Abstract
Endoplasmic Reticulum Aminopeptidase 2 (ERAP2) trims HLA class I-binding peptides, determining the peptide repertoire presented for immune recognition. Variation in the ERAP2 amino acid sequence could affect the ability of some fetuses and tumors to achieve immune evasion. For example, homozygosity for an ERAP2 variant that has increased trimming efficiency for hydrophobic molecules has never been detected in mothers and fetuses. Thus, it is possible that this single nucleotide polymorphism (SNP) in the ERAP2 gene has been selected against in order to prevent alteration of the immune privileged uterine environment, and to allow tumors to escape immune recognition. Currently, there are no immunological treatments or prophylactic approaches to ensure a healthy pregnancy outcome, and the success of cancer immunotherapies is variable. Understanding the role of ERAP2 in immune evasion mechanisms in pregnancy and cancer may improve fetal survival and tumor clearance. This review summarizes current knowledge about ERAP2 and its N392 variant, and their relationship to pregnancy outcomes and cancer immune evasion/recognition.
Keywords: ERAP2, Pregnancy, Cancer, Immune Evasion, Immune Surveillance
Introduction
Immune evasion is good for fetal and tumor growth. Immune detection, on the other hand, is bad for both the fetus and tumors. Evidence is accumulating to support the pivotal role of ERAP2 in immune evasion mechanisms. A better understanding of its role in immune evasion or recognition may allow the development of prophylactic and therapeutic treatments for fetal rejection and tumor clearance. This review will address three main objectives; 1) to introduce genetic studies that suggest how the N392 ERAP2 variant is selected against in populations for potential detrimental effects on pregnancy; 2) to provide an overview of ERAP2 in pregnancy and cancer, and their roles in immune evasion; and 3) to document the need to explore how N392 ERAP2 may be involved in fetal rejection, and how this knowledge can be applied to tumor clearance.
ERAP
Endoplasmic Reticulum Aminopeptidase (ERAP)1 and ERAP2 are zinc-metallopeptidases of the oxytocinase M1 subfamily, which share consensus zinc-binding motifs essential for their enzymatic activity. Most of the antigenic protein degradation occurs in cytosolic proteasome complexes [1]. However, when additional processing of peptides is necessary to increase and to refine the variety available for binding to Major Histocompatibility Complex (MHC), also known as Human Leukocyte Antigen (HLA) class I molecules, the peptides are transported to the endoplasmic reticulum (ER) by a transporter associated with antigen processing protein (TAP) [2–4]. ERAP enzymes then trim amino acid residues from the NH2 terminus of polypeptides to generate the optimal length antigenic peptides for loading onto HLA class I molecules [5, 6].
The human ERAP1 and ERAP2 genes are located on chromosome 5q15 in the opposite orientation. Human ERAP2 has no orthologues in rodents, and evolutionary studies suggest that ERAP2 originates from a relatively recent duplication of the ERAP1 gene [7]. It is not known exactly when the ERAP gene evolved, and equally uncertain is the functional consequence its evolution has for human pregnancy and cancer immunology. However, ERAP2 protein expression is detected in many tissues, and is induced by type I and type II interferon (IFNs) and tumor necrosis factor-alpha (TNF-α), suggesting that ERAP2 is essential for immune control, and that further investigation is needed to clarify its roles [8, 9].
ERAP2 and Pregnancy
A genome-wide association study (GWAS) suggested a role of the ERAP2 locus in preeclampsia (PE) [10]. PE affects 3–8% of pregnancies worldwide, with the highest (10%) among African Americans. It leads to potentially devastating complications for both the mother and fetus [10–12]. Johnson et al. were the first investigators to report that the ERAP2 gene was associated with PE in Australian and Norwegian populations [10]. Recently, our group reported the fetal ERAP2 SNP rs2549782 genotype is associated with a higher risk for PE in African American women (P=0.009) [13]. These findings are supported by a recent study, which revealed elevated levels of ERAP2 transcripts in the decidua of PE patients, which contradicts earlier observations of expression of this gene being down-regulated in first trimester placentas of women who subsequently developed PE [14–16]. One potential explanation for the different observations relates to when and which ERAP2 variant transcripts are expressed and translated to affect the maternal immune response. PE is first diagnosed around 20 weeks of gestation, but it is thought that the problem arises as early as during placentation. The determination of aberrant gene expression prior to development of maternal symptoms suggests ERAP2 is involved in the early disease course.
