Abstract
Neutrophils accumulate in many types of human and murine tumors and represent a significant portion of tumor-infiltrating myeloid cells. Our current understanding of the role of neutrophils in tumor development has depended primarily on murine models of cancer. However, there are crucial species differences in the evolution of tumors, genetic diversity, immune and inflammatory responses, and intrinsic biology of neutrophils that might have a profound impact on the tumor development and function of neutrophils in mouse versus human tumors. To date, the majority of experimental approaches to study neutrophils in cancer patients have been limited to the examination of circulating blood neutrophils. The phenotype and function of tumor-associated neutrophils (TANs) in humans, particularly in the early stages of tumor development, have not been extensively investigated. Thus, the long-term goal of our work has been to characterize human TANs and determine their specific role in tumor development. Here, we summarize our findings on human TANs obtained from human early stage lung cancer patients. We will describe the phenotypes of different TAN subsets identified in early stage lung tumors, as well as their functional dialog with T cells.
Keywords: Human lung cancer, Human tumor microenvironment, Tumor-associated neutrophils, Regulatory myeloid suppressor cells, Neutrophil and T-cell interaction
Introduction
Neutrophils accumulate in many types of human and murine tumors and regulate nearly all steps of tumor progression [1–4]. It is becoming apparent that neutrophils are able to shape and regulate immune and inflammatory responses against tumor cells. Our current understanding of neutrophil function in tumor progression has been inferred from various types of mouse tumor models. These mouse models suggest that neutrophils can exert both pro-tumor and anti-tumor effects on tumor development [1, 5]. Given these varied effects of neutrophils, the concept of neutrophil diversity and plasticity has begun to emerge in murine tumor models, leading to the paradigm of anti-tumoral “N1 neutrophils” versus pro-tumoral “N2 neutrophils” proposed by Fridlender and colleagues [5]. Currently, a pro-tumoral and immunosuppressive role of neutrophils in transplanted mouse tumor has become the dominant view in the field.
It should be noted that there are substantial species differences in tumor progression, host genetic diversity, immune and inflammatory responses, and the intrinsic biology of neutrophils that likely have a profound impact on both tumor development and neutrophil function in mice versus humans [6, 7]. In contrast to human tumors, the majority of mouse tumor models use tumor cell lines that have been derived from spontaneously arising advanced tumors that have already been subjected to immune selection in vivo. These cell lines have been selected to grow rapidly in vivo (usually in the flanks) and have thus already undergone cancer immunoediting and “Darwinian” selection [8]. Hence, the majority of mouse tumor models lack prolonged the initial phases of multistage tumor evolution, such as elimination and equilibrium phases that would be expected to occur in humans. Data obtained from these transplantable mouse models mostly reflect the immune response as it occurs during the advanced stage of tumor development, at which time pro-tumoral mechanisms already prevail. At this stage, the accumulation of immunosuppressive N2 neutrophils and/or polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC) might be expected. In contrast, human tumors are slower to evolve. During the first steps of tumor evolution in humans, the processes of tumor clone initiation, proliferation, and diversification occur concomitantly with selective pressure on tumor clones by anti-tumoral immune responses [9]. Most clones likely elicit an immune response and are killed, while other clones mediate immune tolerance and survive during the sustained selective pressure by the host’s anti-tumor immune response. At these early stages of tumor evolution, anti-tumoral neutrophils (N1) would be expected to predominate. Surviving clones will be increasingly resistant to the adaptive immune system’s attacks and will gradually come to dominate within the tumor. These early stage human tumors have entered the escape phase of the immunoediting process and appear to develop an increasingly immunosuppressive environment. The functional state of TANs at these early stages of tumor development has not been well studied yet. As the tumor progresses, the composition of the tumor microenvironment and its cytokine milieu likely become more suppressive for the anti-tumor host responses. Thus, it is very possible that human and murine neutrophils represent cells with different functional states that exist during the early and advanced stages of tumor evolution.
To date, data on the functional role of neutrophils in human cancer are still relatively scarce and have largely been obtained from experiments relying on circulating peripheral blood neutrophils. A recent summary of these studies has described neutrophils as versatile and heterogeneous cells that exert various effects on T-cell migration, activation, differentiation, and effector functions [10–12]. It has been suggested that one way to segregate and classify these heterogeneous populations of neutrophils is by their sedimentation properties in density gradients: i.e., normal density neutrophils (NDNs) versus low-density neutrophils (LDNs) [11, 13]. However, even these LDN and NDN groups contain diverse cell populations with different functions. LDNs contain immature neutrophils and activated mature neutrophils that perform immunosuppressive and pro-inflammatory functions [11, 13]. The T-cell immunosuppressive LDNs are also known as granulocytic-myeloid-derived suppressor cells (G-MDSCs) [14]. NDNs mainly consist of resting neutrophils. However, under certain disease conditions, smaller subsets of immature neutrophils (mostly band cells) and activated mature neutrophils with different immunoregulatory effects toward T cells have also been detected [11, 15, 16]. The precise phenotypes and functions of all these neutrophil subpopulations within LDNs and NDNs still require further examination. Thus, although the concept of neutrophil diversity and plasticity has begun to emerge in both human and mouse tumor models, uncertainty regarding the phenotypes, functional roles, and relationships between different granulocytic cell populations during tumor progression persists.
