization | No immunization | No immunization | |
Anopheles stephensi P. yoelii 17XNL infected mosquitoes were obtained from the NYU School of Medicine insectary core facility. At day 80 after the priming immunization, experimental challenges were done intravenously using 100 freshly isolated sporozoites. Follow-up of parasitemia and hemoglobin (Hb) was performed as previously reported [14]. Hemoglobin levels below 5 g/dl were considered severe anemia and animals with this condition were euthanized. All procedures were approved by Emory University’s IACUC and followed accordingly.
2.3 ELISA assays
The antibodies elicited by immunization with the hybrid protein and their IgG isotype profiles were determined by ELISA as described [13].The avidity of antibodies was assessed by a thiocyanate elution ELISA as described previously [30, 32, 33] and calculated as described by Perciani et al. [34].
2.4 Flow cytometry assays
To measure PyCMP-specific CD8+ and CD4+ T cells, peripheral blood was collected into 3.7% sodium citrate tubes at days 10, 20, 40, 50, and 70 post priming. Erythrocytes were lysed with ACK buffer (Life Technologies). After washing, the cells were incubated with LIVE/DEAD fixable yellow stain (Life Technologies) per the manufacturer’s protocol. PBMCs were then incubated with the following antibodies for 30 minutes for analysis by flow cytometry: α-CD3ε-PerCP, α-CD4-Alexa Fluor 700 (eBioscience), α-CD11a-PerCP-Cy5.5, α-CD49d-FITC, α-CD8-APC-Cy7, and the H-2Kd/SYVPSAEQI/APC tetramer. All antibodies were from Biolegend unless noted otherwise. The cells were initially gated on SSC/FSC, and the frequency of tetramer-positive cells was determined on the gated CD11α+CD8+ population. The co-expression of CD11a and CD49d were used to identify antigen-specific effector CD4+ T cells as previously described (Supplementary Figure 1) [35].
Cellular immune responses in the spleen were measured by ICS to simultaneously analyze IL-2, IFN-γ, and TNF-α at the single-cell level in T cells 5 days after the final boosting immunization as described [30]. Cells were stimulated with either PyCMP or one of three peptide pools of 15-mer synthetic peptides overlapped by 10 amino acids peptides representing PyCMP. Pool 1 contained 14 peptides representing the PyCSP chimeric antigen [12]; pool 2 contained 24 peptides representing the MSP1 derived promiscuous T-cell epitopes, and pool 3 contained 20 peptides representing the PyMSP119 sequence [14]. Cells were stimulated for 6 hours with the protein or pools at 2 μg/ml at 37°C in the presence of GolgiPlug (BD Biosciences). Cells were then incubated for 15 min with CD16/CD32 before surface staining for 30 min with α-CD3ε-PerCP-Cy5.5, α-CD8α-BV605, and α-CD4-Pacific Blue. Permeabilized cells were then stained intracellularly for 30 min with α-IFN-γ-FITC, α-IL-2-APC, and α-TNF-α-PE. Flow cytometry acquisition was performed using an LSRII (BD). Data were analyzed using FlowJo V10. The leukocytes were initially gated on CD3+CD4+ and CD3+CD8+; then antigen-specific cytokine-secreting T cells were identified (Supplementary Figure 2). The frequency of antigen-specific cytokine-producing cells was determined by subtracting the percentage of cytokine-producing T cells after incubation with medium alone from the percentage of cytokine-producing T cells after incubation with PyCMP or peptide pools. A threshold for a positive cytokine response was set above the background, and samples that did not meet this requirement were set to zero. Analyses of multifunctional T cell responses were performed using a Boolean analysis in FlowJo and SPICE software [36].
2.5 Statistics
Statistical analysis and graphs were made using GraphPad Prism software. Antibody responses and tetramer staining data were log-transformed to achieve normality, permitting parametric testing and comparison using one-way ANOVA with Bonferroni’s post-test. For cytokine-secreting cells, Kruskal-Wallis test with Dunns post-test was used. In experimental challenges, parasitemia differences between groups were evaluated by comparing areas under the curve of parasitemia versus time and parasitemia peak values using Kruskal-Wallis test with Dunns post-test.
3. Results
3.1 Infectivity of Simian Adenovirus 36 compared to Ad5
SAd36 exhibits characteristics that make it desirable as a vector beyond its resistance to anti-Ad5 antibodies [19]. The AB loop within the knob domain of the SAd36 fiber, which mediates binding of CAR-binding human Ad serotypes, is identical to human Ad4 (species E) and is very similar to Ad2, Ad5 (species C) and Ad12 (species A) [37]. This suggests that the fiber knob could mediate SAd36 binding to CAR similar to chimpanzee adenovirus CV-68 (species E) [38]. In addition, the SAd36 penton base includes an RGD motif in one of the two surface loops, suggesting that integrins may also play a role in virus internalization giving this vector different infection routes which could potentially make it a more efficient infectious agent than Ad5.
To evaluate the infectivity of SAd36 derivatives with respect to Ad5-based vectors, we tested a generated SAd36-GFP vector along with previously described Ad5-GFP vector using gene transfer assay in human A549 and murine N2A cells. The levels of gene transfer mediated by SAd36-GFP were about 100-fold lower in both human and mouse epithelial cells when compared to the Ad5 vector. (Supplementary Figure 3).