ERAP2 is a good candidate to contribute to the development of PE and other pregnancy related diseases because of its involvement in the regulation of the immune response, pro-inflammatory cytokine production, and blood pressure [5, 17–20]. PE is associated with predominant presence of T Helper Cell Type 1 (Th1), and is considered to be a failure of immune tolerance resulting in poor placentation, exacerbated inflammation, and endothelial dysfunction [21]. Thus, it is critical to determine how and when paternal antigen presentation on HLA is altered by ERAP2 variants, and its subsequent immune modulation that could determine the pregnancy outcomes.
The following observations provide evidence for racial differences in the role of ERAP2 in risk for PE association. In most populations, the major T allele of rs2549782 (gain-of-function) haplotype structure of ERAP2 N392 pairs with the G allele of rs2248374 which causes nonsense-mediated RNA decay (loss-of-function), such that the N392 ERAP2 isoform would never be expressed [10]. However, the strong linkage disequilibrium described above and ERAP2/PE association was not detected in a Chilean population, suggesting that N392 ERAP2 could be detected. However, we found that homozygosity for the T/T N392 ERAP2 (“gain-of-function”) allele is never detected in the mother or fetus in normal pregnancies in the Chilean population studied (n=528 dyads) [22]. One hypothesis to explain these observations is that “hyper” trimming capability for hydrophobic peptides alters the peptide and HLA class I repertoire in cells triggering immune activation, creating a non-permissive uterine environment for trophoblast cell/fetal survival [23].
ERAP2 and Reproductive/Cancer Immunology
Interest in the immunology of pregnancy was inspired by the realization that expression of paternal histocompatibility antigens by the fetus and placenta should provoke a tissue rejection response similar to that observed following organ transplantation [24]. ERAP2 link to the immune system derives from its ability to trim HLA class I-binding antigens, determining the peptide repertoire presented for T cell recognition [8, 19, 25–27]. The concerted effort of ERAP1 and ERAP2 determines the efficiency of peptide editing [26]. However, as it is mentioned above a single nucleotide polymorphism (SNP) rs2549782 in ERAP2 results in an amino acid change of Lysine (K392) to Asparagine (N392-isoform) that increases ERAP2 protein interaction with hydrophobic peptides by 165-fold, altering the peptide and HLA class I repertoire in cells [23]. We hypothesize that this ERAP2 variant could either generate or destroy immunodominant epitopes to modulate immune recognition.
On the other hand, ERAP2 deficiency can contribute to immune evasion by tumor cells in vivo [7, 15]. Loss of the ERAP2 protein has been observed in renal carcinoma, colon adenocarcinoma, melanoma, and ovarian cancer, suggesting that the lack of ERAP2 benefits cancer growth and/or maintenance [15]. Cancer cells can avoid inducing an immune response by any combination of these steps: 1) inhibiting tumor antigen transport to endoplasmic reticulum (ER), 2) improper trimming of peptides in the ER and/or 3) inadequate peptide presentation on HLA molecule. Currently, there is evidence that disruptions in antigen transportation and antigen presentation are used in immune evasion [28, 29]. However, the role of ERAP2 in immune evasion has yet to be fully elucidated. The first observation of the low expression level of ERAP2, ERAP1 and HLA class I was made in the most aggressive form of neuroblastoma [9].
