Abstract
Drug interactions may dictate the failure or success of a treatment. Patients undergoing hematopoietic stem cell transplantation (HSCT) are exposed to various types of drugs and it is of utmost importance to understand how these drugs interact. The pharmacokinetics of busulfan, melphalan and cyclophosphamide, commonly used drugs in HSCT, are known to be affected by a variety of drugs with differing molecular structures. We hypothesized that these structurally-unrelated drugs affect the transport of DNA alkylating agents. To test this hypothesis, we developed a flow cytometry assay that used 5-carboxyfluorescein diacetate acetoxymethyl ester, which is cleaved by non-specific intracellular esterases to 5-carboxyfluorescein (5-CF), a fluorescent ligand for the drug transporter MRP1. A decreased 5-CF efflux in the presence of a test compound suggests competitive inhibition. We demonstrated that chlorambucil, 4-hydroperoxycyclophosphamide, ketoconazole, ethacrynic acid, everolimus and sirolimus strongly inhibited the 5-CF efflux in lymphoma and leukemia cell lines. The efflux of these drugs partially depends on the glutathione (GSH) level and their cytotoxicity is synergistic with inhibited GSH synthesis. This is consistent with the hypothesis that their GSH-conjugated products are ligands of a common cellular drug transporter. Our results may explain some clinical observations on the effects of various drugs on the pharmacokinetics and pharmacodynamics of alkylating agents and the assay may be used to deduce interaction mechanisms of drugs transported by a common system.
Keywords: carboxyfluorescein, drug efflux method, drug interactions, drug transporter, flow cytometry, stem cell transplantation
Introduction
Cancer patients are exposed to various antineoplastic and non-antineoplastic drugs during their treatments. These drugs are not necessarily given concurrently and prior exposure to one drug may affect the efficacy and toxicity of the succeeding agents by altering their pharmacokinetics and pharmacodynamics [1-6]. This scenario is especially true for patients undergoing hematopoietic stem cell transplantation (HSCT) where various types of medications are utilized.
One group of drugs commonly used in HSCT is DNA alkylating agents (AAs) such as busulfan (Bu), which is used in the pretransplant conditioning regimen. It is used in combination with other drugs with similar or different mechanisms of action. However, combination of Bu with other drugs sometimes results in undesirable drug interactions. For example, metronidazole, itraconazole and deferasirox were found to change Bu pharmacokinetics resulting in high plasma levels and possible toxicity [2,4-6]. The mechanistic nature of these interactions has not been fully characterized and the possible role of glutathione (GSH), known to be involved in Bu metabolism, remains undetermined. Melphalan (Mel), another DNA alkylating agent, was found to accumulate in a multiple myeloma patient taking sulfamethoxazole [7]. In an earlier report, flunarizine, a diphenylpiperazine calcium channel blocker, enhanced Mel cytotoxicity against rhabdomyosarcoma xenograft, which the authors attributed to possible modification of transport mechanisms responsible for cellular Mel retention, but without providing supporting data [8]. Increased Mel cytotoxicity was observed in the K562 cell line exposed to imatinib; the authors attributed this synergism to inhibition of BCR/ABL kinase but they did not determine the effects of imatinib on Mel efflux [9]. Thus, while sulfamethoxazole, flunarizine and imatinib are structurally unrelated, they all affect the cytotoxic efficacy of Mel.
Based on these pre-clinical and clinical observations, we hypothesized that these drug interactions may be attributed to the drug-induced alteration of a common mechanism of cellular drug efflux. To test our hypothesis we, therefore, developed a flow cytometry assay based on the inhibition of efflux of a fluorescent ligand by the test pharmaceutically active agent(s). Using this assay, we identified structurally unrelated drugs that may be transported by the ABC transporter protein MRP1, and we showed the relevance of GSH involvement in their transport of AAs. We conclude that although these drugs may have different functional groups their GSH-conjugated products may compete for the same transport mechanism as non-cytotoxic agents. These observations may explain some drug interactions observed in a clinical setting.
