Abstract
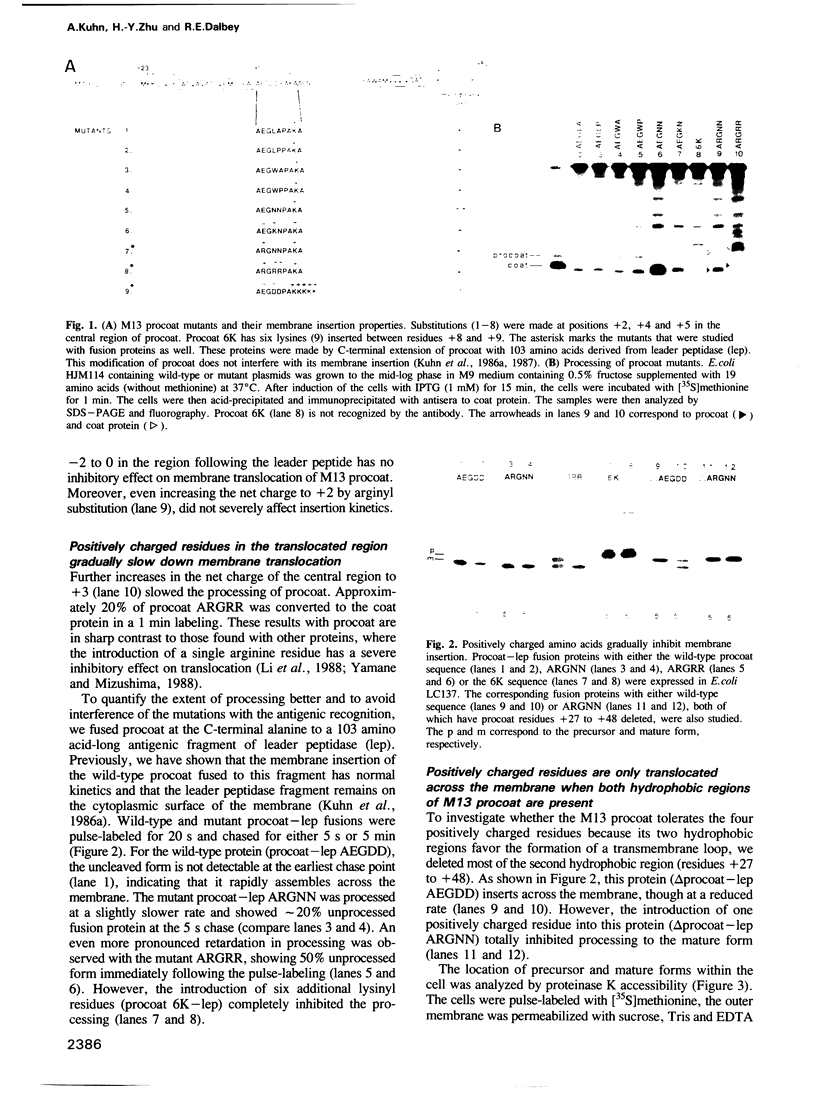
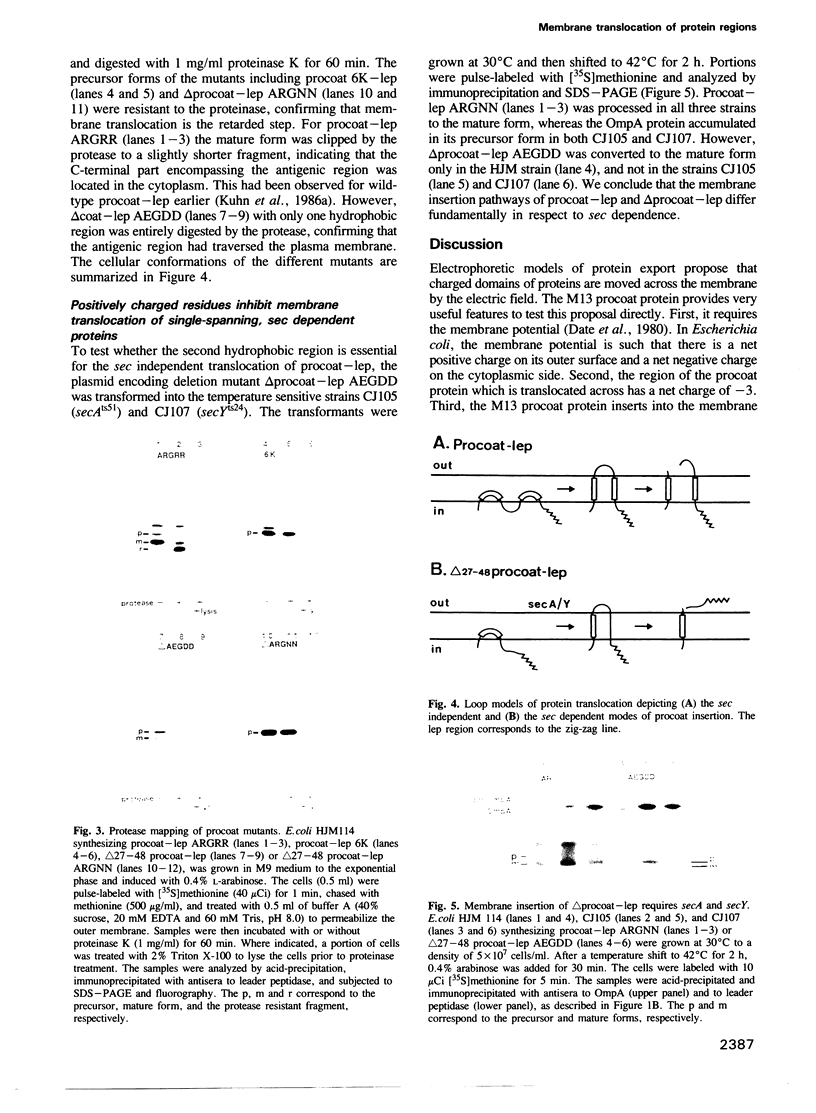
The coat protein of bacteriophage M13 is inserted into the Escherichia coli plasma membrane as a precursor protein, termed procoat, with a typical leader peptide of 23 amino acid residues. Its membrane insertion requires the electrochemical potential but not the cellular components SecA and SecY. Since the electrochemical gradients result in the periplasmic side of the membrane being positively charged, the membrane potential could contribute to the transfer of the negatively charged central region of procoat across the membrane. Here we demonstrate that the central domain following the leader peptide can be translocated across the membrane even when the net charge of the region is changed from -3 to +3. This rules out an electrophoresis-like insertion mechanism for procoat. We also show that the sec independence of procoat insertion is linked to the presence of the second apolar domain. The deletion of most of the second apolar domain from a procoat fusion protein results in sec dependent membrane insertion of the hybrid protein. Moreover, like other proteins that require the sec genes, translocation of this sec dependent procoat protein is inhibited when positively charged residues are introduced after the leader peptide. Loop models involving one or two hydrophobic regions are presented that account for the differences in tolerance of positively charged residues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Hidaka H. Vasodilatory action of HA1004 [N-(2-guanidinoethyl)-5-isoquinolinesulfonamide], a novel calcium antagonist with no effect on cardiac function. J Pharmacol Exp Ther. 1984 Oct;231(1):141–145. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Aksamit R. R., Unson C. G., Goldsmith P., Spiegel A. M., Milligan G. Immunochemical and electrophoretic characterization of the major pertussis toxin substrate of the RAW264 macrophage cell line. Biochemistry. 1988 Mar 22;27(6):2040–2046. doi: 10.1021/bi00406a034. [DOI] [PubMed] [Google Scholar]
- Becker S., Warren M. K., Haskill S. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J Immunol. 1987 Dec 1;139(11):3703–3709. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Cuatrecasas P. Phorbol esters rapidly stimulate amiloride-sensitive Na+/H+ exchange in a human leukemic cell line. J Cell Biol. 1984 Jul;99(1 Pt 1):340–343. doi: 10.1083/jcb.99.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Jelsema C., Axelrod J. Cholera toxin and pertussis toxin stimulate prostaglandin E2 synthesis in a murine macrophage cell line. J Pharmacol Exp Ther. 1988 Feb;244(2):765–773. [PubMed] [Google Scholar]
- Casey P. J., Gilman A. G. G protein involvement in receptor-effector coupling. J Biol Chem. 1988 Feb 25;263(6):2577–2580. [PubMed] [Google Scholar]
- Church J. G., Buick R. N. G-protein-mediated epidermal growth factor signal transduction in a human breast cancer cell line. Evidence for two intracellular pathways distinguishable by pertussis toxin. J Biol Chem. 1988 Mar 25;263(9):4242–4246. [PubMed] [Google Scholar]