3.2 Immunization with SAd36 Induces Comparable Quantity and Quality of Antibodies as Ad5
We previously determined that heterologous adenoviral prime-protein boost regimens are the most efficacious where the combination of adenovirus and protein is used [15, 30]. We, therefore, used one of three doses of the recombinant simian adenoviral vector at either 106, 107, or 1010 v.p for priming to determine the optimal dose to induce protective efficacy. Mice primed with recombinant Ad5 at 107 v.p., as well as mice receiving no immunizations, were used as controls (Table 1). Following an intramuscular adenoviral prime at day 0, mice were boosted with 20 μg of the PyCMP protein emulsified in a 1:1 volume ratio of Montanide 51 ISA VG adjuvant subcutaneously at days 30 and 60 post priming.
To determine the effect of SAd36 prime-PyCMP protein boost regimens on antibody responses and the impact of the dosage, antibody titers were measured by ELISA against the PyCMP protein. Antibody titers determined from sera obtained at day 20 post prime revealed that immunization regimens that included a priming immunization with SAd36 at doses of 106 and 107 v.p. elicited antibody titers comparable to mice primed with 107 v.p. of Ad5 (Figure 1A). In addition, mice primed with SAd36 at 1010 v.p. had significantly higher antibody titers compared to both SAd36 106 and 107 v.p. priming groups (Figure 1A). No significant differences between groups were observed at later time points, and analysis of antibody titers 20 days after the final immunization revealed that all regimens produced over 105 log titers, with the SAd36 106 and 107 v.p. priming doses being the most immunogenic (Figure 1B). Although the SAd36 1010 regimen initially displayed the highest antibody titers at day 20 after the final immunization, by day 80 the antibody titers induced by this regimen were the lowest compared to the SAd36 106 and 107 v.p. regimen and the Ad5 regimen.
Figure 1. SAd36PyCMP elicits comparable antibody responses compared to Ad5PyCMP.
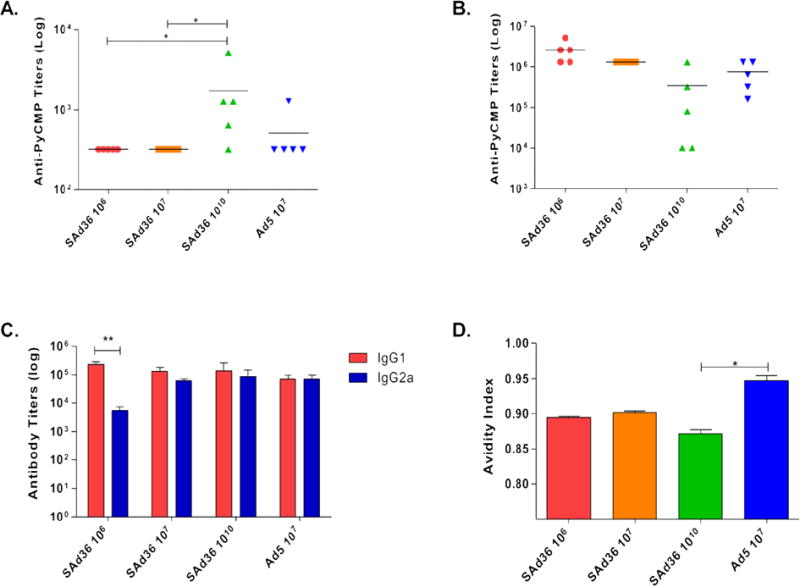
Female CB6F1/J mice (n = 5 per group) were vaccinated according to the regimens described in Table 1. (A) Anti-PyCMP antibody titers measured 20 days after the priming immunization. Each symbol represents the values for an individual mouse. The horizontal lines represent the arithmetic mean for each group. Statistical analysis was conducted using Kruskal-Wallis and Dunn’s post-test, *p<0.05. (B) Anti-PyCMP antibody titers measured 20 days after the final immunization. (C) Comparative titers of IgG1 and IgG2a antibody subclasses induced by each immunization regimen measured 20 days after the final immunization. Endpoint ELISA titers were measured as the highest dilution of sera that resulted in an optical density of 0.5 (OD0.5) and were determined using the recombinant protein PyCMP and serum from mice immunized with Ad5 or SAd36. (D) Avidity of antibodies induced by the different vaccinated groups calculated as defined by Perciani, et al. [34]. Statistical analysis was conducted using Kruskal-Wallis and Dunn’s post-test, *p<0.05.
Since cytophilic antibody subclasses directed against MSP119 [39] and CSP [40] have been found to be correlated with protection against Plasmodium infection, we assessed the distribution of anti-PyCMP IgG1 and IgG2a induced by the immunization regimens. This approach also allows us to indirectly assess Th1 and Th2 responses as cytokines produced by Th1 cells induce antibody class switching to produce cytophilic antibodies. When titers of IgG1 and IgG2a subclasses were evaluated by ELISA 20 days after the final immunization, we observed no significant difference between IgG1 and IgG2a anti-PyCMP antibody titers within the SAd36 107, SAd36 1010 or Ad5 107 regimens indicating a more balanced induction of Th1/Th2 response (Figure 1C). However, we observed significantly higher IgG1 response in mice immunized with the SAd36 106 regimen. When we assessed the differences in the IgG1 or the IgG2a response between the groups, we observed no significant differences suggesting that the Ad5 and SAd36 vectors elicit a similar subclass profile.