Methods
Cells and drugs
The J45.01 T-cell line and Toledo B-cell line were obtained from American Type Culture Collection (Manassas, VA). KBM3/Bu2506 is a busulfan-resistant AML cell line established in our laboratory as previously described [10]. MOLM14 is an AML cell line obtained from Dr. Michael Andreeff's laboratory (UT MD Anderson Cancer Center, Houston, TX). All cells were cultured in RPMI 1640 (Mediatech, Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum (FBS: Sigma-Aldrich, St. Louis, MO) and 100 U/mL penicillin and 100 μg/mL streptomycin (Mediatech) at 37°C in a humidified atmosphere of 5% CO2 in air. 5-Carboxyfluorescein diacetate acetoxymethyl ester (5-CFDA) was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA). Verapamil and MK571 were purchased from Selleckchem (Houston, TX). Chlorambucil, busulfan, melphalan, bendamustine, cyclophosphamide, ifosfamide, ketoconazole, posaconazole, fluconazole, itraconazole, metronidazole, ethacrynic acid, everolimus, sirolimus, phenytoin, levetiracetam, and buthionine sulphoximine (BSO) were obtained from Sigma-Aldrich Corp. (St. Louis, MO). 4-Hydroperoxycyclophosphamide (4-HC) and 4-hydroperoxyifosfamide (4-HI) were generous gifts from from Dr. Scott Rowley (Hackensack University Medical Center, Hackensack, New Jersey), and Dr. Robert F. Struck (Southern Research Institute, Birmingham, Alabama) respectively.
Cellular efflux assay by flow cytometry
A 24-hr cell culture was centrifuged for 4 min at 200 × g at 4°C and the resulting cell pellet was resuspended in culture medium at a cell density of 5 × 105 cells/mL. 5-CFDA was added to a final concentration of 0.1 μM and kept on ice for 20 min to allow its cellular uptake. Cells were washed, resuspended in culture medium (5 × 105 cells/mL), divided into two aliquots (0.5 mL) and kept either on ice (inactive drug transporter) or at 37°C (active drug transporter) for 30 min. A test drug was added during the incubation to determine its effect on the efflux of 5-CF. Cells were centrifuged as above at 4°C, washed with cold culture medium and resuspended in 0.5 mL culture medium containing 0.1 μg/mL propidium iodide. Cells were kept on ice for 20 min prior to flow cytometry analysis.
Determination of cellular GSH level
Cells were incubated with BSO for 48 hrs and the level of cellular GSH was determined using the Glutathione Assay kit from Cayman Chemical (Ann Arbor, MI) following the manufacturer's protocol.
Determination of the level of reactive oxygen species (ROS)
The effects of BSO on the early production of ROS were determined by incubating cells with BSO for 24 hrs. Cells were aliquoted (0.5 ml) into 5 ml tubes and 1 μl of 1.5 mM CM-H2DCFDA (5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester, dissolved in dimethyl sulfoxide), an ROS indicator that diffuses into cells where it is oxidized to a fluorescent product (Life Technologies, Eugene, OR). Cells were incubated at 37 °C for 1 h and immediately analyzed with a Gallios Flow Cytometer (Beckman Coulter, Brea, CA, USA) using excitation/emission wavelengths of 492/520 nm. Geometric means of the fluorescence intensities were compared, and the relative fold increase in ROS production was calculated.
Western blot analysis
Cells exposed to BSO for 48 h were collected by centrifugation, washed with ice-cold phosphate-buffered saline (PBS) and lysed with cell lysis buffer (Cell Signaling Technology, Danvers, MA). The protein concentrations were determined using a BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). Proteins were resolved on polyacrylamide-SDS gels and blotted onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Western blot analyses were done by chemiluminescence using the Immobilon Western Chemiluminescent HRP Substrate (EMD Mil lipore, Danvers, MA). Antibodies to MDR1, MRP1 and BCRP were obtained from Abeam (Cambridge, MA). Antibody to β-ACTIN was obtained from Sigma-Aldrich.
Cytotoxicity assay
Cells exposed to drugs were aliquoted (100 μl) into 96-well and analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay [11]. Briefly, 50 μl of 5 μlg/mL MTT reagent in PBS was added per well and incubated for 4 h at 37°C. The solid reaction product was dissolved by adding 100 μl solubilization solution (0.1 N HC1 in isopropanol containing 10% Triton X) to each well, mixing, and incubating at 37°C overnight. Absorbance at 570 nm was measured using a Victor® ×3 (Perkin Elmer Life and Analytical Sciences, Shelton, CT) plate reader. Cell survival was determined relative to the control cells exposed to solvent alone.