As mentioned above, the majority of experimental approaches to studying neutrophils in cancer patients rely on circulating peripheral blood neutrophils (PBNs) and thus make the assumption that peripheral neutrophils function similarly to those found within the tumor. This assumption may not be valid, since the phenotype and function of tumor-associated neutrophils (TANs) in human subjects, particularly in the early stages of tumor development, have not been extensively investigated. Importantly, in contrast to PBNs, the interplay between TANs and the tumor microenvironment likely causes phenotypic and functional changes in TAN populations during tumor progression. For this reason, to understand the role of neutrophils during human tumor progression, TANs, themselves, should be investigated and compared with PBNs. The primary challenges that have limited progress in this area include technical difficulties in obtaining fresh human tumors, inefficient digestion of human tumor tissue, failure to isolate TANs while preserving cell surface markers and functionality, the fragility of human TANs (i.e. they do not survive freezing and thawing), and the lack of cognate mouse myeloid-cell markers in humans. In contrast to PBNs, isolation of TANs from tumors requires a more prolonged multi-step procedure, where technical issues might have some effect on the phenotype and function of TANs. The logistical, ethical, and regulatory difficulties in obtaining human tumor tissue for research also act to discourage such studies.
By having a close collaboration between the surgeon and the laboratory, we have overcome these difficulties and have begun the initial studies with the goal of phenotypic and functional characterization of human TANs. Here, we will summarize and discuss our latest findings regarding the TANs and their role in the regulation of the T-cell responses in patients with early stage lung cancer. Understanding the role of TANs in regulating T-cell responses in cancer patients is particularly important, because cytotoxic T lymphocytes are the chief effector cells mediating antigen-driven anti-tumor immunity.
Optimized disaggregation of tumor tissue is the first critical step in studying human TANs
Generation of high-quality single-cell suspensions from human tumor tissues is required to study human TAN function. To date, specific disaggregation techniques that are tailored to specific types of human tumors have not been developed. Rather, a wide variety of enzymes have been used to digest solid tumors, under the assumption that almost any particular enzymatic dissociation technique will provide high cell yield without affecting the functional activity of the cell populations under study. In general, methods of enzymatic digestion which produce higher cell yields are more harsh and tend to induce more artifacts through the cleavage of cell surface markers [17, 18]. Without careful assessment of the effects of enzymatic digestion on phenotype, a particular method may cause alterations in the true immune cell profile and thus provide misleading results. We have conducted a study in which we critically evaluated the current techniques available in the literature used to prepare human tumors for immunologic studies and found that many approaches used an unbalanced composition of enzymes that inadvertently cleaved multiple cell surface markers [18]. Such digestion–induced effects might lead to false conclusions about the presence or absence of specific cellular populations and their biologic characteristics. Our group investigates the tumor microenvironment of human lung cancer, so we rigorously tested and validated various techniques to process human non-small cell lung carcinoma (NSCLC) specimens. Our objective was to balance high immune cell yield and high cell viability with maintenance of key surface markers and functional characteristics. Our final approach to prepare human lung tumors used a combination of non-traumatic, gentle mechanical manipulation, and an optimized cocktail of specific enzymes used at low doses. We have established that this disaggregation approach optimized cell yield and cell viability, retrieved all major tumor-associated cell populations, and maintained the expression of cell surface markers for both lineage definition and in vivo effector functions [18]. Using this methodology, we have been able to develop a complete phenotypical and functional description of TANs [19, 20].
Characteristics of TANs in early stage human lung cancer
In mice, antibodies to the CD11b and Ly-6G antigens are well-established tools to identify granulocytes. In humans, granulocytes do not express the Ly-6G antigen, making the direct comparison of murine and human granulocytes impossible. To date, the characterization of neutrophils within human tumor tissue has largely been limited to the detection of only single or double granulocytic markers [for example, CD15, CD66b or myeloperoxidase (MPO)] by immunohistochemistry. However, there had been no reports that extensively evaluated the phenotype of TANs in human tumors. Thus, the first goal of our investigation was to develop a complete phenotypical description of TANs in humans. For this purpose, we used fresh, surgically resected early stage lung tumor tissue.