Antibody responses against PyCMP were further characterized by assessment of antibody avidity using sodium thiocyanate antibody displacement ELISAs 20 days after the final immunization. All immunization regimens induced antibodies with a mean avidity over 0.85, with the Ad5 107 immunization group producing antibodies with the highest index. The only statistically significance difference observed was the higher antibody avidity of the Ad5 when compared to SAd36 at 1010 v.p. (Figure 1D).
3.3 Immunization with SAd36 is Capable of Inducing High Levels of CSP-Specific CD8+ T cells
To determine if the SAd36 regimens could elicit PyCMP specific-CD8+ T cells, the frequency of tetramer-reactive CD8+ T cell in peripheral blood was compared. All immunization regimens induced robust CD8+ T cell responses (Figure 2A). By day 40, the mice immunized with Ad5 107 v.p. had the lowest numbers of PyCMP specific CD8+ T cells compared to the mice immunized with SAd36 at any dose, with the frequency of tetramer-positive CD8+ T cells induced by the Ad5 107 regimen at day 40 being significantly lower than that induced by SAd36 1010 (p < 0.05). Although not significant at later time points, lower frequencies of antigen-specific CD8+ T cells induced by the Ad5 107 regimen were observed through day 70 (Figure 2C).
Figure 2. Induction of PyCMP-Antigen-Specific T cells by SAd36PyCMP and Ad5PyCMP Immunization.
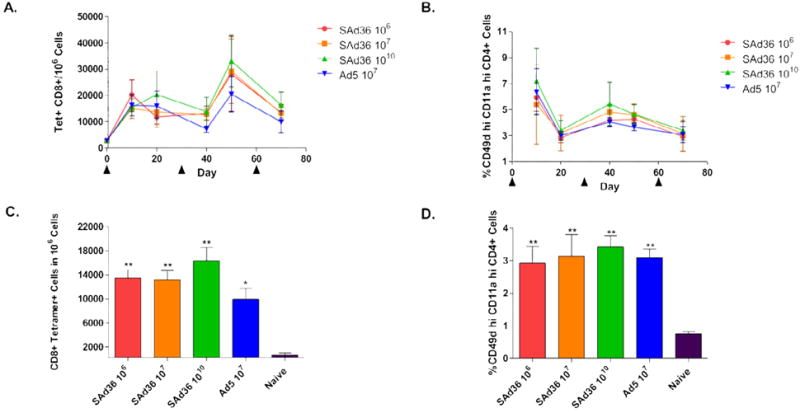
Female CB6F1/J mice (n = 5 per group) were immunized according to the regimens described in Table 1. PBMCs were obtained from mouse whole blood samples at days 10, 20, 40, 50, and 70-post priming and were processed for flow cytometry. (A) Kinetics of CD8+ T cells capable of recognizing the H-2Kd/SYVPSAEQI/APC tetramer induced over the course of the immunization regimen. (B) Kinetics of the percentage of CD4+ T cells expressing high levels of CD11a and CD49d, indicating antigen-experienced cells, in the course of the immunizations. (C) Average number of tetramer-specific CD8+ T cells induced by each immunization group were determined ten days after the final immunization (day 70). (D) Percentage of activated CD4+ T cells, determined by high levels of expression of CD49d and CD11a, at day 70, ten days after the final immunization. Results are presented as the percentages of positive cells per 106 PBMCs. Statistical analysis was conducted by Kruskal-Wallis test with Dunns post-test, statistically significant differences are denoted by *(p < 0.05) and ** (p < 0.01).
When the CD4+ T cell response was analyzed, the highest level of antigen-experienced CD4+ T cells was observed after priming and the first boosting (Figure 2B). No significant differences were observed at any time point, with all the SAd36 immunization regimens displaying ~3% or more antigen-experienced CD4+ T cells, similar to the levels induced by the Ad5 regimen (Figure 2B and D). Nonetheless, since this response includes both CD4+ T cells induced by the transgene product and the vector, the results demonstrate a higher induction of CD4+ T cells by SAd36 but not necessarily transgene-specific.
3.4 Multi-functionality of T cells induced by Immunization
To assess CD4+ and CD8+ T cell functionality, their ability to produce the cytokines IFN-γ, IL-2, and TNF-α was measured 5 days after the final immunization. When compared to other immunization regimens, SAd36 at 1010 v.p. produced a significantly higher proportion of triple cytokine producing CD8+ T cells (Figure 3A). In addition, the SAd36 1010 v.p. regimen also induced a significantly higher proportion of single producing IL-2 CD4+ T cells and the highest percentage of IFN- γ and TNF-α double producing, and IFN-γ single-producing CD8+ T cells compared to all other regimens (Figure 3A).
Figure 3. Cytokine production by CD8+ and CD4+ T cells stimulated ex vivo with the PyCMP protein.