- Clark M. A., Littlejohn D., Conway T. M., Mong S., Steiner S., Crooke S. T. Leukotriene D4 treatment of bovine aortic endothelial cells and murine smooth muscle cells in culture results in an increase in phospholipase A2 activity. J Biol Chem. 1986 Aug 15;261(23):10713–10718. [PubMed] [Google Scholar]
- Daniel T. O., Ives H. E. Cyclosporin A inhibits kinase C-independent activation of the Na+/H+ exchanger by PDGF and vanadate. Biochem Biophys Res Commun. 1987 May 29;145(1):111–117. doi: 10.1016/0006-291x(87)91294-0. [DOI] [PubMed] [Google Scholar]
- Didsbury J. R., Ho Y. S., Snyderman R. Human Gi protein alpha-subunit: deduction of amino acid structure from a cloned cDNA. FEBS Lett. 1987 Jan 26;211(2):160–164. doi: 10.1016/0014-5793(87)81428-x. [DOI] [PubMed] [Google Scholar]
- Didsbury J. R., Snyderman R. Molecular cloning of a new human G protein. Evidence for two Gi alpha-like protein families. FEBS Lett. 1987 Jul 13;219(1):259–263. doi: 10.1016/0014-5793(87)81228-0. [DOI] [PubMed] [Google Scholar]
- Downing J. R., Rettenmier C. W., Sherr C. J. Ligand-induced tyrosine kinase activity of the colony-stimulating factor 1 receptor in a murine macrophage cell line. Mol Cell Biol. 1988 Apr;8(4):1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hamilton J. A., Vairo G., Lingelbach S. R. Activation and proliferation signals in murine macrophages: stimulation of glucose uptake by hemopoietic growth factors and other agents. J Cell Physiol. 1988 Mar;134(3):405–412. doi: 10.1002/jcp.1041340311. [DOI] [PubMed] [Google Scholar]
- He Y. X., Hewlett E., Temeles D., Quesenberry P. Inhibition of interleukin 3 and colony-stimulating factor 1-stimulated marrow cell proliferation by pertussis toxin. Blood. 1988 May;71(5):1187–1195. [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Horiguchi J., Sariban E., Kufe D. Transcriptional and posttranscriptional regulation of CSF-1 gene expression in human monocytes. Mol Cell Biol. 1988 Sep;8(9):3951–3954. doi: 10.1128/mcb.8.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi J., Warren M. K., Kufe D. Expression of the macrophage-specific colony-stimulating factor in human monocytes treated with granulocyte-macrophage colony-stimulating factor. Blood. 1987 Apr;69(4):1259–1261. [PubMed] [Google Scholar]
- Imamura K., Kufe D. Colony-stimulating factor 1-induced Na+ influx into human monocytes involves activation of a pertussis toxin-sensitive GTP-binding protein. J Biol Chem. 1988 Oct 5;263(28):14093–14098. [PubMed] [Google Scholar]
- Irving H. R., Exton J. H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987 Mar 15;262(8):3440–3443. [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Rock C. O. Prostaglandin E2 inhibition of growth in a colony-stimulating factor 1-dependent macrophage cell line. J Biol Chem. 1990 Apr 25;265(12):6611–6616. [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Sherr C. J., Rock C. O. A guanine nucleotide-dependent phosphatidylinositol 4,5-diphosphate phospholipase C in cells transformed by the v-fms and v-fes oncogenes. J Biol Chem. 1986 Apr 15;261(11):4978–4985. [PubMed] [Google Scholar]