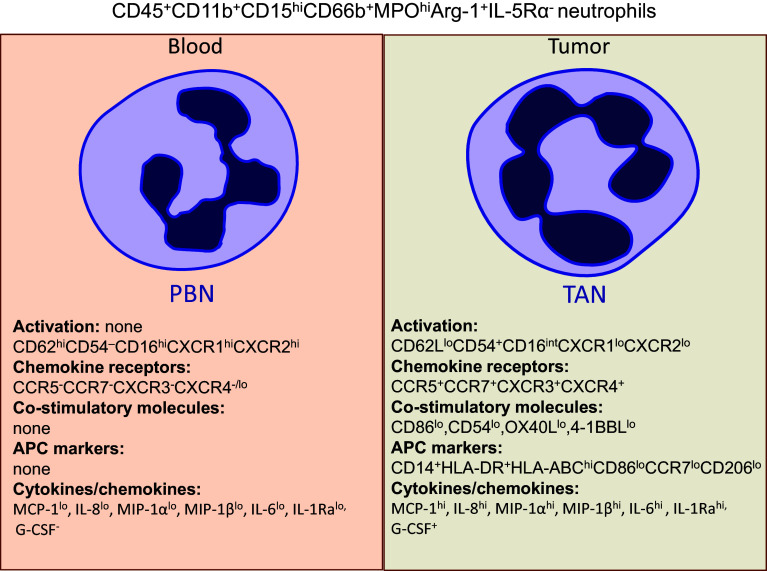
We performed an extensive phenotypic analysis of tumor-associated neutrophils in high-quality, single-cell suspensions obtained from fresh NSCLCs using our optimized disaggregation method. In multicolor flow cytometry, TANs could be defined as CD11b+CD15hiCD66b+MPOhiArg1+CD16intIL-5Ra− cells and were found in varying frequency, ranging from 2 to 20% of live cells in the tumor microenvironment [19]. We showed that neutrophils recruited into lung tumors exhibited an activated phenotype when compared with circulating peripheral blood neutrophils (Fig. 1) [19]. For example, recruited TANs expressed the “classic” activation markers characterized by up-regulation of the adhesion molecule CD54 (ICAM-1) and down-regulation of CD62L (L-selectin), CXCR1, CXCR2, and CD16 [21]. CD54 plays an important role in cellular adhesion, endothelial transmigration, and stabilizing cell–cell interactions. Recent studies have shown that CD54 can also be actively involved in the neutrophil-dependent potentiation of IL-12 and IFN-γ release by DCs and NK cells, respectively [22]. Of note, CD54 (ICAM-1) was found to be an N1 neutrophil marker in murine tumor models [5].
Fig. 1.
Phenotypic characteristic of peripheral blood neutrophils (PBNs) and tumor-associated neutrophils (TANs) in early stage human lung cancer. Both PBNs and TANs express the canonical neutrophil markers CD45+CD11b+CD15hiCD66b+MPOhiArg-1+IL-5Rα−. In addition to canonical neutrophil phenotype, TANs express an activated phenotype characterized by up-regulation of the adhesion molecule CD54 and down-regulation of CD62L (L-selectin), CXCR1, CXCR2, and CD16. TANs up-regulate co-stimulatory molecules, which acquire a novel repertoire of chemokine receptors and a new cytokine/chemokine profile. In some small-sized, early stage tumors that produce IFN-γ and GM-CSF, TANs could differentiate into a subset of cells exhibiting a partial phenotype of professional APCs (CD14+HLA-DR+HLA-ABChiCCR7lowCD86lowCD206low)
In addition to changes in activation, we also found that activated TANs expressed a new chemokine receptor profile in the tumor microenvironment (Fig. 1). We observed that TANs up-regulate CCR5, CCR7, CXCR3, and CXCR4, and down-regulate CXCR1 and CXCR2, compared to peripheral blood neutrophils in lung cancer patients [19]. Interestingly, the expression of CCR5 and CXCR1 was modulated on all TANs, whereas the changes in CCR7, CXCR3, and CXCR4 expression were seen only on subsets of TANs. The elevated expression of CCR1, CCR2, CCR3, CCR5, CXCR3, and CXCR4 on neutrophils has also been reported for cells directly isolated from bronchoalveolar lavage fluid of patients with chronic inflammatory lung diseases and from synovial fluid of patients with rheumatoid arthritis [23]. It appears that, in addition to playing a role in neutrophil recruitment, these newly expressed chemokine receptors, which are up-regulated in neutrophils at sites of inflammation, can also regulate many other neutrophil functions. For instance, the binding of CXCL11 to CXCR3 stimulated the release of α-defensin and induced strong bacterial cytotoxicity by neutrophils, whereas the binding of CXCL12 to CXCR4 reduced the respiratory burst in pulmonary neutrophils [23]. Furthermore, a recent study found that CCR7 is involved in the migration of murine neutrophils to lymph nodes, where they may induce and/or modulate adaptive immune responses [24]. Finally, the high expression of CCR5 on neutrophils has been found to play a role in sequestering CCR5 ligands during the resolution of inflammation in murine peritonitis [25].
TANs also express co-stimulatory molecules such as CD86, CD54, OX40L, and 4-1BBL at low levels; however, these are dramatically up-regulated during interactions with activated T cells (Figs. 1, 2) [19, 20]. Normally, resting neutrophils do not express these co-stimulatory molecules on the cell surface, but rather store them in cytoplasmic granules [26]. During the activation process, neutrophils are able to transfer these stored molecules onto the surface and then synthesize them de novo [26]. Surprisingly, in some early stage lung cancer patients, we identified a subset of activated TANs with atypical expression of surface markers which normally belong to the professional antigen-presenting cells (APCs) [20]. This subset of TANs displayed a combination of canonical neutrophil markers (CD11b/CD66b/CD15) and APC markers (CD14/HLA-DR/CCR7/CD86). Given this unique hybrid phenotype, we termed this subset ‘‘APC-like hybrid TANs’’. The frequency of this hybrid population declined as tumors grew larger and they were almost completely absent in large (but still early stage) tumors. Importantly, in addition to a unique phenotype, these hybrid TANs also acquired new functions compared with canonical TANs and PBNs, which will be discussed further below.