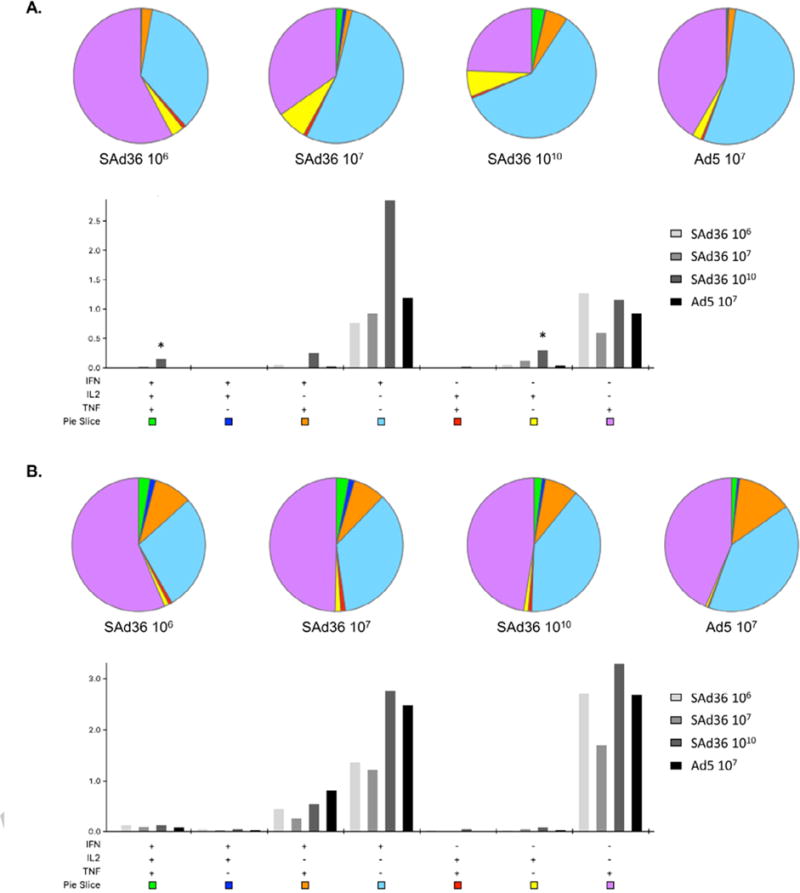
Female CB6F1/J mice (n = 5 per group) were vaccinated according to the regimens described in Table 1. (A) Pie Charts: Percentage of multifunctional and single cytokine producing CD8+ T cells following ex vivo stimulation with PyCMP protein at day 70 post priming. Bar Graph: Percentage of total CD8+ T cells capable of producing one, two, or three cytokines following ex vivo stimulation with PyCMP protein. Results are presented as the percentages of positive cells per 106 CD8+ T cells. (B) Pie Charts: Percentage of multifunctional and single cytokine producing CD4+ T cells following ex vivo stimulation with PyCMP protein at day 70 post priming. Bar Graph: Percentage of total CD4+ T cells capable of producing one, two, or three cytokines following ex vivo stimulation with PyCMP protein. Results are presented as the percentages of positive cells per 106 CD4+ T cells. Graphs were produced and data was analyzed using SPICE software [36].
When the multi-functionality of CD4+ T cells was assessed, the SAd36 1010 regimen induced the most triple cytokine producing CD4+ T cells compared to all other regimens, as well as the most IFN-γ single producers followed closely by the Ad5 regimen. Furthermore, multi-functionality of CD4+ T cells was observed in all groups (Figure 3B).
3.5 Cytokine Production in Response to PyCMP
The breadth of the cytokine response induced by immunization was assessed in splenocytes obtained 5 days after the final immunization and stimulated with peptide pools. We observed that the SAd36 1010 immunization regimen induced the highest level of IFN-γ producing CD8+ T cells, and this recognition was significantly higher than that of the SAd36 107 regimen for pool 1 (Figure 4A). Furthermore, the SAd36 1010 regimen induced a significantly higher IFN-γ production by CD4+ T cells in response to pool 3 stimulation (PyMSP119) when compared to all other immunization regimens, and higher IFN-γ production of IFN-γ by CD4+ T cells in response to pool 1 when compared to SAd36 107 (Figure 4D).
Figure 4. Cytokine production following ex vivo stimulation with PyCMP peptide pools.
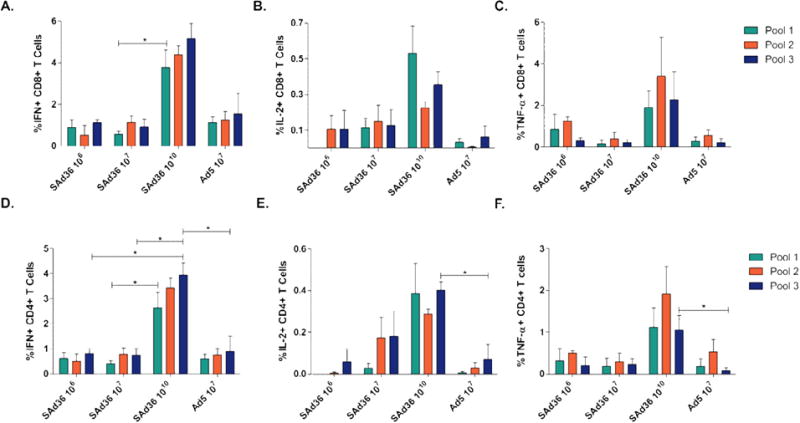
Female CB6F1/J mice (n = 5 per group) were immunized according to the regimens described in Table 1. Splenocytes were obtained five days after the final immunization and incubated with peptide pools containing pools of 15AA overlapping peptides representing PyCMP. Following 6 hours of stimulation, cells were intracellularly stained and processed for flow cytometry. Results are presented after background subtraction. Percentage of CD8+ T cells capable of producing IFN-γ (A), IL-2 (B), and TNF-α (C) after stimulation. Percentage of CD4+ T cells capable of producing IFN-γ (D), IL-2 (E), and TNF-α (F) following stimulation. Statistical analysis was conducted using Kruskal-Wallis test to determine differences between the immunization regimens. Statistically significant differences (p < 0.05) are indicated by a single asterisk.
