Abstract
Background
The risk of hepatocellular carcinoma (HCC) in patients with autoimmune hepatitis (AIH) is unclear. We conducted a systematic review and meta-analysis of the incidence of HCC and associated risk factors among patients with AIH.
Methods
We searched PubMed, Embase, and reference lists from relevant articles through June 2016 to identify cohort studies that examined the incidence of HCC in patients with AIH. We used random effects models to estimate pooled incidence rates overall and in subgroup of patients with cirrhosis. The between-study heterogeneity was assessed using I2 statistic.
Results
A total of 25 studies (20 papers and 5 abstracts) including 6,528 patients met the eligibility criteria. The median cohort size was 170 AIH patients (range 25–1721 patients) followed for a median of 8.0 years (range 3.3–16.0 years). The pooled incidence rate for HCC in patients with AIH was 3.06 per 1,000 patient-years (95% CI 2.22–4.23, I2 51.5%, p 0.002). The pooled incidence of HCC in patients with cirrhosis at AIH diagnosis was 10.07 per 1,000 patient-years (95% CI 6.89–14.70, I2 48.8%, p 0.015). In addition, 92 of 93 patients that had HCC had evidence of cirrhosis prior to or at time of HCC diagnosis. The risk of HCC appears to be lower in patients with AIH cirrhosis compared to that reported in patients with hepatitis B cirrhosis, hepatitis C cirrhosis, and primary biliary cholangitis.
Conclusions
Based on elevated HCC risk shown in this meta-analysis, there may be a role for HCC surveillance in patients with AIH cirrhosis.
Keywords: hepatocellular carcinoma, autoimmune hepatitis, systematic review, meta-analysis
Introduction
Autoimmune hepatitis (AIH) occurs globally and is characterized by interface hepatitis, hypergammaglobulinemia, and autoantibodies. 1–7 Clinical manifestations of AIH range from no symptoms to severe acute hepatitis, and rarely fulminant hepatic failure. 5,8 Approximately one third of patients with AIH have evidence of cirrhosis at the time of initial diagnosis. 3,4 Additionally, patients with AIH progress to cirrhosis at variable rates (ranging from 0.1 to 8.1% annually) despite treatment with immunosuppressive therapies. 9–12
In the United States, the combination of rising incidence of hepatocellular carcinoma (HCC) in both men and women and a low 5-year survival rate has made HCC the fastest growing cause of cancer-related death. 13,14,15 The American Association for the Study of Liver Diseases (AASLD) guidelines recommends HCC surveillance of high-risk patient groups (defined as those with an annual HCC risk of 1.5% or higher).16 Cirrhosis is a known precursor for HCC in over 80–90% of cases.13 AIH with cirrhosis has been associated with an increase in HCC risk; however, estimates of HCC risk among patients with AIH in cohort studies have varied widely.17–20 The European Association for the Study of Liver Diseases guidelines for autoimmune hepatitis recommend HCC screening in patients with underlying cirrhosis.21 However, AASLD guidelines while acknowledging HCC risk may be present make no recommendation for surveillance for patients with AIH cirrhosis per AASLD guidelines.16
There has not been a systematic effort to consolidate and critically evaluate the evidence regarding the risk of HCC in AIH patients. Furthermore, studies have reported different and inconsistent findings of determinants other than cirrhosis in increasing the risk for HCC. 19 We performed a systematic review and meta-analysis to estimate the incidence of HCC in patients with AIH, and to examine demographic, clinical, biochemical, and treatment-related factors associated with HCC risk in AIH.
Methods
We performed the study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA) guidelines and MOOSE guidelines with an a priori study protocol. 22,23
Search strategy
We searched PubMed and Embase databases from inception up to June 2016 for original research studies regardless of language or design using a combination of MESH and free text terms for AIH and liver cancer (search strategy in Appendix Table 1). Additionally, we manually searched reference lists of relevant articles for further studies.