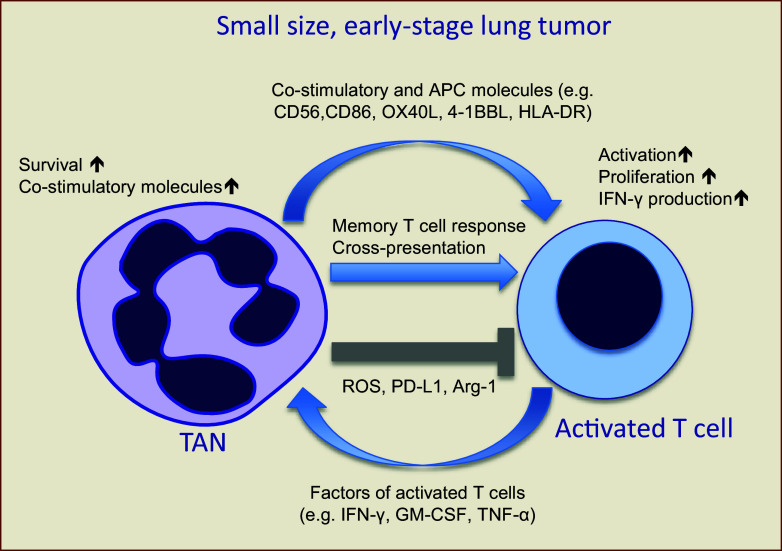
Fig. 2.
Cross talk between TANs and activated T cells in small, early stage human lung cancers. IHC staining of lung tumor sections for TANs and T cells reveals frequent contact between these cells, suggesting that they may interact in the tumor microenvironment of lung cancer patients. This interaction of TANs with activated T cells results in mutual cell activation. Activated T cells produce pro-inflammatory factors (e.g., IFN-γ, GM-CSF, and TNF-α) that increase the activation of TANs, their survival time, and up-regulate co-stimulatory molecules. TAN-affected activated T cells, in turn, further up-regulate their activation markers, produce more IFN-γ, and proliferate. In some patients, TANs can acquire new characteristics of “professional APCs” and become APC-like hybrid TANs. These APC-like hybrid TANs are able to directly stimulate antigen-specific autologous memory and effector T-cell responses, as well as cross-present tumor antigens bound with specific antibodies. It seems that neutrophil-mediated suppression of T-cell response through the ROS, PD-L1, and arginine depletion mechanisms is not active in early stages of tumor development, but might be turned on at more advanced stages
Neutrophils with a composite phenotype of APCs, particularly those with the same phenotype as dendritic cells, have recently been described in many non-cancerous inflammatory conditions and were termed ‘‘neutrophil-DC hybrids’’ [27–30]. However, the APC-like hybrid TANs which we have identified in early stage human lung tumors are not quite similar to these “neutrophil-DC hybrid” cells. We have found that neutrophils in some early stage lung tumors do not exhibit the full phenotype of dendritic cells, but rather acquire only a partial dendritic cell phenotype, as well as a partial monocyte/macrophage phenotype. The APC-like hybrid TANs do not express many other macrophage- and DC-lineage defining markers such as CD209, CD204, CD83, CD80, CD1c, CD163, and CCR6 [20]. Thus, the differentiation of neutrophils in early stage lung tumors results in the formation of activated canonical TANs (CD11b+CD66b+CD15hiHLA-DR−CD14−), as well as a unique rare subset of TANs with composite characteristics of neutrophils and APCs (CD11b+CD66b+CD15hiHLA-DR+CD14+).
In addition to the phenotypic changes observed in TANs, the tumor microenvironment can also prolong the survival of recruited short-lived circulating neutrophils. We found that TANs were able to survive in cell culture longer than circulating neutrophils. In the presence of tumor-conditioned media in vitro, both TAN and naïve blood neutrophils exhibited prolonged survival [19]. This effect is likely due to the fact that pro-inflammatory factors (such as IFN-γ, IL-6, IL-8 and GM-CSF) present in tumor-conditioned media are known to prolong the lifespan of human neutrophils in vitro by delaying apoptosis [19, 31, 32]. For instance, we found that TANs are able to produce a large amount of IL-8 as compared to PBNs [19]. This TAN-derived IL-8 can increase neutrophil survival as well as recruit more neutrophils.
In turn, the cytokines and chemokines produced by TAN subsets within the lung tumor are critical in mediating their effects on the tumor microenvironment and the local inflammatory processes. We found that, in contrast to circulating neutrophils, TANs produce a variety of pro-inflammatory mediators such as CCL2 (MCP-1), CCL3 (MIP-1α), and IL-6, as well as anti-inflammatory IL-1Ra [19]. MCP-1 is a classic monocyte chemoattractant that can also promote adaptive immune changes, particularly Th1-type responses [33]. TANs also secrete CCL3 (MIP-1α). The role of neutrophil-derived CCL3 is critical in the recruitment of immature DCs to sites of inflammation, as well as in the initiation of a protective Th1 response [34]. The cross talk that occurs between neutrophils and dendritic cells has been described previously [35]. As a result of this interaction, neutrophil-activated DCs produce the pro-inflammatory cytokine IL-12, which induces T-cell proliferation. As we often see co-localization of TANs with HLA-DR+ APCs in lung tumors [19, 20], we infer that TANs create a favorable environment for T-cell activation and differentiation at the early stages of tumor development. On the other hand, TAN-secreted MIP-1α may act as a growth, survival, and chemotactic factor for tumor cells and thus could be considered pro-tumorigenic [36]. In our study, we did not see high levels of pro-angiogenic VEGF, but there were other growth factors that might support angiogenesis, such as FGF, HGF, and EGF. Thus, these data suggest a complex role for TANs in early stage human lung cancer.