When the IL-2 and TNF-α response was analyzed, SAd36 1010 regimen immunization also induced the highest IL-2 (Figure 4B and E) and TNF-α (Figure 4C and F) production by CD8+ and CD4+ T cells compared to all other immunization regimens, with a significantly higher CD4+ T cell production of IL-2 and TNF-α in recognition of the Pool 3 when compared to the Ad5 regimen (Figures 4E and 4F).
In addition to differences in cytokine production between the immunization regimens, cytokine production by CD4+ and CD8+ T cells within each immunization regimen was not significantly different in response to the three peptide pools, suggesting that there was no immunodominant epitope within the chimeric protein.
3.6 Protective Efficacy of SAd36 Prime-PyCMP Protein Boost Immunization Regimens
At day 80, mice were challenged intravenously with ~100 freshly isolated P. yoelii sporozoites isolated from Anopheles stephensi mosquitoes. All immunization regimens had significantly reduced parasitemia levels compared to naïve mice when the parasitemia kinetics were expressed as area under the curve (Figure 5A). Although not statistically significant, SAd36 106 and 1010 v.p. regimens were the most protective regarding the reduction of parasitemia and all regimens that included an SAd36 prime displayed a trend for lower parasitemia when compared to Ad5 in comparison to the naïve group (Figure 5A).
Figure 5. SAd36-PyCMP Protective Efficacy.
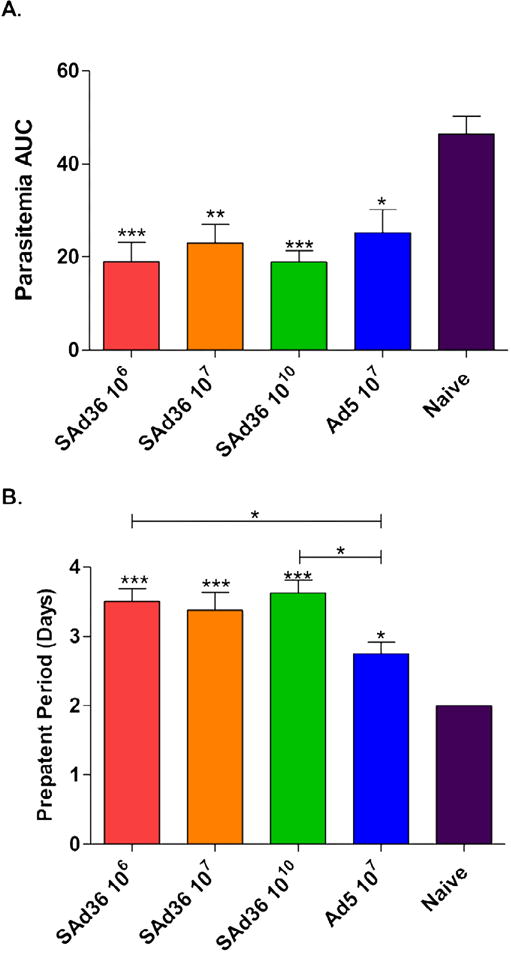
Female CB6F1/J mice (n = 10 per group) were immunized according to the regimens described in Table 1. 30 days after the final immunization mice were challenged with 100 freshly Isolated Plasmodium yoelii sporozoites isolated from Anopheles stephensi. (A) Kinetics of parasitemia expressed as Area Under the Curve (AUC) and (B) the length of the pre-patency period were analyzed by Kruskal-Wallis test with Dunns post-test. Statistically significant differences are denoted by *(p < 0.05), ** (p < 0.01), and ***(p < 0.001).
When pre-patency periods were compared, all immunization regimens showed an increased pre-patency period compared to the naïve group, indicating that all regimens reduced parasite load in the liver and delayed progression to blood stage infection. The SAd36 106 and 1010 immunization regimens displayed significantly longer pre-patency periods when compared to the Ad5 immunization regimen, however the SAd36 107 regimen also displayed a trend towards increased pre-patency period compared to the Ad5 regimen (Figure 5B).
4. Discussion
Both humoral and cellular immune responses are necessary to provide protection against Plasmodium infection [10, 41–43]. To induce an optimal cellular response against Plasmodium, viral vectors have been used to increase the numbers of CD8+ T cells capable of recognizing Plasmodium antigens [15, 24–26, 30, 44, 45]. Adenoviral vectors are easily adaptable to vaccine studies because of their ability to incorporate large transgene inserts, high levels of transgene expression for periods up to one year [46], and their ability to be mass-produced at vaccine quality under good manufacturing practice [47, 48]. The most commonly used adenoviral vector, Ad5, shows an excellent immunogenicity and safety profile. Nonetheless, studies of populations living in malaria endemic areas have revealed that approximately 50% of the adult population has high titers of neutralizing antibodies against this virus [49]. To circumvent the pre-existing immunity to Ad5, while maintaining the many benefits of adenoviral vectors, we tested SAd36 a novel simian adenovirus vector, which is resistant to anti-Ad5 antibodies [19]. We evaluated the relevance of using a recombinant SAd36 to deliver a transgene that includes chimeric P. yoelii CSP, and MSP1 proteins denoted PyCMP [14].