Results
5-Carboxyfluorescein (5-CF) as a drug transporter ligand and flow cytometry tracer
5-CFDA passively enters cells and when hydrolyzed by non-specific esterases yields negatively-charged 5-carboxyfluorescein (5-CF), which is retained in cells (Fig. 1A). 5-CF is transported out of cells via drug transporter MRP1 ([12]. Its fluorescence (excitation/emission at 492/517 nm) enables visualization of labeled cells. These properties make 5-CF an excellent flow cytometry tracer to assay the drug transport activity of MRP1.
Fig. 1.
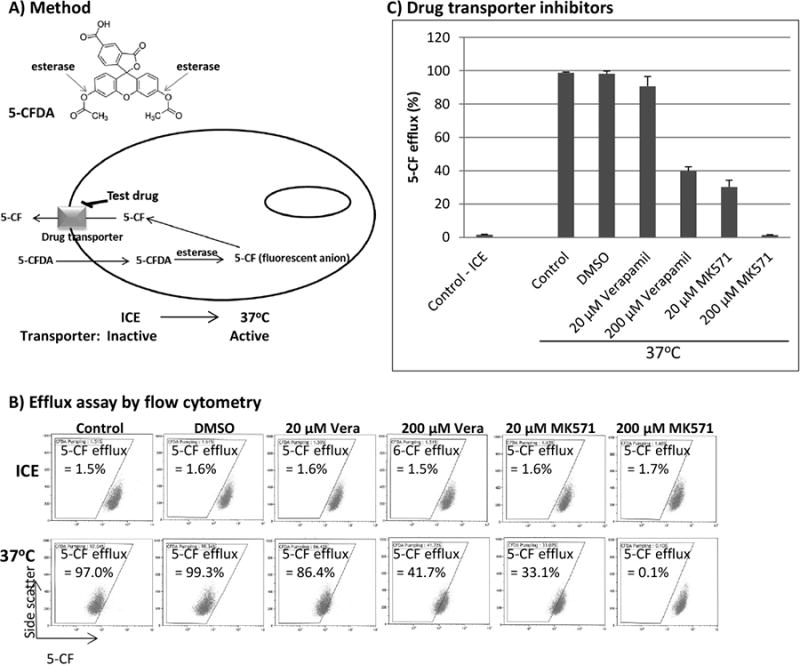
Assay for cellular efflux of 5-CF by flow cytometry. (A) Chemical structure of 5-carboxyfluorescein diacetate acetoxymethyl ester (5-CFDA) and illustration of the assay. (B and C) J45.01 cells were suspended in a growth medium containing 0.1 μM 5-CFDA and kept on ice for 20 min to allow its uptake as described under Materials and Methods. The effects of verapamil (MDR1 inhibitor) and MK571 (MRP1 inhibitor) on the efflux of 5-CF at 37°C was determined by flow cytometry (B) and the average values±SD from three independent experiments were graphed as shown (C).
We initially optimized the conditions for cellular efflux assay of 5-CF by flow cytometry. Cells that take up 5-CFDA, which is eventually converted to 5-CF, were kept on ice to inhibit 5-CF energy-dependent MRP 1-mediated efflux; shifting the cells to 37°C activated MRP1 and caused efflux of 5-CF as suggested by a downward shift in fluorescence intensity (Fig. 1B). Changes in fluorescence intensity were tracked by drawing an arbitrary line (Fig. 1B); cell population with fluorescence below this line would indicate 5-CF efflux. Based on this method, control cells kept on ice showed an efflux value of 1.5% which increased to 97.0% when shifted to 37°C; addition of 0.2% DMSO did not significantly affect the 5-CF efflux. The addition of 20 μM verapamil (stock solution was prepared in DMSO), a known inhibitor of the drug transporter MDR1 ([13], slightly decreased the 5-CF efflux to 86.4% (Fig. 1B). A more significant inhibition of the 5-CF efflux to 33.1% was observed in the presence of 20 μM MK571, a known inhibitor of the drug transporter MRP 1 [14]. The efflux of 5-CF was totally obliterated (0.1 %) in the presence of 200 μM MK571 (Fig. 1B). These results are graphically represented in Fig. 1C. The data suggest that 5-CF can be used as a tracer to identify drugs which modulate the activity of MRP 1.