Dialog between TAN and T cells in early stage lung cancer
Given that cytotoxic T cells are the major effector cells mediating anti-tumor immunity, there is a great deal of emphasis placed on understanding the regulation of T-cell responses by tumor-infiltrating myeloid cells, including neutrophils. In general, neutrophils are extremely versatile cells and depending on environmental cues may exert diverse effects on T-cell response. Numerous studies have convincingly demonstrated that activated neutrophils are able to stimulate T-cell responses by providing co-stimulatory signals [16, 37, 38]. Moreover, in some circumstances, activated neutrophils can even present antigens and thus function as professional APCs [38–40]. Neutrophils can also exert inhibitory effects on T-cell responses via the production of reactive oxygen species and the depletion of extracellular arginine, suggesting a degree of effector function plasticity [15, 41, 42].
These studies, however, have only evaluated peripheral neutrophils and have not examined the interaction of TANs with T lymphocytes in the human tumor microenvironment. In cancer, immunosuppressive function of neutrophils is often associated with a population of circulating low-density granulocytes termed PMN-MDSC [14]. However, there has been some uncertainty whether PMN-MDSC are present in human tumors, particularly at early stages of development, and whether the majority of TANs are actually PMN-MDSC. Given the dual functionality of neutrophils in the regulation of T-cell responses, one of our goals was to determine whether lung tumors at an early stage of development can convert recruited granulocytes into cells with T-cell suppressive activity or whether neutrophils represent a part of anti-tumoral host response with T-cell stimulatory activity.
First, we found the frequent co-localization of TANs and T cells in tumor tissue in lung cancer patients, suggesting that TANs can functionally interact with T cells [19]. Although the presence of a minor suppressive subpopulation of TANs cannot be excluded, our data suggest that in patients with early stage lung cancer, TANs do not significantly contribute to the inhibition of T cell responses [19]. Freshly isolated TANs from early stage lung cancer patients were not able to suppress IFN-γ production or proliferation of T cells that had been activated with anti-CD3/CD28 antibodies or allogeneic dendritic cells (DC). In fact, the TANs isolated from a vast majority of small-sized, early stage tumors were actually able to stimulate T-cell response to varying degrees [19]. Direct cell–cell contact was important for this neutrophil-mediated stimulation of T-cell proliferation. The important feature of this interaction is cross talk between TANs and T cells resulting in mutual cell activation (Fig. 2). During their interaction, T cells further up-regulated activation markers and produced more IFN-γ, whereas TANs showed increased longevity and expressed the co-stimulatory molecules CD86, CD54, OX40L, and 4-1BBL [19]. Typically, these co-stimulatory molecules are expressed on antigen-presenting cells, including mature dendritic cells, activated macrophages, and B cells [43]. Our data suggest that the CD86, CD54, 4-1BBL and OX40L co-stimulatory molecules can also be up-regulated on activated TANs as a result of their interaction with activated T cells. The ability of human neutrophils to up-regulate OX40L has also been reported in human sepsis [44]. The OX40L/OX40 and 4-1BBL/4-1BB pathways could have the potential to enhance anti-tumor immunity and break tumor-induced immune suppression and immunological tolerance. Indeed, the administration of soluble OX40L or gene transfer of OX40L into tumors has been shown to strongly enhance anti-tumor immune function in mice [45]. Furthermore, co-stimulation through 4-1BB/CD137 protects from activation-induced cell death and enhances the anti-tumor effector function of CD8+ melanoma tumor-infiltrating lymphocytes [46]. Our data are in good concordance with the previous studies demonstrating the ability of granulocytes to provide accessory signals for T cell activation [16, 39]. For example, Radsak et al. have shown that PMNs activated with IFN-γ and GM-CSF are able to augment T-cell proliferation by providing co-stimulatory signals through MHC class II, CD86, and CD54 co-stimulatory molecules. Our findings demonstrated that some lung tumors can secrete IFN-γ and GM-CSF, as well as trigger the expression of co-stimulatory molecules, including CD86 and CD54, on the surface of TANs. Furthermore, using an in vitro functional assay, we found that blocking CD86, CD54, OX40L or 4-1BBL with neutralizing antibodies significantly reduced the ability of TANs to stimulate T cells [19]. Thus, the early stage tumors have a potential to create a favorable environment in which TANs can bolster T-cell response.