Priming with either SAd36PyCMP and Ad5PyCMP followed by two protein boosts, induced comparable antibody titers, levels of IgG1 and IgG2a subclasses, and similar antibody avidity for the SAd36 106 and 1010 regimens, indicating that SAd36 exerts effects on the humoral immune responses similar to that of the Ad5 vector. Furthermore, all immunization regimens induced antibodies with avidity indices above 0.85. These results are promising as previous reports have cited that antibody avidity above 0.80 is correlated with sterilizing immunity to Plasmodium infection [50].
Despite promising humoral responses, the goal of adenoviral immunization is to induce robust cellular immune responses to the transgene product. Tetramer analysis revealed that SAd36 induces more anti-CSP specific CD8+ T cells compared to Ad5 in response to the CTL epitope included in PyCMP. The lower induction of CD8+ T cells by the Ad5 107 regimen is consistent with previous reports that vaccination with Ad5 leads to T cell exhaustion or anergy [51]. Additionally, both vectors induced comparable levels of CD4+ T cell activation. The ability of simian adenoviral vectors to induce more robust CD8+ T cells responses is a desirable feature for pre-erythrocytic malaria vaccines, as high numbers of antigen-specific CD8+ T cells are necessary for protection [52], while high CD4+ T cells levels provide protection against blood stage parasite challenge in humans [42]. Furthermore, the higher immunogenicity of simian vectors has also been observed by other groups using chimpanzee-derived adenoviruses [25, 53–56], making simian adenoviruses particularly attractive as rare human adenoviruses have shown lower immunogenicity than Ad5 in preclinical trials [49, 57, 58]. However, despite the improved anti-CSP specific CD8+ T cell responses observed, analysis of memory responses following priming immunizations with either SAd36 or Ad5 is required to determine differences in long-term immunogenicity between these vectors.
The use of SAd36 as a vector for malaria vaccination is further supported by the results obtained from assessment of CD4+ and CD8+ T cells functionality in response to adenoviral prime-protein boost immunization regimens. T cell functionality was assessed as the ability of these cells to produce IFN-γ, IL-2, and TNF-α in response to the PyCMP recombinant protein since these cytokines have been associated with protection against malaria infection [2]. We found that immunization regimens incorporating SAd36 induced equivalent, if not higher, levels of IFN-γ from both CD4+ and CD8+ T cells. IFN-γ is involved in promoting more efficient phagocytosis and presentation of foreign peptides by macrophages [2, 59]. Relevantly, the protective effect of IFN-γ production was also observed during the phase IIa clinical trial of RTS,S, as prolonged IFN-γ production by CD4+ and CD8+ T cells was associated with protection [41].
In addition to T cell functionality, the breadth of the immune responses induced by vaccination must be considered since immunodominance of one or a small set of epitopes may promote the immune escape of the parasite over time. When we assessed the breadth of the immune response to three peptide pools representing the length of PyCMP, we found no indication of immunodominance. The lack of immunodominance suggests that the SAd36 vector should be considered for improving the cellular immunogenicity of protein-based malaria vaccine candidates, as immunodominance has been reported by other groups when using Simian Ad vectors and multi-antigen vaccine candidates [11, 25].
The infectious challenge with P. yoelii demonstrated that heterologous Ad prime-protein boost regimens including SAd36 priming provided both liver stage protection by increasing the length of the pre-patency period and blood stage protection by reducing parasitemia levels. This also further confirms the role of heterologous Ad-Protein regimens as one of the best strategies to obtain multistage malaria protection [15, 30]. Based on the similar protective efficacy of the regimens including a high and low dosage of SAd36, we can consider that the lower-dose regimen overcame the negative impact of the low adenoviral dosage on cellular immune responses through the induction of high titers of high avidity antibodies against the PyCMP transgene product, which further suggests that an effective malaria vaccine will depend on both humoral and cellular responses. However, the dosage-effect described here requires further characterization. Furthermore, it is likely that the humoral immunogenicity of SAd36 can be improved with hexon-modifications to present T helper epitopes, as we have recently described [30].
It is also important to note that a lower infectivity of SAd36 compared to Ad5 can explain the capacity of SAd36 to induce protective responses in a wider dose range than Ad5, as we have demonstrated that high Ad5 doses (higher than 1010 v.p.) significantly reduce the protective efficacy of heterologous Ad-regimens, an event mediated by a significant reduction in antigen-specific responses [15].
In conclusion, we have found that SAd36 offers many advantages over the commonly used Ad5, beyond its resistance to anti-Ad5 antibodies, as it shows an improved humoral and cellular immune responses profile when delivered as part of an adenoviral prime-protein boost regimen that correlates with improved protection from infectious challenge in a stringent murine malaria model.
Supplementary Material
Highlights.
SAd36 enhances T cell responses against a chimeric multistage P. yoelii vaccine in comparison to Ad5.
SAd36PyCMP elicits similar antibody titers when compared to Ad5PyCMP.
SAd36PyCMP reduced parasitemia during P. yoelii challenge when compared to Ad5PyCMP.
Acknowledgments
The authors would like to thank Dr. James Wilson for providing the SAd36 vector, and Monica Cabrera-Mora for technical support with flow cytometry and experimental challenge.