Various pharmaceutically active agents inhibit the efflux of 5-CF
We next sought to determine which drugs inhibit the drug transport activity of MRP1. We initially examined DNA-alkylating agents which are used to treat blood disorders. As shown in Fig. 2A, chlorambucil inhibited the 5-CF efflux in a concentration-dependent manner in J45.01 cell line; using 50 μM chlorambucil 88.9% 5-CF efflux was observed which decreased to 40.9% and 5.2% in the presence of 100 μM and 200 μM chlorambucil, respectively.
Fig. 2.
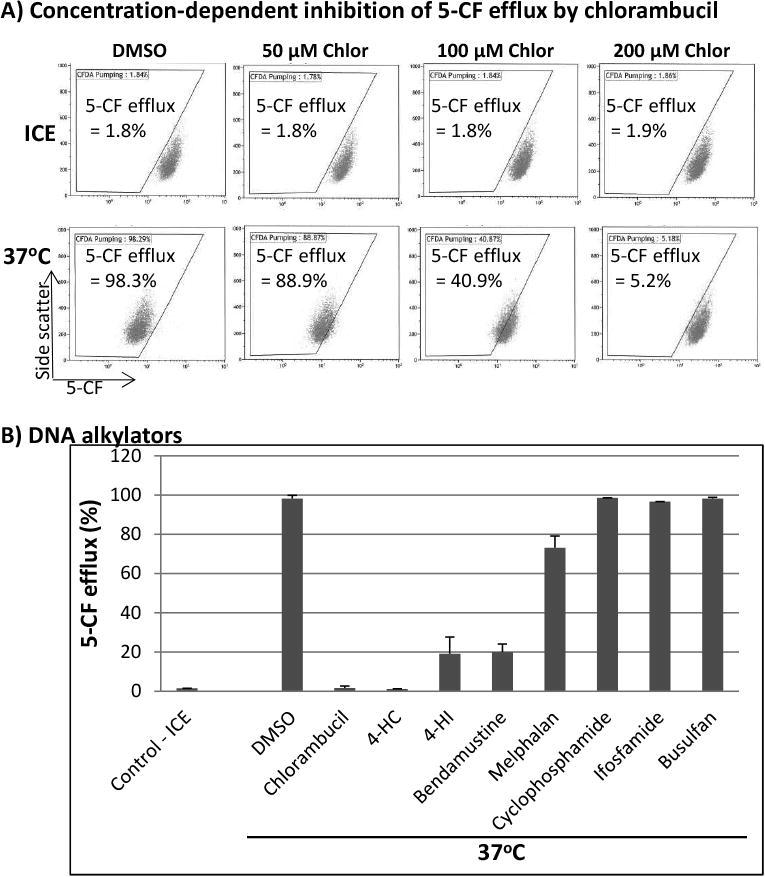
Effects of DNA alkylating agents on the efflux of 5-CF. (A) J45.01 cells were incubated with 5-CFDA in the presence of DMSO (solvent control) or various concentrations of chlorambucil (Chlor) on ice or at 37°C, and 5-CF efflux was measured by flow cytometry. (B) Different DNA alkylators (200 μM each) were examined for their effects on the efflux of 5-CF.
4-HC: 4-hydroperoxycyclophosphamide; 4-HI: 4-hydroperoxyifosfamide.
4-hydroperoxycyclophosphamide (4-HC) and its analog 4-HI are two nitrogen mustard alkylating agents effectively found to inhibit the 5-CF efflux (Fig. 2B). At 200 μM, both drugs inhibited the 5-CF efflux to less than 20%. Their pro-drug precursors cyclophosphamide and ifosfamide, however, did not show significant inhibitory activity (Fig. 2B) whereas melphalan (200 μM) slightly inhibited the 5-CF efflux.
Among the antifungal triazole drugs, ketoconazole was the most effective at inhibiting the 5-CF efflux (∼20 % efflux); the presence of posaconazole resulted in ∼78% 5-CF efflux (Fig. 3A). At equimolar concentrations of 200 μM, fluconazole, itraconazole and metronidazole, did not cause any significant inhibition of the 5-CF efflux.
Fig. 3.