Previously, researchers have demonstrated that ERAP1-mediated trimming is necessary for the generation of many antigenic epitopes but can also lead to destruction by trimming them too small for HLA binding [27]. Based on this observation, Stratikos and colleagues attempted to develop a potent ERAP1 inhibitor using a structure-guided design approach, focusing on the Zn-containing active enzyme site. They demonstrated that one of the inhibitors, DG013A, enhances antigen presentation by HeLa cells and elicits a potent Cytotoxic T Lymphocyte (CTL) response against murine colon carcinoma cells [30]. In this mouse model, inhibition of ERAP1 was an effective way to improve immunodominant epitope process, presentation, and CTL response. However, this model does not address if the response will be the same if ERAP2 was present and inhibited. Any aberrant generation of antigenic peptides and alteration of MHC repertoire can lead to either immune evasion or activation. Thus, we need to better understand the role of ERAP2 in either to inhibit or enhance its action to develop more effective cancer treatments. Unfortunately, determining ERAP2’s role in cancer targeting and cell clearance has been confounded because animal models lack ERAP2 orthologues.
The fine balance of epitope destruction/generation by ERAP1 and ERAP2 is necessary for healthy fetal and cancer cells. On the contrary, if we understand how those peptide-processing functions are affected by these enzymatic activities, the fetal rejection can be prevented and tumor clearance can be promoted. Especially, by attempting to understand the evolutionary selection against N392 ERAP2 and its mechanism of action in the immune system, we might obtain new insight into ERAP2’s role in immune recognition in both reproductive and cancer immunology.
HLA-C association in Pregnancy and Cancer
Decidual natural killer (dNK) cells are the most abundant lymphocytes (70%) during early gestation [31, 32] and are virtually absent by the end of pregnancy [31, 33, 34]. These NK cells are phenotypically and functionally different from the systemic circulating NK cells [35]. Several studies suggest that the primary role of dNK cells is to encourage those changes in uterine vasculature necessary for efficient maternal blood flow through the placenta permitting nutrient and gas exchange. The failure to establish a proper vasculature results in placental hypo-perfusion which is believed to trigger complications such as PE, intrauterine growth restriction, unexplained stillbirth, recurrent miscarriage, or preterm birth [36].
The recent work of Hiby et al. showed that placentation is regulated by the interactions between maternal killer immunoglobulin-like receptors (KIRs) on dNK cells and HLA-C molecules on invading fetal trophoblast cells [37]. The trophoblast cells that invade the uterus have a unique pattern of HLA class I expression consisting of invariant HLA-G, HLA-E and the polymorphic HLA-C, and no expression of highly polymorphic HLA-A or HLA-B [38]. Therefore, only HLA-C presents polymorphic forms necessary for the recognition of fetal alloantigens. Due to high variability and genetic diversity of KIR in different populations, certain combinations of maternal KIRs and fetal HLA-C ligands could determine the success or failure of a pregnancy.
There are 2 basic KIR haplotypes, A and B. The only difference between the two is that haplotype B has additional activating receptors. Similarly, HLA-C ligands for KIRs are divided into 2 groups. Group 1 HLA-C (C1) allotypes bind inhibitory KIR, while group 2 HLA-C (C2) allotype binds both inhibitory and activating KIRs on uNK cells. Specifically, this study concluded that the maternal activating KIR AA frequencies are increased in affected pregnancies only when the fetus has more HLA-C2 gene expression than the mother [37]. Furthermore, Hackmon et al. confirmed the distinct expression of HLA-F, HLA-G, HLA-E, and HLA-C in placental tissue, especially on extravillous trophblast cells (EVT). This study provided the first evidence of increased HLA-C expression in parturition that may indicate modulation of the immune-inflammatory role of HLA-C [39]. These combined studies raise the possibility of the deleterious allogeneic effect stemming from paternal HLA-C, specifically C2 group. Altogether, the missing piece of the puzzle is the functional mechanism to explain the pregnancy outcomes determined by the KIR/HLA-C interactions due to hyper trimming effect of paternal antigens by N392 ERAP2 and how this translates into different uNK cell functions, which is illustrated in Figure 1. Trophoblast cells expressing the N392 ERAP2 isoform, which alters class I antigen expression, may now become target cells triggering NK cell-mediated lysis, and thus results in fetal “rejection,” and may explain why this particular genotype is never detected in populations studied to date.