Importantly, our study also showed that this T-cell stimulatory activity was related to the size of early stage tumors [19]. In contrast to the stimulatory interactions described above, we observed that when TANs were isolated from large early stage tumors, they attenuated T-cell stimulatory ability. It appears that the anti-tumoral potential of neutrophils diminishes during tumor progression, supporting the concept of an immunogenic ‘‘switch’’ from anti-tumor to pro-tumor phenotype [47, 48]. We speculate that, while small-sized early stage tumors in humans have already reached a considerable size and represent the final escape stage of the immunoediting process, the process of tumor evolution is still ongoing, because the profound immunosuppressive environment is not fully developed yet and anti-tumoral neutrophils still prevail in small-size tumors (early escape stage). The resistant tumor clones, which survived the immunoediting process, likely develop further immunosuppressive mechanisms that sustain tumor growth into even more advanced stages. At these advanced tumor stages, the suppressive environment appears to disable anti-tumoral N1 neutrophils and perhaps even convert them into the pro-tumoral N2 type (late escape stage). Thus, it is very possible that N2 pro-tumoral TANs represent the consequence of tumor progression, rather than the cause. This hypothesis has not been experimentally confirmed, but we are currently testing it by studying TANs from patients with advanced lung cancer (stages III and IV). However, obtaining these cells is logistically challenging, since these individuals do not routinely undergo tumor resection and are managed with chemotherapy and radiation therapy. In support of this concept, in studies using murine TANs with a very compressed time scale compared to human tumors, Mishalian et al. reported that TANs from small early tumors (Days 1–7 after implantation) were cytotoxic to tumor cells and produced higher levels of TNF-α, NO, and H2O2 compared with TANs in larger, established tumors [48]. It appears that TANs isolated from small-size, early stage lung cancers resemble the anti-tumor N1 TANs from small, early murine tumors, and as tumors become larger, the TANs become less stimulatory and lose this N1 phenotype.
TANs with composite characteristics of neutrophils and APCs
As mentioned above, in some lung cancer patients, we identified a specialized subpopulation of TANs that exhibited hybrid characteristics of canonical neutrophils and APCs (APC-like hybrid TANs) [20]. These APC-like hybrid TANs accumulated only in small, early stage tumors that produced low amounts of IFN-γ and GM-CSF. These APC-like hybrid neutrophils originated from immature CD15hiCD66b+CD10−CD16−/low/int progenitors and were driven to differentiate into the hybrid phenotype by IFN-γ and GM-CSF present within the tumor microenvironment in a subset of patients with early stage lung cancers [20]. These soluble factors, at the very low concentrations found in tumor-conditioned media, synergistically exert their APC-promoting effect on immature neutrophils via the down-regulation of the transcription factor Ikaros [20]. Interestingly, the development of APC-like hybrid neutrophils was inhibited under hypoxic conditions [20]. This observation might explain their absence in large tumors. APC-like hybrid TANs acquire new functions compared to canonical TANs and are able to: (1) augment both antigen non-specific and tumor-specific T- cell responses, (2) directly stimulate antigen-specific autologous memory and effector T-cell responses to virus and tumor-derived antigens, respectively, and (3) uptake, degrade, and cross-present tumor antigens [20]. Interestingly, in our model antigen system, we found that antigen cross-presentation is triggered in hybrid neutrophils only when tumor antigen (in this case, NY-ESO-1 protein) was delivered as an IgG-immune complex, although this cross-presentation occurred at a relatively low level [20]. Therefore, our data demonstrate that APC-like hybrid neutrophils likely utilize their up-regulated FcγRI and FcγRII receptors for efficient antigen uptake and cross-presentation. It is also possible that these hybrid neutrophils may ‘‘regurgitate’’ processed peptide outside of the cell and thus facilitate the antigen uptake and processing by other professional APCs that are frequently co-localized with neutrophils in lung tumors [40]. We also found a small population of APC-like neutrophils in regional lymph nodes of cancer patients (consistent with their expression of the lymph node homing receptor, CCR7), suggesting they play a potential role in directly priming effector T-cell responses outside of the tumor. Our findings are in line with the previous studies demonstrating the ability of activated neutrophils to function as professional APCs in some inflammatory diseases [28–30]. In particular, it has been shown that activated mouse and human neutrophils are able to present viral and bacterial antigens to T cells and prime antigen-specific Th1 and Th17 cells [16, 29, 40, 49].
The strong T-cell stimulatory activity of hybrid neutrophils may contribute to the amplification of anti-tumor effector CD8 responses or the longevity of CD8 T-cell memory in early stage tumors. Thus, it is possible that these hybrid TANs are a primary contributor to the strong T-cell stimulatory effect of all TANs as observed in small-size, early stage tumors. Consistent with this idea, the absence of hybrid TANs and the establishment of an immunosuppressive tumor microenvironment may explain the inability of TANs to stimulate T cells in large tumors as described above.
Interestingly, we found that the differentiation of APC-like neutrophils into T-cell stimulatory cells strictly depends on the concentration of IFN-γ and GM-CSF in the tumor microenvironment. While low doses of IFN-γ and GM-CSF synergistically drove the differentiation of immature neutrophils into highly immunostimulatory hybrid neutrophils, high doses of IFN-γ resulted in the formation of hybrid neutrophils with high expression of PD-L1 and the ability to profoundly suppress T-cell responses [20]. This dual effect of APC-like hybrid neutrophils on T cells suggests a regulatory role for hybrid neutrophils in inflammation, in situations when stimulation of T cells should be followed by subsequent suppression to resolve the inflammatory process. On the other hand, in advanced stage tumors, where a more chronic inflammatory process exists, immature neutrophils might be directed to suppress anti-tumor T-cell response by converting immature granulocytes into G-MDSC, thus facilitating tumor growth.