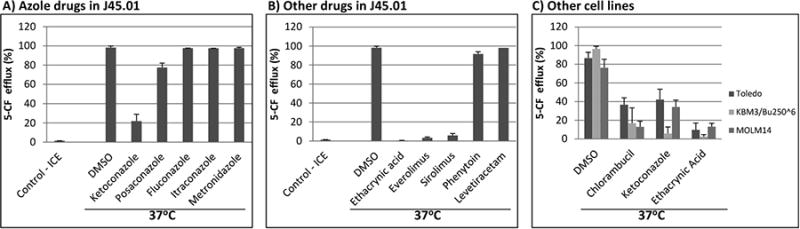
Effects of different drugs on the efflux of 5-CF. (A) J45.01 cells were incubated with 5-CFDA in the presence of DMSO (solvent control) or 200 μM of various azole drugs on ice or at 37°C, and 5-CF efflux was measured by flow cytometry. (B) Drugs (200 μM each) with different mechanisms of action were analyzed for their effects on the efflux of 5-CF. (C) Different cell lines including Toledo, KBM3/Bu2506, and MOLM14 were incubated with 5-CFDA in the presence of DMSO (solvent control), or chlorambucil, ketoconazole or ethacrynic acid (200 μM each) at 37°C and analyzed by flow cytometry.
Ethacrynic acid, everolimus and sirolimus, but not phenytoin and levetiracetam (200 μM each) showed strong inhibition of the 5-CF efflux (Fig. 3B). Further analysis showed that ethacrynic acid inhibited the 5-CF efflux between 50 μM and 100 μM while everolimus and sirolimus were both very effective in inhibiting the 5-CF efflux even at 20 μM concentration (data not shown).
To determine whether the effects of these various drugs on the efflux of 5-CF were specific to J45.01 cells, three other cell lines were analyzed. Toledo, a B-cell lymphoma cell line, efficiently effluxed (87%) 5-CF in the presence of DMSO (solvent control); the efflux decreased to 37%, 42% and 10% in the presence of chlorambucil, ketoconazole, or ethacrynic acid, respectively (Figure 3C). Two AML cell lines, KBM3/Bu2506 and MOLM14, similarly effluxed 5-CF (96% and 76%, respectively), which significantly decreased in the presence of chlorambucil, ketoconazole, or ethacrynic acid (Figure 3C). These results suggest that the effects of these three drugs on the efflux of 5-CF are not cell-line specific.
Inhibition of GSH synthesis enhances the cytotoxicity of DNA alkylators and EA by inhibiting their cellular efflux
Based on our results, it is evident that multiple drugs with differing chemical structures may inhibit the drug transport activity of MRP1. Based on previous reports, cellular transports of these drugs may require conjugation with GSH [15, 16]. We therefore determined the importance of GSH in the efficacy and transport of these drugs. We inhibited the synthesis of GSH using BSO and examined its effects on ROS production, cell survival and levels of drug transporter proteins. Exposure of cells to 0.01 mM and 1 mM BSO resulted in ∼80% and ∼95% inhibition of glutathione synthesis, respectively (Fig. 4A). Since GSH is an anti-oxidant that neutralizes ROS [17], we sought to determine if the BSO-mediated decrease in GSH concentration would result in increased ROS production. Indeed, 24-hr exposure of J45.01 cells to BSO increased the level of ROS (Fig. 4B) in a dose-dependent manner. Similar results were obtained in Toledo and MOLM14 cells (data not shown). Despite an early increase in the level of ROS cell survival was not significantly affected (Fig. 4C).
Fig. 4.
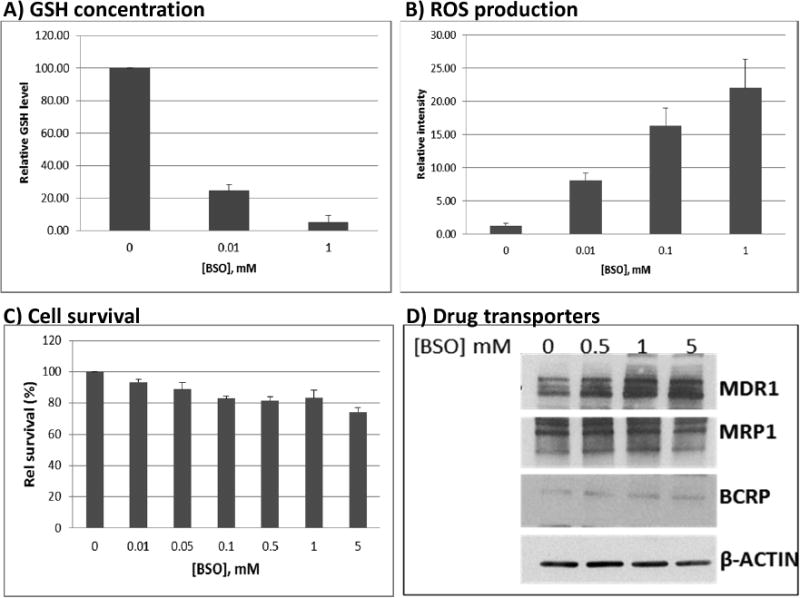
Effects of buthionine sulfoximine (BSO) on GSH concentration, ROS production, cell survival, and expression of drug transporters. J45.01 cells were incubated with BSO for 48 hrs and the level of cellular GSH (A), cell survival (C), and changes in the expression of drug transporters (D) were determined. Early changes in the level of ROS were determined in J45.01 cells exposed to BSO for 24 hrs (B). The results are averages±SD (A, B, C) or a representative (D) of three independent experiments.