Figure 1.
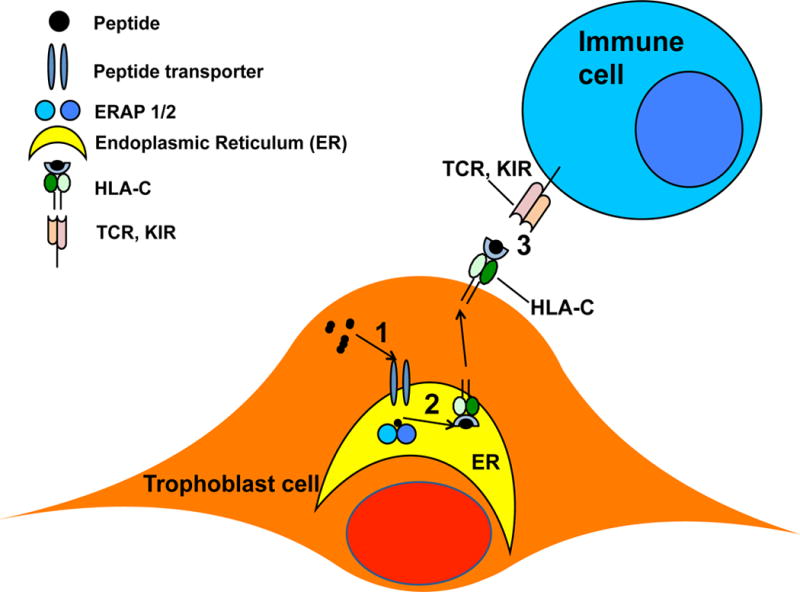
Illustration of peptide 1. transport, 2. process, and 3. presentation by trophoblast.
Furthermore, it is not surprising that trophobast cells only express HLA-C to be protected from the maternal immune system. Similarly, HLA-C has been shown to be associated with protection of nasopharygeal carcinoma and cervical neoplasia, as well from the host immune system [40]. Nature possibly meant HLA-Cs main role to be protective, but the evidence of biological function supporting HLA-C-restriced CTL responses suggests that when this tolerance is dfisrupted by an intrinsic factor such as a ERAP2 SNP (gain-of-function), the host immune response could be detrimental to fetal and tumorigenic cells.
The main question is if HLA-C restricted allogeneic paternal or tumorigenic antigens are generated by ERAP2 to induce T and NK cell response. This is becoming a feasible hypothesis since the biological significance of HLA-C restricted responses was confirmed as the HLA-C antigen restricted alloreactivity CTL response was established particularly in chronic infection of Epstein-Barr virus ad HIV infections [41, 42]. To date, the HLA-C alleles have been involved in several human diseases, emphasizing its critical role, but it is not clear if the relationship is the result of HLA-C function as a T-cell restricted response, or as a consequence of its interaction with KIRs on NK cells.
Conclusion
Fetal and tumor growth have many similarities such as cellular invasion, angiogenesis, rapid growth, and immune modulation [43]. In this review, we have briefly summarized additional similarities based on recent knowledge of the involvement of ERAP2 in immune recognition. Genetic evidence provided by the association of the ERAP2 variant with a pregnancy related disorder (PE), and the fact that this variant is evidently selected against to protect the uterine environment warrants further investigation. Conversely, this knowledge could be used to develop targeted immunotherapies for cancer. If N392 ERAP2 expression is 100 percent successful in preventing full term fetal development, resulting in zero detection, what if N392 ERAP2 is introduced into tumors? Could it promote efficient immune clearance of tumor cells?
Highlights.
Current knowledge of Endoplasmic Reticulum Aminopeptidase 2 (ERAP2) in pregnancy and cancer is presented.