Study selection
Two investigators (AT and LK) independently determined study eligibility and any disagreements were resolved after discussion with a senior investigator (FK). Studies were eligible for inclusion if they fulfilled the following criteria: (1) cohort study design (prospective or retrospective), (2) study population included at least 20 patients with AIH, (3) provided incidence rate of HCC or sufficient raw data to calculate incidence rates, and (4) AIH patients did not have additional etiologies for liver disease (e.g., hepatitis B, hepatitis C). If multiple articles were published using the same cohort, we used the study with the most complete data on the largest number of patients.
Data extraction and study quality assessment
Two independent reviewers (AT and LK) used standardized data collection forms to extract the following from each study: publication date, authors, country where the study was performed, follow-up years, number of participants, study design, sample size, mean age, sex, methods and intervals for HCC screening if performed, methods used for diagnosing AIH and HCC, liver disease severity or cirrhosis at AIH diagnosis and at the time of HCC diagnosis (if available), and risk factors associated with HCC. We classified patients as subjects with cirrhosis based on authors’ definition (of cirrhosis) or histology with stage 3 or 4 fibrosis. Further classification of decompensated cirrhosis (ascites, variceal bleeding, or hepatic encephalopathy) was recorded when available. Any data discrepancy was resolved by referring back to the original studies. Authors of studies were contacted for further clarification when necessary.
Two investigators (AT and LK) independently determined study eligibility and assessed quality with a modified version of the Newcastle-Ottawa quality assessment scale in three main areas: (1) selection process of cohorts, (2) similarity and comparability between cohorts, and (3) ascertainment of the outcome of interest. Disagreements were resolved with a third investigator (FK).
Statistical methods
For each study, we recorded or calculated incidence rates of HCC per 1,000 person-years with 95% confidence intervals (CI) based on the event rates in relation to the duration of follow up. We calculated pooled incidence rates and 95% CI using a Poisson distribution. Meta-analysis models were conducted using the log incidence rates of HCC and corresponding CIs with a random effects model. We assessed the between-study heterogeneity using I2 statistic, and used I2 values of 25%, 50% and 75% as evidence of low, moderate, or high levels of heterogeneity, respectively. We used a correction factor of 0.5 for both the number of HCC cases and total person-years of follow-up in instances when HCC counts were zero.24 We assessed for publication bias with the funnel plot, Begg’s test, and Egger’s test.
We conducted subgroup analyses based on presence of cirrhosis, sex, continent, and source population (i.e., single center, multiple centers, general population). We conducted sensitivity analyses by excluding low quality studies and abstracts, studies with zero HCC cases, and retrospective studies.
We conducted statistical analyses with Stata, version 13.1 (StataCorp, College Station, Texas, US). Tests were two tailed and p values <0.05 were considered significant.
Results
Our search strategy identified 811 articles. Of these, 59 were retrieved for detailed evaluation.
We identified 15 articles that used data from 5 cohorts (1 abstract and 2 papers from a cohort in Sweden;11,25,26 1 abstract and 2 papers from a cohort in Japan;27,28 2 papers from a cohort in the UK;19,29 1 abstract and 1 paper from a different cohort in the UK;30,31 and 4 papers from a cohort in the US).17,32–34 In each instance, we used the most recent paper that met criteria for inclusion in our analysis; these studies reported the most complete data on the largest number of patients from each cohort.9,15,17,11,28
A total of 25 studies, involving 6,425 patients with AIH, met our selection criteria and were included in the meta-analysis (between reviewer kappa=0.92). A total of 20 studies were published as full papers and 5 were reported only as abstracts (Figure 1). Of the 25 studies, five were performed in North America, twelve in Europe, seven in Asia, and one in Australia. We did not find any studies from South America or Africa. Four studies were prospective, four were both retrospective and prospective and the remaining 17 were retrospective cohort in design (Table 1). Most studies used liver biopsy and the International Autoimmune Hepatitis guidelines to establish the diagnosis of AIH, and radiology or pathology for HCC diagnosis. The method of AIH diagnosis was not mentioned in five out of six studies published only as abstracts. None of the included studies reported external funding sources.
Figure 1.
PRISMA Flow Diagram. AIH – autoimmune hepatitis. HCC-hepatocellular cancer
Table 1.