Changes in the expression of drug transporters may also affect drug efflux. While MDR1 protein increased in the presence of 1 mM BSO, the level of drug transporters MRP1 and BCRP did not change significantly (Fig. 4D).
If GSH were required for cellular efflux of alkylating agents and ethacrynic acid, we presumed that inhibition of GSH synthesis would result in synergistic cytotoxicity. Indeed, the presence of 0.1-1 mM BSO synergistically increased the cytotoxicity of chlorambucil, bendamustine, 4-HC and ethacrynic acid in J45.01 cells (Fig. 5A). These results are consistent with the reported synergistic cytotoxicity of BSO and melphalan in preclinical models of multiple myeloma [18].
Fig. 5.
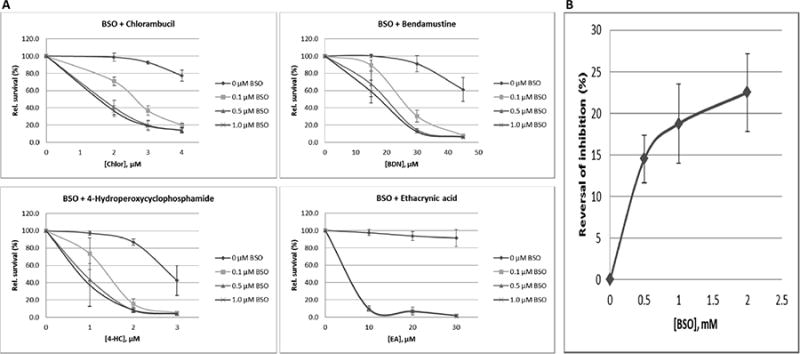
Synergistic cytotoxicity of BSO with various chemotherapeutic drugs. J45.01 cells were pre-exposed to BSO for 16 hrs, added the indicated drug, and MTT assay was performed after 48 hrs (A). Efflux of 5-CF in the presence of 200 μM chlorambucil was analyzed after overnight exposure of J45.01 cells to BSO (B). The results are averages±SD of at least three independent experiments.
To further determine the role of GSH in drug transport, we examined the effects of BSO on the ability of chlorambucil to inhibit 5-CF efflux. While 200 μM chlorambucil effectively inhibited 5-CF efflux, pre-exposure of cells to 0.5 mM - 2 mM BSO reversed this inhibition by 14-23%, suggesting the importance of glutathione in chlorambucil-mediated inhibition of the 5-CF efflux (Fig. 5B).
Discussion
We describe in this study a flow cytometry assay which can be used to analyze cellular efflux of various pharmaceutically active agents, including cytotoxic drugs. This method uses 5-CFDA which, when cleaved by intracellular esterases, results in a negatively charged fluorescent product, 5-CF, that can be exported from the cell by the MRP1 protein. Any drug that inhibits the 5-CF efflux would suggest competition for a common transport mechanism and implies that the test drug is a ligand for the MRP1 drug transporter. Further, we can infer the importance of GSH in the transport of these drugs based on our results, which suggest a coordinated action of GSH metabolic enzymes (eg. glutathione S-transferases) and MRP1.