We believe that early stage lung tumors exert diverse effects on the differentiation and function of TANs resulting in the formation of two subsets of TANs: canonical and hybrid [20]. Although recruited mature neutrophils acquire the phenotype of activated canonical TANs, immature neutrophils can change their differentiation program depending on the tumor microenvironment. We, therefore, envision a system in which, if tumors produce a sufficient amount of IFN-γ and GM-CSF, the immature neutrophils will differentiate into hybrid neutrophils. In the absence of these factors, or if other inhibitory conditions become dominant in the tumor microenvironment, then the immature neutrophils use a default pathway and become canonical TANs. Given that APC-like TAN frequency correlates inversely with tumor size, their role in modulating the host anti-tumor response in early tumor stages may be critical in limiting disease progression. It is tempting to speculate that by encouraging the development of APC-like TANs pharmacologically, these cells might provide an even more potent augmentation of the host anti-tumor T-cell response and reduce overall tumor burden.
Conclusion
In contrast to observations in murine model systems using rapidly growing, highly immunosuppresive tumor cells, human tumor development represents a process of slow and gradual evolution. Therefore, it is critical to understand the complex interaction of TANs with the tumor microenvironment at all stages of tumor evolution, since their relationship appears to change over time. Our findings characterize tumor-infiltrating neutrophils and their subsets in patients with early stage lung cancer for the first time. Areas of active investigation in our lab focus on the determination of the specific roles of canonical and APC-like hybrid TANs during early and advanced stages of tumor development in lung cancer patients. Deciphering the functional role of TANs in early versus advanced stages of lung cancer will add new knowledge to our understanding of TAN plasticity during tumor progression and may help us to develop different therapeutic strategies to regulate the function of TANs depending on tumor stage. Understanding how to direct and maintain human TANs towards anti-tumor effector cells will open new therapeutic options and aid in the future design of active immunotherapy to boost natural or vaccine induced anti-tumor immunity.
Acknowledgements
Thanks to Steven Albelda, Jason Stadanlick, Abhishek Rao, and Michael Annunziata for critical reading of this review. This work was supported by the Department of Defense (LC140199 # W81XWH-15-1-0717 to Evgeniy Eruslanov), National Institutes of Health (NIH)/National Cancer Institute (NCI) (RO1 CA187392-01A1 to Evgeniy Eruslanov), and the Lung Cancer Translation Center of Excellence of the Abramson Cancer Center at the University of Pennsylvania.
Abbreviations
- EGF
Epidermal growth factor
- FGF
Fibroblast growth factors
- G-MDSCs
Granulocytic-myeloid-derived suppressor cells
- HGF
Hepatocyte growth factor
- ICAM-1
Intercellular adhesion molecule 1
- LDN
Low-density neutrophils
- MCP-1
Monocyte chemotactic protein 1
- MPO
Myeloperoxidase
- NDN
Normal density neutrophils
- NSCLC
Non-small cell lung carcinoma
- NY-ESO-1
New York-esophageal cancer-1
- PBNs
Peripheral blood neutrophils
- PMN-MDSC
Polymorphonuclear myeloid-derived suppressor cells
- TANs
Tumor-associated neutrophils
- VEGF
Vascular endothelial growth factor
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the conference Regulatory Myeloid Suppressor Cells: From Basic Discovery to Therapeutic Application which was hosted by the Wistar Institute in Philadelphia, PA, USA, 16th—19th June, 2016. It is part of a Cancer Immunology, Immunotherapy series of Focussed Research Reviews.
References
- 1.Brandau S. The dichotomy of neutrophil granulocytes in cancer. Semin Cancer Biol. 2013;23:139–140. doi: 10.1016/j.semcancer.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8:125–158. doi: 10.1007/s12307-014-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granot Z, Jablonska J. Distinct functions of neutrophil in cancer and its regulation. Mediators Inflamm. 2015;2015:701067. doi: 10.1155/2015/701067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 5.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 7.Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol. 2014;34:433–454. [PubMed] [Google Scholar]
- 8.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 9.Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 10.Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28:187–196. doi: 10.1016/j.smim.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev. 2016;273:48–60. doi: 10.1111/imr.12448. [DOI] [PubMed] [Google Scholar]
- 12.Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z, Fridlender ZG. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17—a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135:1178–1186. doi: 10.1002/ijc.28770. [DOI] [PubMed] [Google Scholar]
- 13.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, Ariel A, Hovav AH, Henke E, Fridlender ZG, Granot Z. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, Kropf P, Mueller I, Kolb A, Modolell M, Ho AD. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 16.Radsak M, Iking-Konert C, Stegmaier S, Andrassy K, Hansch GM. Polymorphonuclear neutrophils as accessory cells for T-cell activation: major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;101:521–530. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulder WM, Koenen H, van de Muysenberg AJ, Bloemena E, Wagstaff J, Scheper RJ. Reduced expression of distinct T-cell CD molecules by collagenase/DNase treatment. Cancer Immunol Immunother. 1994;38:253–258. doi: 10.1007/BF01533516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quatromoni JG, Singhal S, Bhojnagarwala P, Hancock WW, Albelda SM, Eruslanov E. An optimized disaggregation method for human lung tumors that preserves the phenotype and function of the immune cells. J Leukoc Biol. 2015;97:201–209. doi: 10.1189/jlb.5TA0814-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, Conejo-Garcia JR, Feldman M, Albelda SM, Singhal S. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singhal S, Bhojnagarwala PS, O’Brien S, Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L, Deshpande C, Feldman MD, Hancock WW, Conejo-Garcia JR, Albelda SM, Eruslanov EB. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30:120–135. doi: 10.1016/j.ccell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch V. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009;155:559–566. doi: 10.1111/j.1365-2249.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costantini C, Calzetti F, Perbellini O, Micheletti A, Scarponi C, Lonardi S, Pelletier M, Schakel K, Pizzolo G, Facchetti F, Vermi W, Albanesi C, Cassatella MA. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFN{gamma}: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–1686. doi: 10.1182/blood-2010-06-287243. [DOI] [PubMed] [Google Scholar]
- 23.Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, Hector A, Eber E, Marcos V, Bittmann I, Eickelberg O, Griese M, Roos D. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol. 2008;181:8053–8067. doi: 10.4049/jimmunol.181.11.8053. [DOI] [PubMed] [Google Scholar]
- 24.Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, Magistrelli G, Masternak K, Chevailler A, Delneste Y, Jeannin P. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood. 2011;117:1196–1204. doi: 10.1182/blood-2009-11-254490. [DOI] [PubMed] [Google Scholar]
- 25.Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandilands GP, McCrae J, Hill K, Perry M, Baxter D. Major histocompatibility complex class II (DR) antigen and costimulatory molecules on in vitro and in vivo activated human polymorphonuclear neutrophils. Immunology. 2006;119:562–571. doi: 10.1111/j.1365-2567.2006.02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng S, Matsushima H, Okamoto T, Yao Y, Lu R, Page K, Blumenthal RM, Ward NL, Miyazaki T, Takashima A. Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood. 2013;121:1690–1700. doi: 10.1182/blood-2012-07-445197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, Mayuzumi N, Kotol PF, Chojnacki BJ, Miyazaki T, Gallo RL, Takashima A. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood. 2013;121:1677–1689. doi: 10.1182/blood-2012-07-445189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol. 2011;23:317–326. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansch GM, Wagner C. Expression of MHC class II antigen and coreceptor molecules in polymorphonuclear neutrophils. Chem Immunol Allergy. 2003;83:45–63. doi: 10.1159/000071556. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi SD, Voyich JM, Whitney AR, DeLeo FR. Spontaneous neutrophil apoptosis and regulation of cell survival by granulocyte macrophage-colony stimulating factor. J Leukoc Biol. 2005;78:1408–1418. doi: 10.1189/jlb.0605289. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura T, Takahashi M. IFN-gamma-mediated survival enables human neutrophils to produce MCP-1/CCL2 in response to activation by TLR ligands. J Immunol. 2007;179:1942–1949. doi: 10.4049/jimmunol.179.3.1942. [DOI] [PubMed] [Google Scholar]
- 33.Rand ML, Warren JS, Mansour MK, Newman W, Ringler DJ. Inhibition of T cell recruitment and cutaneous delayed-type hypersensitivity-induced inflammation with antibodies to monocyte chemoattractant protein-1. Am J Pathol. 1996;148:855–864. [PMC free article] [PubMed] [Google Scholar]
- 34.Charmoy M, Brunner-Agten S, Aebischer D, Auderset F, Launois P, Milon G, Proudfoot AE, Tacchini-Cottier F. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 2010;5:6. doi: 10.1371/journal.ppat.1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Gisbergen KP, Sanchez-Hernandez M, Geijtenbeek TB, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY. Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood. 2003;101:3568–3573. doi: 10.1182/blood-2002-08-2383. [DOI] [PubMed] [Google Scholar]
- 37.Beauvillain C, Delneste Y, Scotet M, Peres A, Gascan H, Guermonprez P, Barnaba V, Jeannin P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–2973. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- 38.Thewissen M, Damoiseaux J, van de Gaar J, Tervaert JW. Neutrophils and T cells: bidirectional effects and functional interferences. Mol Immunol. 2011;48:2094–2101. doi: 10.1016/j.molimm.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Ashtekar AR, Saha B. Poly’s plea: membership to the club of APCs. Trends Immunol. 2003;24:485–490. doi: 10.1016/S1471-4906(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 40.Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J Immunol. 2001;167:2538–2546. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- 41.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 43.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 44.Karulf M, Kelly A, Weinberg AD, Gold JA. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol. 2010;185:4856–4862. doi: 10.4049/jimmunol.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andarini S, Kikuchi T, Nukiwa M, Pradono P, Suzuki T, Ohkouchi S, Inoue A, Maemondo M, Ishii N, Saijo Y, Sugamura K, Nukiwa T. Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res. 2004;64:3281–3287. doi: 10.1158/0008-5472.CAN-03-3911. [DOI] [PubMed] [Google Scholar]
- 46.Chacon JA, Wu RC, Sukhumalchandra P, Molldrem JJ, Sarnaik A, Pilon-Thomas S, Weber J, Hwu P, Radvanyi L. Co-stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy. PLoS ONE. 2013;8:e60031. doi: 10.1371/journal.pone.0060031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Granot Z, Fridlender ZG. Plasticity beyond cancer cells and the “immunosuppressive switch”. Cancer Res. 2015;75:4441–4445. doi: 10.1158/0008-5472.CAN-15-1502. [DOI] [PubMed] [Google Scholar]
- 48.Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother. 2013;62:1745–1756. doi: 10.1007/s00262-013-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tvinnereim AR, Hamilton SE, Harty JT. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J Immunol. 2004;173:1994–2002. doi: 10.4049/jimmunol.173.3.1994. [DOI] [PubMed] [Google Scholar]