In some cancers, lack of ERAP2 expression may benefit tumor immune evasion.
In pregnancy, hyper trimming of an ERAP2 variant may cause fetal rejection.
Speculation on how knowledge of ERAP2 function in pregnancy can be translated into translational tumor clearance.
Acknowledgments
The author thanks Dr. Jerome F. Strauss and Dr. Bennett B Lee for editorial assistance in the preparation of the manuscript
Funding
The National Institutes of Health grant R01 HD073555 supported this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There is no conflict of interest.
References
- 1.Rock KL, Goldberg AL. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annual review of immunology. 1999;17:739–79. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 2.Reits E, Neijssen J, Herberts C, Benckhuijsen W, Janssen L, Drijfhout JW, Neefjes J. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity. 2004;20(4):495–506. doi: 10.1016/s1074-7613(04)00074-3. [DOI] [PubMed] [Google Scholar]
- 3.Neisig A, Roelse J, Sijts AJ, Ossendorp F, Feltkamp MC, Kast WM, Melief CJ, Neefjes JJ. Major differences in transporter associated with antigen presentation (TAP)-dependent translocation of MHC class I-presentable peptides and the effect of flanking sequences. J Immunol. 1995;154(3):1273–9. [PubMed] [Google Scholar]
- 4.Daniel S, Brusic V, Caillat-Zucman S, Petrovsky N, Harrison L, Riganelli D, Sinigaglia F, Gallazzi F, Hammer J, van Endert PM. Relationship between peptide selectivities of human transporters associated with antigen processing and HLA class I molecules. J Immunol. 1998;161(2):617–24. [PubMed] [Google Scholar]
- 5.Hattori A, Matsumoto H, Mizutani S, Tsujimoto M. Molecular cloning of adipocyte-derived leucine aminopeptidase highly related to placental leucine aminopeptidase/oxytocinase. J Biochem. 1999;125(5):931–8. doi: 10.1093/oxfordjournals.jbchem.a022371. [DOI] [PubMed] [Google Scholar]
- 6.Evnouchidou I, Papakyriakou A, Stratikos E. A new role for Zn(II) aminopeptidases: antigenic peptide generation and destruction. Curr Pharm Des. 2009;15(31):3656–70. doi: 10.2174/138161209789271816. [DOI] [PubMed] [Google Scholar]
- 7.Andres AM, Dennis MY, Kretzschmar WW, Cannons JL, Lee-Lin SQ, Hurle B, Schwartzberg PL, Williamson SH, Bustamante CD, Nielsen R, Clark AG, Green ED. Balancing selection maintains a form of ERAP2 that undergoes nonsense-mediated decay and affects antigen presentation. PLoS Genet. 2010;6(10):e1001157. doi: 10.1371/journal.pgen.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saric T, Chang SC, Hattori A, York IA, Markant S, Rock KL, Tsujimoto M, Goldberg AL. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat Immunol. 2002;3(12):1169–76. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- 9.Forloni M, Albini S, Limongi MZ, Cifaldi L, Boldrini R, Nicotra MR, Giannini G, Natali PG, Giacomini P, Fruci D. NF-kappaB, and not MYCN, regulates MHC class I and endoplasmic reticulum aminopeptidases in human neuroblastoma cells. Cancer Res. 2010;70(3):916–24. doi: 10.1158/0008-5472.CAN-09-2582. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MP, Roten LT, Dyer TD, East CE, Forsmo S, Blangero J, Brennecke SP, Austgulen R, Moses EK. The ERAP2 gene is associated with preeclampsia in Australian and Norwegian populations. Hum Genet. 2009;126(5):655–66. doi: 10.1007/s00439-009-0714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anoymous, ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 12.Caughey AB, Stotland NE, Washington AE, Escobar GJ. Maternal ethnicity, paternal ethnicity, and parental ethnic discordance: predictors of preeclampsia. Obstet Gynecol. 2005;106(1):156–61. doi: 10.1097/01.AOG.0000164478.91731.06. [DOI] [PubMed] [Google Scholar]