Among the DNA-alkylating agents tested, chlorambucil, 4-HC, 4-HI and bendamustine had the highest activity of inhibiting the 5-CF efflux. These results are consistent with the known in vitro antineoplastic activity of 4-HC and 4-HI, which are active metabolic products of cyclophosphamide and ifosfamide, respectively [19, 20]. 4-HC and 4-HI are known to be partly metabolized through GSH-conjugation in human cells [21-23]. Cyclophosphamide and ifosfamide, on the other hand, did not show significant activity. These observations are expected since both cyclophosphamide and ifosphamide are stable prodrugs with negligible in vitro activity.
The observed inhibition of the 5-CF efflux by these various drugs was partly dependent on GSH. Inhibition of GSH synthesis by BSO partly reversed chlorambucil-mediated inhibition of the 5-CF efflux (Fig. 5B) and made J45.01 more sensitive to chlorambucil, 4-HC, bendamustine and ethacrynic acid (Fig. 5A). These results are consistent with the coordinated action of glutathione S-transferases and MRP1 in chlorambucil and ethacrynic acid detoxicification in MCF7 breast cancer cells [24, 25].
In summary, the method we described in this study can be used to screen for drugs that may be transported out of the cell through the MRP1 system. The results will be useful in investigating drug interactions; drugs that use similar transport mechanisms may affect each other's pharmacokinetics, resulting in altered cytotoxicity and differentially influence the clinical safety profile of cytotoxic agents, especially when used in high-dose chemotherapy. In fact, the pharmacokinetics of busulfan and melphalan, two DNA alkylating drugs used as part of pretransplant conditioning regimens, were altered in patients taking deferasirox and sulfamethoxazole [4, 7]. Based on our present study, the observed low clearance of busulfan and melphalan in these patients may be due to the inhibition of their efflux mediated by deferasirox and sulfamethoxazole. The assay we described in this report also may be used to study drug resistance through drug transport-mediated mechanisms.
Highlights.
Various drugs are used in combination chemotherapy.
Drug interactions may decrease or increase efficacy/toxicity.
Assay methods for cellular drug interactions are limited.
5-carboxyfluorescein is an MRP1 substrate and tracer for flow cytometry.
A method to determine drug interaction using efflux of 5-CF is described.
Acknowledgments
Part of this research was performed in the Flow Cytometry & Cellular Imaging Facility, which is supported in part by the National Institutes of Health through M.D. Anderson's Cancer Center Support Grant CA016672. This work was also supported by the Stephen L. and Lavinia Boyd Fund for Leukemia Research, and by funds donated by grateful patients.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hassan M, Oberg G, Björkholm M, Wallin I, Lindgren M. Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer Chemo Pharm. 1993;33:181–186. doi: 10.1007/BF00686213. [DOI] [PubMed] [Google Scholar]
- 2.Buggia I, Zecca M, Alessandrino EP, et al. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow transplantation. GITMO (Gruppo Italiano Trapianto di Midollo Osseo) Anticancer Res. 1996;16:2083–2088. [PubMed] [Google Scholar]
- 3.Hassan M, Ljungman P, Ringdén O, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. 2000;25:915–924. doi: 10.1038/sj.bmt.1702377. [DOI] [PubMed] [Google Scholar]
- 4.Sweiss K, Patel P, Rondelli D. Deferasirox increases Bu blood concentrations. Bone Marrow Transplant. 2012;47:315–316. doi: 10.1038/bmt.2011.75. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson C, Aschan J, Hentschke P, Ringden O, Ljungman P, Hassan M. The effect of metro-nidazole on busulfan pharmacokinetics in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31:429–435. doi: 10.1038/sj.bmt.1703896. [DOI] [PubMed] [Google Scholar]
- 6.Gulbis AM, Culotta KS, Jones RB, Andersson BS. Busulfan and metronidazole: an often forgotten but significant drug interaction. Annals Pharma. 2011;45:e39. doi: 10.1345/aph.1Q087. [DOI] [PubMed] [Google Scholar]
- 7.Jolivot PA, Poinsignon V, Paci A, et al. A case of melphalan sustained accumulation in an 80-year old patient. Inter J Clin Pharm. 2015;37:984–987. doi: 10.1007/s11096-015-0197-x. [DOI] [PubMed] [Google Scholar]