Characteristics of included studies
| Authors (Year) | Country | Years of study | Study type | Source of study population | Study N | Mean age at AIH | % Women | % Cirrhosis at Index AIH diagnosis | Mean follow up in months | Incidence HCC per 1,000 person-years |
|---|---|---|---|---|---|---|---|---|---|---|
| Sonavane (2016)**64 | West India | Not provided | R | Single | 242 | 44.7 | 61.6 | 71.9 | 39.6 | 10.02 |
| Danielsson Borssen (2015)11 | Sweden | Not provided | R | Multiple | 634 | 46* | 73.0 | 28.0 | 152.8 | 1.24 |
| Grønbæk (2014)18 | Denmark | 1994–2012 | R | General Population | 1721 | - | 72.0 | 6.3 | 82.5 | 0.68 |
| Umemura (2014)35 | Japan | 1999–2013 | R | Single | 156 | 62 | 89.0 | - | 118.0* | 3.91 |
| Van Gerven (2014)36 | Netherlands | 1967–2011 | R | Multiple | 730 | 48 | 78.0 | 12.0 | 96.0* | 1.37 |
| Martens (2013)**65 | Germany | 1980–2010 | R | Single | 170 | - | - | 8.2 | 64.0 | 1.10 |
| Zachou (2013)**12 | Greece | 2000–2012 | P | Single | 159 | 47 | 69.8 | 32.7 | 49.0 | 6.16 |
| Yoshizowa (2012)46 | Japan | 1974–2010 | R/P | Multiple | 203 | 55.5 | 87.2 | 12.3 | 131.0 | 3.16 |
| Ngu (2012)66 | New Zealand | 1980–2006 | R/P | General Population | 130 | 50 | 70.8 | - | 106.7 | 2.60 |
| Migita (2012)47 | Japan | 1995–2008 | P | Multiple | 193 | 56.6 | 91.7 | 10.9 | 96.0 | 4.53 |
| Hino-Arinaga (2012)27 | Japan | 11/1978–4/2008 | R/P | Multiple | 180 | 59.9 | 88.3 | 18.9 | 80.2 | 5.00 |
| Wong (2011)49 | USA | 1999–2009 | R | Single | 322 | 51.8 | 79.6 | 1.6 | 75.0 | 0.00 |
| Macaron (2010)**40 | USA | 2001–2007 | R | Single | 49 | - | - | 100.0 | 43.2 | 11.34 |
| Hoeroldt (2009)30 | UK | 1971–2007 | R | Single*** | 248 | 57 | 83.5 | 36.7 | 117.0 | 3.31 |
| Ng (2009)**41 | Hong Kong | 1/1996–5/2008 | R | Single | 25 | 56 | - | 32.0 | 60* | 0.00 |
| Teufel (2009)50 | Germany | 1970–2008 | R | Single | 278 | - | - | 11.0 | 84.2 | 0.26 |
| Muratori (2009)67 | Italy | 1992–2007 | R | Single | 163 | 36 | 82.2 | 55.0 | 80 | 0.92 |
| Montano-Loza (2008)17 | USA | Not provided | P | Single | 227 | 46 | 81.5 | 43.2 | 134.0 | 3.55 |
| Seo (2008)44 | USA | 4/1979–7/2007 | R | Single | 72 | 50.5 | 84.0 | 56.9 | 98.0* | 3.40 |
| Yeoman (2008)19 | UK | 1971–2007 | R | Single | 243 | 42 | 80.7 | 50.2 | 149.6 | 4.95 |
| Miyake (2006)43 | Japan | 11/1988–06/2005 | P | Multiple | 69 | 54 | 89.9 | 42.0 | 96.0* | 5.44 |
| Floreani (2006)68 | Italy | 1981–2004 | R/P | Multiple | 73 | 47.7 | 86.3 | 38.4 | 91 | 1.81 |
| Seela (2005)42 | USA | Not provided | R | Single | 42 | 47 | 76.2 | 42.6 | 192.0 | 0.74 |
| Poulsen (2002)38 | Denmark | 1977–1993 | R | General Population | 96 | - | - | - | 90.0 | 2.78 |
| Kanzler (2001)39 | Germany | Not provided | R | Single | 103 | 45.8 | 83.0 | 29.1 | 98 | 1.19 |
abstract, R=retrospective, P=prospective, R/P=retrospective and prospective,
represents median,
represents nontransplant center
Eight of the 25 studies were comparative (comparative groups included national population, local population, and patients with PBC).11,18,35–40 We assessed study quality with a modified Newcastle Ottawa scale. Scores ranged from 3 to 7 (with a maximum score of 9). The median scores were 6 and 5 for comparative and non-comparative studies, respectively (Appendix Table 2).