- Johnson R. M., Connelly P. A., Sisk R. B., Pobiner B. F., Hewlett E. L., Garrison J. C. Pertussis toxin or phorbol 12-myristate 13-acetate can distinguish between epidermal growth factor- and angiotensin-stimulated signals in hepatocytes. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2032–2036. doi: 10.1073/pnas.83.7.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Kozawa O., Kaibuchi K., Katada T., Ui M., Takai Y. Direct evidence for involvement of a guanine nucleotide-binding protein in chemotactic peptide-stimulated formation of inositol bisphosphate and trisphosphate in differentiated human leukemic (HL-60) cells. Reconstitution with Gi or Go of the plasma membranes ADP-ribosylated by pertussis toxin. J Biol Chem. 1986 Sep 5;261(25):11558–11562. [PubMed] [Google Scholar]
- L'Allemain G., Pouysségur EGF and insulin action in fibroblasts. Evidence that phosphoinositide hydrolysis is not an essential mitogenic signalling pathway. FEBS Lett. 1986 Mar 3;197(1-2):344–348. doi: 10.1016/0014-5793(86)80354-4. [DOI] [PubMed] [Google Scholar]
- Macara I. G. Oncogenes, ions, and phospholipids. Am J Physiol. 1985 Jan;248(1 Pt 1):C3–11. doi: 10.1152/ajpcell.1985.248.1.C3. [DOI] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Browning P. J., White M. F., Roberts T. M. Tyrosine phosphorylations in vivo associated with v-fms transformation. Mol Cell Biol. 1988 Jan;8(1):176–185. doi: 10.1128/mcb.8.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Bunn H. F., Turner P. H., Gopal T. V., Nash W. G., O'Brien S. J., Sherr C. J. Expression of the human c-fms proto-oncogene in hematopoietic cells and its deletion in the 5q- syndrome. Cell. 1985 Sep;42(2):421–428. doi: 10.1016/0092-8674(85)90099-6. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Rothenberg P. L., Kahn C. R. Insulin inhibits pertussis toxin-catalyzed ADP-ribosylation of G-proteins. Evidence for a novel interaction between insulin receptors and G-proteins. J Biol Chem. 1988 Oct 25;263(30):15546–15552. [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature. 1985 Jul 4;316(6023):64–66. doi: 10.1038/316064a0. [DOI] [PubMed] [Google Scholar]
- Sengupta A., Liu W. K., Yeung Y. G., Yeung D. C., Frackelton A. R., Jr, Stanley E. R. Identification and subcellular localization of proteins that are rapidly phosphorylated in tyrosine in response to colony-stimulating factor 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8062–8066. doi: 10.1073/pnas.85.21.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Stone R. M., Weber B. L., Spriggs D. R., Kufe D. W. Phospholipase C activates protein kinase C and induces monocytic differentiation of HL-60 cells. Blood. 1988 Aug;72(2):739–744. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Teitelbaum I. The epidermal growth factor receptor is coupled to a phospholipase A2-specific pertussis toxin-inhibitable guanine nucleotide-binding regulatory protein in cultured rat inner medullary collecting tubule cells. J Biol Chem. 1990 Mar 15;265(8):4218–4222. [PubMed] [Google Scholar]