Funding Sources
This research was supported by the National Institutes of Health, NIAID grants R56-AI103382-01A1, R21-AI095718-01, and R21-AI117459-01, and the Yerkes National Primate Research Center Base Grant No RR00165 awarded by the National Center for Research Resources of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or the materials discussed in the manuscript.
References
- 1.World Health Organization. World malaria report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 2.Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, et al. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol. 2014;32:157–87. doi: 10.1146/annurev-immunol-032713-120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menard R. Gliding motility and cell invasion by Apicomplexa: insights from the Plasmodium sporozoite. Cell Microbiol. 2001;3:63–73. doi: 10.1046/j.1462-5822.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- 4.Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, Nussenzweig V, et al. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell. 1997;90:511–22. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 5.Dodoo D, Aikins A, Kusi KA, Lamptey H, Remarque E, Milligan P, et al. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malaria journal. 2008;7:142. doi: 10.1186/1475-2875-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. The New England journal of medicine. 2011;365:1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 7.Bejon P, White MT, Olotu A, Bojang K, Lusingu JPA, Salim N, et al. Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis. 2013;13:319–27. doi: 10.1016/S1473-3099(13)70005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malaria journal. 2009;8:312. doi: 10.1186/1475-2875-8-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertolino P, Bowen DG. Malaria and the liver: immunological hide-and-seek or subversion of immunity from within? Front Microbiol. 2015;6:41. doi: 10.3389/fmicb.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, et al. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PloS one. 2013;8:e61395. doi: 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauza K, Atcheson E, Malinauskas T, Blagborough AM, Reyes-Sandoval A. Tailoring a Combination Preerythrocytic Malaria Vaccine. Infection and immunity. 2015;84:622–34. doi: 10.1128/IAI.01063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva-Flannery LM, Cabrera-Mora M, Jiang J, Moreno A. Recombinant peptide replicates immunogenicity of synthetic linear peptide chimera for use as pre-erythrocytic stage malaria vaccine. Microbes and infection / Institut Pasteur. 2009;11:83–91. doi: 10.1016/j.micinf.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh B, Cabrera-Mora M, Jiang J, Galinski M, Moreno A. Genetic linkage of autologous T cell epitopes in a chimeric recombinant construct improves anti-parasite and anti-disease protective effect of a malaria vaccine candidate. Vaccine. 2010;28:2580–92. doi: 10.1016/j.vaccine.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh B, Cabrera-Mora M, Jiang J, Moreno A. A hybrid multistage protein vaccine induces protective immunity against murine malaria. Infection and immunity. 2012;80:1491–501. doi: 10.1128/IAI.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabrera-Mora M, Fonseca JA, Singh B, Zhao C, Makarova N, Dmitriev I, et al. A Recombinant Chimeric Ad5/3 Vector Expressing a Multistage Plasmodium Antigen Induces Protective Immunity in Mice Using Heterologous Prime-Boost Immunization Regimens. Journal of immunology. 2016;197:2748–61. doi: 10.4049/jimmunol.1501926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy S, Zhi Y, Kobinger GP, Figueredo J, Calcedo R, Miller JR, et al. Generation of an adenoviral vaccine vector based on simian adenovirus 21. The Journal of general virology. 2006;87:2477–85. doi: 10.1099/vir.0.81989-0. [DOI] [PubMed] [Google Scholar]
- 17.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–83. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 18.Hayton EJ, Rose A, Ibrahimsa U, Del Sorbo M, Capone S, Crook A, et al. Safety and tolerability of conserved region vaccines vectored by plasmid DNA, simian adenovirus and modified vaccinia virus ankara administered to human immunodeficiency virus type 1-uninfected adults in a randomized, single-blind phase I trial. PloS one. 2014;9:e101591. doi: 10.1371/journal.pone.0101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy S, Medina-Jaszek A, Wilson MJ, Sandhu A, Calcedo R, Lin J, et al. Creation of a panel of vectors based on ape adenovirus isolates. J Gene Med. 2011;13:17–25. doi: 10.1002/jgm.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobinger GP, Feldmann H, Zhi Y, Schumer G, Gao G, Feldmann F, et al. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology. 2006;346:394–401. doi: 10.1016/j.virol.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 21.Ledgerwood JE, DeZure AD, Stanley DA, Novik L, Enama ME, Berkowitz NM, et al. Chimpanzee Adenovirus Vector Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2014 doi: 10.1056/NEJMc1505499. [DOI] [PubMed] [Google Scholar]
- 22.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Science translational medicine. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, et al. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infection and immunity. 2010;78:145–53. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehy SH, Duncan CJ, Elias SC, Biswas S, Collins KA, O’Hara GA, et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PloS one. 2012;7:e31208. doi: 10.1371/journal.pone.0031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheehy SH, Duncan CJ, Elias SC, Choudhary P, Biswas S, Halstead FD, et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol Ther. 2012;20:2355–68. doi: 10.1038/mt.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehy SH, Duncan CJ, Elias SC, Collins KA, Ewer KJ, Spencer AJ, et al. Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:2269–76. doi: 10.1038/mt.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seki T, Dmitriev I, Kashentseva E, Takayama K, Rots M, Suzuki K, et al. Artificial extension of the adenovirus fiber shaft inhibits infectivity in coxsackievirus and adenovirus receptor-positive cell lines. J Virol. 