Baseline characteristics
The median cohort size was 170 patients (range 25–1721). Patients were followed for a median of 8.0 years (range 3.3–16.0). The majority of AIH patients were women (80.4%, range 61.6–91.7%). The mean age of patients at the time of study enrollment was 46.1 years (range 36.0–62.0 years). In 22 studies that reported cirrhosis status, 32.4% (median, range 6.3–100%) of patients had cirrhosis at the time of AIH diagnosis. Based on six studies that reported concurrent autoimmune disease, 29.1% patients with AIH had concurrent autoimmune disease at AIH diagnosis.12,41–45
HCC incidence rate in AIH
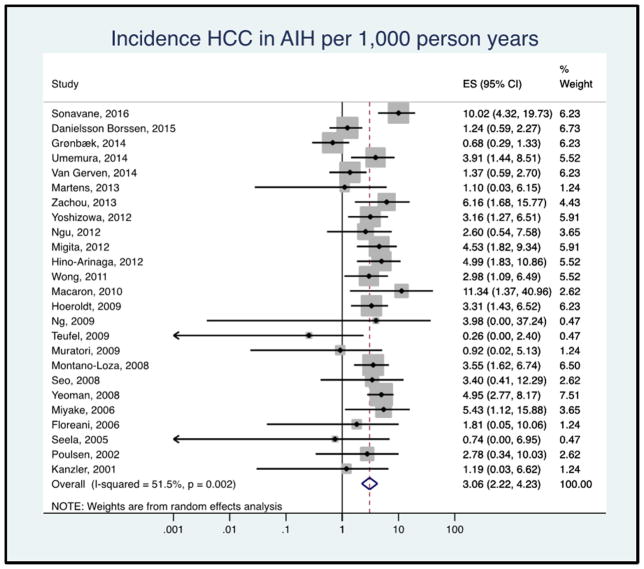
Of the 6,528 patients included in the analysis, 118 patients developed HCC during a median follow up of 8.0 years (53,026 total person-years). A total of nine patients from three studies had HCC at the time of initial AIH diagnosis or cohort entry and were excluded from HCC incidence calculations.18,41,39 The pooled HCC incidence rate was 3.06 (95% CI 2.22–4.23) per 1,000 person-years, with moderate heterogeneity between the studies (I2 = 49.5%, Figure 2). The range of HCC incidence in individual studies was from 0 to 11.34 per 1,000 person-years.
Figure 2.
Forest plot of incidence of hepatocellular cancer in patients with autoimmune hepatitis. Incidence rate reported per 1,000 person-years.
HCC incidence rate in patients with AIH cirrhosis
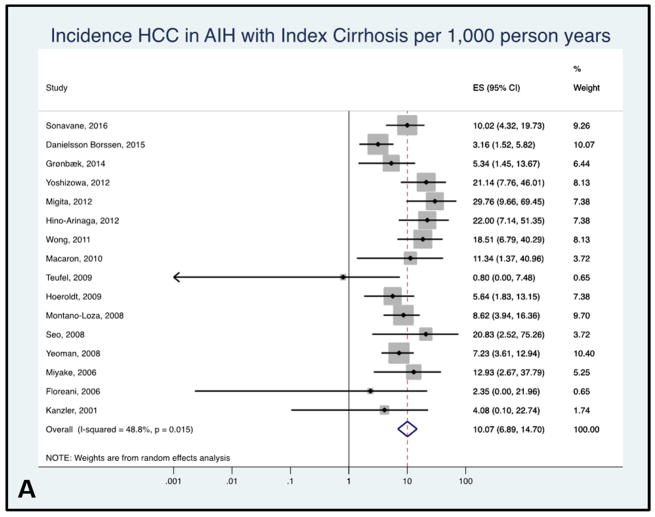
A total of 16 studies examined HCC incidence in patients with evidence of cirrhosis at the time of AIH diagnosis. The pooled HCC incidence rate in patients with AIH cirrhosis was 10.07 per 1,000 person-years with low between-study heterogeneity (95% CI 6.90–14.70, I2 = 29.3%, Figure 3A). The range of HCC incidence for this subgroup was from 0.80 to 29.76 per 1,000 person-years.