- 13.Hill LD, Hilliard DD, York TP, Srinivas S, Kusanovic JP, Gomez R, Elovitz MA, Romero R, Strauss JF., 3rd Fetal ERAP2 variation is associated with preeclampsia in African Americans in a case-control study. BMC Med Genet. 2011;12:64. doi: 10.1186/1471-2350-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yong HE, Murthi P, Borg A, Kalionis B, Moses EK, Brennecke SP, Keogh RJ. Increased decidual mRNA expression levels of candidate maternal preeclampsia susceptibility genes are associated with clinical severity. Placenta. 2014;35(2):117–24. doi: 10.1016/j.placenta.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fruci D, Giacomini P, Nicotra MR, Forloni M, Fraioli R, Saveanu L, van Endert P, Natali PG. Altered expression of endoplasmic reticulum aminopeptidases ERAP1 and ERAP2 in transformed non-lymphoid human tissues. J Cell Physiol. 2008;216(3):742–9. doi: 10.1002/jcp.21454. [DOI] [PubMed] [Google Scholar]
- 16.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30(1):15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsujimoto M, Hattori A. The oxytocinase subfamily of M1 aminopeptidases. Biochim Biophys Acta. 2005;1751(1):9–18. doi: 10.1016/j.bbapap.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Taylor A. Aminopeptidases: structure and function. Faseb J. 1993;7(2):290–8. doi: 10.1096/fasebj.7.2.8440407. [DOI] [PubMed] [Google Scholar]
- 19.Tanioka T, Hattori A, Masuda S, Nomura Y, Nakayama H, Mizutani S, Tsujimoto M. Human leukocyte-derived arginine aminopeptidase. The third member of the oxytocinase subfamily of aminopeptidases. J Biol Chem. 2003;278(34):32275–83. doi: 10.1074/jbc.M305076200. [DOI] [PubMed] [Google Scholar]
- 20.Tanioka T, Hattori A, Mizutani S, Tsujimoto M. Regulation of the human leukocyte-derived arginine aminopeptidase/endoplasmic reticulum-aminopeptidase 2 gene by interferon-gamma. Febs J. 2005;272(4):916–28. doi: 10.1111/j.1742-4658.2004.04521.x. [DOI] [PubMed] [Google Scholar]
- 21.Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59(2):161–73. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 22.Vanhille DL, Hill LD, Hilliard DD, Lee ED, Teves ME, Srinivas S, Kusanovic JP, Gomez R, Stratikos E, Elovitz MA, Romero R, Strauss JF., 3rd A Novel Haplotype Structure in a Chilean Population: Implications for ERAP2 Protein Expression and Preeclampsia Risk. Mol Genet Genomic Med. 2013;1(2):98–107. doi: 10.1002/mgg3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evnouchidou I, Birtley J, Seregin S, Papakyriakou A, Zervoudi E, Samiotaki M, Panayotou G, Giastas P, Petrakis O, Georgiadis D, Amalfitano A, Saridakis E, Mavridis IM, Stratikos E. A common single nucleotide polymorphism in endoplasmic reticulum aminopeptidase 2 induces a specificity switch that leads to altered antigen processing. J Immunol. 2012;189(5):2383–92. doi: 10.4049/jimmunol.1200918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;(7):320–38. [Google Scholar]
- 25.Kamphausen E, Kellert C, Abbas T, Akkad N, Tenzer S, Pawelec G, Schild H, van Endert P, Seliger B. Distinct molecular mechanisms leading to deficient expression of ER-resident aminopeptidases in melanoma. Cancer Immunol Immunother. 2010;59(8):1273–84. doi: 10.1007/s00262-010-0856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saveanu L, Carroll O, Lindo V, Del Val M, Lopez D, Lepelletier Y, Greer F, Schomburg L, Fruci D, Niedermann G, van Endert PM. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat Immunol. 2005;6(7):689–97. doi: 10.1038/ni1208. [DOI] [PubMed] [Google Scholar]
- 27.York IA, Chang SC, Saric T, Keys JA, Favreau JM, Goldberg AL, Rock KL. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat Immunol. 2002;3(12):1177–84. doi: 10.1038/ni860. [DOI] [PubMed] [Google Scholar]