- 8.Castellino SM, Friedman HS, Elion GB, et al. Flunarizine enhancement of melphalan activity against drug-sensitive/resistant rhabdomyosarcoma. Br J Cancer. 1995;71:1181–1187. doi: 10.1038/bjc.1995.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giallongo C, La Cava P, Tibullo D, et al. Imatinib increases cytotoxicity of melphalan and their combination allows an efficient killing of chronic myeloid leukemia cells. Eur J Haemat. 2011;86:216–225. doi: 10.1111/j.1600-0609.2010.01570.x. [DOI] [PubMed] [Google Scholar]
- 10.Valdez BC, Murray D, Ramdas L, et al. Altered gene expression in busulfan-resistant human myeloid leukemia. Leuk Res. 2008;32:1684–1697. doi: 10.1016/j.leukres.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Dogan AL, Legrand O, Faussat AM, Perrot JY, Marie JP. Evaluation and comparison of MRP1 activity with three fluorescent dyes and three modulators in leukemic cell lines. Leuk Res. 2004;28:619–622. doi: 10.1016/j.leukres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Tsuruo T, Iida H, Yamashiro M, Tsukagoshi S, Sakurai Y. Enhancement of vincristine- and adriamycin-induced cytotoxicity by verapamil in P388 leukemia and its sublines resistant to vincristine and Adriamycin. Biochem Pharma. 1982;31:3138–3140. doi: 10.1016/0006-2952(82)90097-1. [DOI] [PubMed] [Google Scholar]
- 14.Gekeler V, Ise W, Sanders KH, Ulrich WR, Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem Biophys Res Comm. 1995;208:345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- 15.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16:31–42. [PubMed] [Google Scholar]
- 16.Hassan M, Andersson BS. Role of pharmacogenetics in busulfan/cyclophosphamide conditioning therapy prior to hematopoietic stem cell transplantation. Pharmacogen. 2013;14:75–87. doi: 10.2217/pgs.12.185. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong JS, Steinauer KK, Hornung B, et al. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line, Cell Death Differ. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 18.Tagde A, Singh H, Kang MH, Reynolds CP. The glutathione synthesis inhibitor buthionine sulfoximine synergistically enhanced melphalan activity against preclinical models of multiple myeloma. Blood Cancer J. 2014;4:e229. doi: 10.1038/bcj.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brade WP, Seeber S, Herdrich K. Comparative activity of ifosfamide and cyclophosphamide. Cancer Chemo Pharma. 1986;18(Suppl. 2):1–9. doi: 10.1007/BF00647438. [DOI] [PubMed] [Google Scholar]
- 20.Ludeman SM. The chemistry of the metabolites of cyclophosphamide. Current Pharma Design. 1999;5:627–643. [PubMed] [Google Scholar]
- 21.Lind MJ, McGown AT, Hadfield JA, Thatcher N, Crowther D, Fox BW. The effect of ifosfamide and its metabolites on intracellular glutathione levels in vitro and in vivo. Bio-chem Pharm. 1989;38:1835–1840. doi: 10.1016/0006-2952(89)90419-x. [DOI] [PubMed] [Google Scholar]
- 22.Peters RH, Ballard K, Oatis JE, Jollow DJ, Stuart RK. Cellular glutathione as a protective agent against 4-hydroperoxycyclophosphamide cytotoxicity in K-562 cells. Cancer Chemo Pharma. 1990;26:397–402. doi: 10.1007/BF02994088. [DOI] [PubMed] [Google Scholar]
- 23.Meier T, Allenbacher A, Mueller E, et al. Ifosfamide induced depletion of glutathione in human peripheral blood lymphocytes and protection by mesna. Anticancer Drugs. 1995;5:403–409. doi: 10.1097/00001813-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Morrow CS, Smitherman PK, Diah SK, Schneider E, Townsend AJ. Coordinated action of glutathione S-transferases (GSTs) and multidrug resistance protein 1 (MRP1) in antineo-plastic drug detoxification: Mechanism of GST A1-1- and MRP1-associated resistance to chlorambucil in MCF7 breast carcinoma cells. J Biol Chem. 1998;273:20114–20120. doi: 10.1074/jbc.273.32.20114. [DOI] [PubMed] [Google Scholar]
- 25.Morrow CS, Smitherman PK, Townsend AJ. Combined expression of multidrug resistance protein (MRP) and glutathione S-transferase P1-1 (GSTP1-1) in MCF7 cells and high level resistance to the cytotoxicities of ethacrynic acid but not oxazaphosphorines or cisplatin. Biochem Pharma. 1998;56:1013–1022. doi: 10.1016/s0006-2952(98)00240-8. [DOI] [PubMed] [Google Scholar]