Figure 3.
Figure 3A: Forest plot of incidence of hepatocellular cancer in autoimmune hepatitis patients with cirrhosis at baseline. Incidence rate reported per 1,000 person-years.
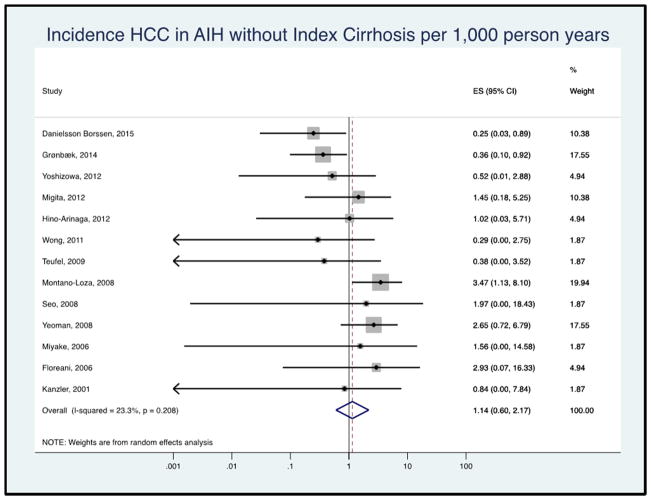
Figure 3B: Forest plot of incidence of hepatocellular cancer in autoimmune hepatitis patients without cirrhosis at baseline. Incidence rate reported per 1,000 person-years.
A total of 13 studies reported HCC incidence among patients without cirrhosis at AIH diagnosis. The pooled HCC incidence rate was 1.14 per 1,000 person-years (95% CI 0.60–2.17, I2 = 15.6%, Figure 3B).
A total of 16 studies reported cirrhosis status at the time of HCC diagnosis. Of 93 patients with HCC, 92 had evidence of cirrhosis. Of these, 22 patients (23.7%) did not have cirrhosis at the time of initial AIH diagnosis but developed cirrhosis during follow up.
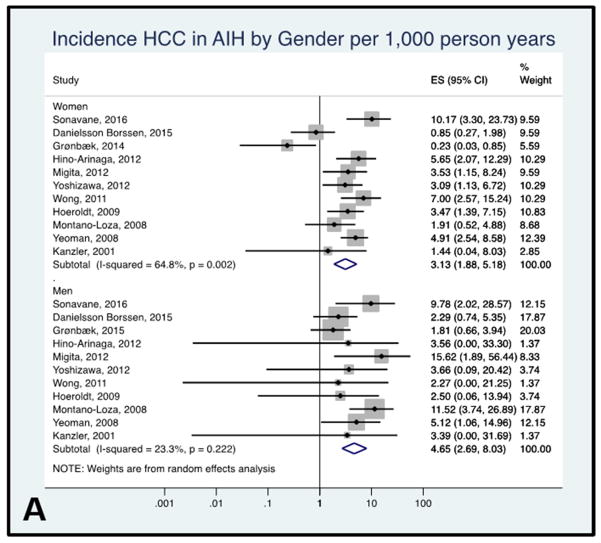
HCC incidence rate in AIH by sex
In the eleven studies examining the incidence of HCC by sex, the pooled HCC incidence rate was 3.13 per 1,000 person-years (95% CI 1.88–5.18) in women and 4.65 per 1,000 person-years (95% CI 2.69–8.03) in men, with low to moderate between-study heterogeneity (I2= 64.8% women, I2= 23.3% men, Figure 4A).
Figure 4.
Figure 4A: Forest plot of incidence of hepatocellular cancer in autoimmune hepatitis patients by sex. Incidence rate reported per 1,000 person-years.