- Tushinski R. J., Stanley E. R. The regulation of macrophage protein turnover by a colony stimulating factor (CSF-1). J Cell Physiol. 1983 Jul;116(1):67–75. doi: 10.1002/jcp.1041160111. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Stanley E. R. The regulation of mononuclear phagocyte entry into S phase by the colony stimulating factor CSF-1. J Cell Physiol. 1985 Feb;122(2):221–228. doi: 10.1002/jcp.1041220210. [DOI] [PubMed] [Google Scholar]
- Vairo G., Argyriou S., Bordun A. M., Whitty G., Hamilton J. A. Inhibition of the signaling pathways for macrophage proliferation by cyclic AMP. Lack of effect on early responses to colony stimulating factor-1. J Biol Chem. 1990 Feb 15;265(5):2692–2701. [PubMed] [Google Scholar]
- Vairo G., Hamilton J. A. Activation and proliferation signals in murine macrophages: stimulation of Na+,K+-ATPase activity by hemopoietic growth factors and other agents. J Cell Physiol. 1988 Jan;134(1):13–24. doi: 10.1002/jcp.1041340103. [DOI] [PubMed] [Google Scholar]
- Vara F., Rozengurt E. Stimulation of Na+/H+ antiport activity by epidermal growth factor and insulin occurs without activation of protein kinase C. Biochem Biophys Res Commun. 1985 Jul 31;130(2):646–653. doi: 10.1016/0006-291x(85)90466-8. [DOI] [PubMed] [Google Scholar]
- Vara F., Schneider J. A., Rozengurt E. Ionic responses rapidly elicited by activation of protein kinase C in quiescent Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2384–2388. doi: 10.1073/pnas.82.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegesna R. V., Wu H. L., Mong S., Crooke S. T. Staurosporine inhibits protein kinase C and prevents phorbol ester-mediated leukotriene D4 receptor desensitization in RBL-1 cells. Mol Pharmacol. 1988 May;33(5):537–542. [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamiya N., Horiguchi J., Kufe D. Detection of c-fms and CSF-1 RNA by in situ hybridization. Leukemia. 1987 Jun;1(6):518–520. [PubMed] [Google Scholar]
- Warden C. H., Friedkin M. Regulation of choline kinase activity and phosphatidylcholine biosynthesis by mitogenic growth factors in 3T3 fibroblasts. J Biol Chem. 1985 May 25;260(10):6006–6011. [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Downes C. P. The haemopoietic growth factors interleukin 3 and colony stimulating factor-1 stimulate proliferation but do not induce inositol lipid breakdown in murine bone-marrow-derived macrophages. EMBO J. 1986 Dec 1;5(12):3281–3286. doi: 10.1002/j.1460-2075.1986.tb04640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Huang S. J., Dexter T. M., Downes C. P. Interleukin 3 stimulates proliferation via protein kinase C activation without increasing inositol lipid turnover. Proc Natl Acad Sci U S A. 1988 May;85(10):3284–3288. doi: 10.1073/pnas.85.10.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Molecular analysis of signal transduction by growth factors. Biochemistry. 1988 May 3;27(9):3113–3119. doi: 10.1021/bi00409a001. [DOI] [PubMed] [Google Scholar]
- Yavin E. Regulation of phospholipid metabolism in differentiating cells from rat brain cerebral hemispheres in culture. Patterns of acetylcholine phosphocholine, and choline phosphoglycerides labeling from (methyl-14C)choline. J Biol Chem. 1976 Mar 10;251(5):1392–1397. [PubMed] [Google Scholar]