2002;76:1100–8. doi: 10.1128/JVI.76.3.1100-1108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashentseva EA, Douglas JT, Zinn KR, Curiel DT, Dmitriev IP. Targeting of adenovirus serotype 5 pseudotyped with short fiber from serotype 41 to c-erbB2-positive cells using bispecific single-chain diabody. J Mol Biol. 2009;388:443–61. doi: 10.1016/j.jmb.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maizel JV, Jr, White DO, Scharff MD. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–25. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca JA, Cabrera-Mora M, Kashentseva EA, Villegas JP, Fernandez A, Van Pelt A, et al. A Plasmodium Promiscuous T Cell Epitope Delivered within the Ad5 Hexon Protein Enhances the Protective Efficacy of a Protein Based Malaria Vaccine. PloS one. 2016;11:e0154819. doi: 10.1371/journal.pone.0154819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caro-Aguilar I, Lapp S, Pohl J, Galinski MR, Moreno A. Chimeric epitopes delivered by polymeric synthetic linear peptides induce protective immunity to malaria. Microbes Infect. 2005;7:1324–37. doi: 10.1016/j.micinf.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Caro-Aguilar I, Rodriguez A, Calvo-Calle JM, Guzman F, De la Vega P, Patarroyo ME, et al. Plasmodium vivax promiscuous T-helper epitopes defined and evaluated as linear peptide chimera immunogens. Infect Immun. 2002;70:3479–92. doi: 10.1128/IAI.70.7.3479-3492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira MU, Katzin AM. The assessment of antibody affinity distribution by thiocyanate elution: a simple dose-response approach. Journal of immunological methods. 1995;187:297–305. doi: 10.1016/0022-1759(95)00186-4. [DOI] [PubMed] [Google Scholar]
- 34.Perciani CT, Peixoto PS, Dias WO, Kubrusly FS, Tanizaki MM. Improved method to calculate the antibody avidity index. J Clin Lab Anal. 2007;21:201–6. doi: 10.1002/jcla.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nature immunology. 2012;13:188–95. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bewley MC, Springer K, Zhang YB, Freimuth P, Flanagan JM. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–83. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 38.Cohen CJ, Xiang ZQ, Gao GP, Ertl HC, Wilson JM, Bergelson JM. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J Gen Virol. 2002;83:151–5. doi: 10.1099/0022-1317-83-1-151. [DOI] [PubMed] [Google Scholar]
- 39.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infection and immunity. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwenk R, DeBot M, Porter M, Nikki J, Rein L, Spaccapelo R, et al. IgG2 antibodies against a clinical grade Plasmodium falciparum CSP vaccine antigen associate with protection against transgenic sporozoite challenge in mice. PloS one. 2014;9:e111020. doi: 10.1371/journal.pone.0111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, et al. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. Journal of immunology. 2003;171:6961–7. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 42.Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360:610–7. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 43.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–77. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 44.Sheehy SH, Duncan CJA, Elias SC, Collins KA, Ewer KJ, Spencer AJ, et al. Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:2269–76. doi: 10.1038/mt.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuldt NJ, Amalfitano A. Malaria vaccines: focus on adenovirus based vectors. Vaccine. 2012;30:5191–8. doi: 10.1016/j.vaccine.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 46.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–23. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butterfield LH, Comin-Anduix B, Vujanovic L, Lee Y, Dissette VB, Yang JQ, et al. Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. J Immunother. 2008;31:294–309. doi: 10.1097/CJI.0b013e31816a8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santosuosso M, McCormick S, Xing Z. Adenoviral vectors for mucosal vaccination against infectious diseases. Viral Immunol. 2005;18:283–91. doi: 10.1089/vim.2005.18.283. [DOI] [PubMed] [Google Scholar]
- 49.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. Journal of virology. 2007;81:4654–63. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter MD, Nicki J, Pool CD, DeBot M, Illam RM, Brando C, et al. Transgenic parasites stably expressing full-length Plasmodium falciparum circumsporozoite protein as a model for vaccine down-selection in mice using sterile protection as an endpoint. Clin Vaccine Immunol. 2013;20:803–10. doi: 10.1128/CVI.00066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penaloza-MacMaster P, Provine NM, Ra J, Borducchi EN, McNally A, Simmons NL, et al. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J Virol. 2013;87:1373–84. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Overstreet MG, Cockburn IA, Chen YC, Zavala F. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an 'unnatural' immune response. Immunol Rev. 2008;225:272–83. doi: 10.1111/j.1600-065X.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. The Journal of infectious diseases. 2015;211:1076–86. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Douglas AD, de Cassan SC, Dicks MD, Gilbert SC, Hill AV, Draper SJ. Tailoring subunit vaccine immunogenicity: maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine. 2010;28:7167–78. doi: 10.1016/j.vaccine.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, et al. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infection and immunity. 2010;78:145–53. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antrobus RD, Coughlan L, Berthoud TK, Dicks MD, Hill AV, Lambe T, et al. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22:668–74. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. Journal of virology. 2003;77:8263–71. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shott JP, McGrath SM, Pau MG, Custers JH, Ophorst O, Demoitie MA, et al. Adenovirus 5 and 35 vectors expressing Plasmodium falciparum circumsporozoite surface protein elicit potent antigen-specific cellular IFN-gamma and antibody responses in mice. Vaccine. 2008;26:2818–23. doi: 10.1016/j.vaccine.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 59.McCall MB, Sauerwein RW. Interferon-gamma–central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol. 2010;88:1131–43. doi: 10.1189/jlb.0310137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