- 28.Tai W, Chen Z, Cheng K. Expression profile and functional activity of peptide transporters in prostate cancer cells. Molecular pharmaceutics. 2013;10(2):477–87. doi: 10.1021/mp300364k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marincola FM, Shamamian P, Rivoltini L, Salgaller M, Cormier J, Restifo NP, Simonis TB, Venzon D, White DE, Parkinson DR. HLA associations in the antitumor response against malignant melanoma. J Immunother Emphasis Tumor Immunol. 1995;18(4):242–52. doi: 10.1097/00002371-199511000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zervoudi E, Saridakis E, Birtley JR, Seregin SS, Reeves E, Kokkala P, Aldhamen YA, Amalfitano A, Mavridis IM, James E, Georgiadis D, Stratikos E. Rationally designed inhibitor targeting antigen-trimming aminopeptidases enhances antigen presentation and cytotoxic T-cell responses. Proc Natl Acad Sci U S A. 2013;110(49):19890–5. doi: 10.1073/pnas.1309781110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Human reproduction. 1991;6(6):791–8. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- 32.King A, Loke YW. On the nature and function of human uterine granular lymphocytes. Immunology today. 1991;12(12):432–5. doi: 10.1016/0167-5699(91)90014-K. [DOI] [PubMed] [Google Scholar]
- 33.Moffett-King A. Natural killer cells and pregnancy. Nature reviews Immunology. 2002;2(9):656–63. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 34.Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol. 2009;82(1):24–31. doi: 10.1016/j.jri.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Colucci F, Boulenouar S, Kieckbusch J, Moffett A. How does variability of immune system genes affect placentation? Placenta. 2011;32(8):539–45. doi: 10.1016/j.placenta.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 37.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, Tower C, Regan L, Moore GE, Carrington M, Moffett A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120(11):4102–10. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127(1):26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackmon R, Pinnaduwage L, Zhang J, Lye SJ, Geraghty DE, Dunk CE. Definitive class I human leukocyte antigen expression in gestational placentation: HLA-F, HLA-E, HLA-C, and HLA-G in extravillous trophoblast invasion on placentation, pregnancy, and parturition. Am J Reprod Immunol ( 2017 doi: 10.1111/aji.12643. [DOI] [PubMed] [Google Scholar]
- 40.Blais ME, Dong T, Rowland-Jones S. HLA-C as a mediator of natural killer and T-cell activation: spectator or key player? Immunology. 2011;133(1):1–7. doi: 10.1111/j.1365-2567.2011.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pei J, Akatsuka Y, Anasetti C, Lin MT, Petersdorf EW, Hansen JA, Martin PJ. Generation of HLA-C-specific cytotoxic T cells in association with marrow graft rejection: analysis of alloimmunity by T-cell cloning and testing of T-cell-receptor rearrangements. Biol Blood Marrow Transplant. 2001;7(7):378–83. doi: 10.1053/bbmt.2001.v7.pm11529487. [DOI] [PubMed] [Google Scholar]
- 42.Hov JR, Lleo A, Selmi C, Woldseth B, Fabris L, Strazzabosco M, Karlsen TH, Invernizzi P. Genetic associations in Italian primary sclerosing cholangitis: heterogeneity across Europe defines a critical role for HLA-C. J Hepatol. 2010;52(5):712–7. doi: 10.1016/j.jhep.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 43.Holtan SG, Creedon DJ, Haluska P, Markovic SN. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin Proc. 2009;84(11):985–1000. doi: 10.1016/S0025-6196(11)60669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