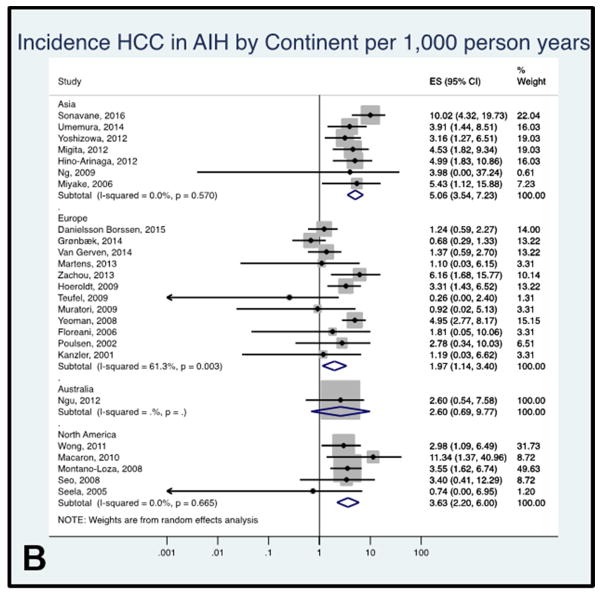
Figure 4B: Forest plot of incidence of hepatocellular cancer in autoimmune hepatitis patients by continent. Incidence rate reported per 1,000 person-years.
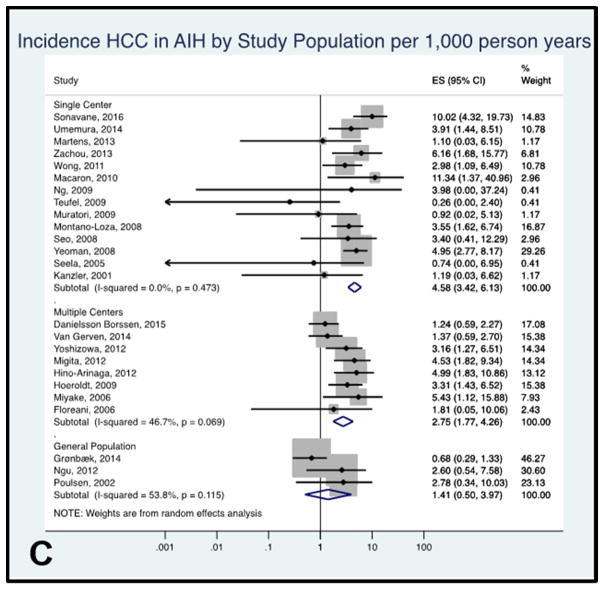
Figure 4C: Forest plot of incidence of hepatocellular cancer in autoimmune hepatitis patients by study population. Incidence rate reported per 1,000 person-years.
HCC incidence rate in AIH by continent
The pooled annual incidence rate of AIH was higher in studies performed in Asia (5.06 per 1,000 person-years, 95% CI 3.54–7.23, I2= 0.0%, 7 studies) compared with Europe (1.97 per 1,000 person-years, 95% CI 1.15–3.40, I2= 64.5%, 12 studies), Australia (2.60 per 1,000 person-years, 95% CI 0.69–9.77, one study) and North America (3.63 per 1,000 person-years, 95% CI 2.20–6.00, I2= 0.0%, 5 studies) (Figure 4B). Patients included in the studies from Asia were older (mean age of 54.7 years) at the time of AIH diagnosis compared to patients in studies from other continents (mean age: Europe 46.1 years, North America 49.4 years, and Australia 50.0 years).
HCC incidence rate in AIH by source population
The pooled annual incidence rate of HCC was higher in single specialized tertiary center studies (4.58 per 1,000 person-years, 95% CI 3.42–6.13, I2= 0.0%, 14 studies) than multicenter studies (2.75 per 1,000 person-years, 95% CI 1.77–4.26, I2= 46.7%, 8 studies) or studies including AIH patients identified from general populations (1.41 per 1,000 person-years, 95% CI 0.50–3.97, I2= 53.8%, 4 studies, Figure 4C).
Sensitivity analysis
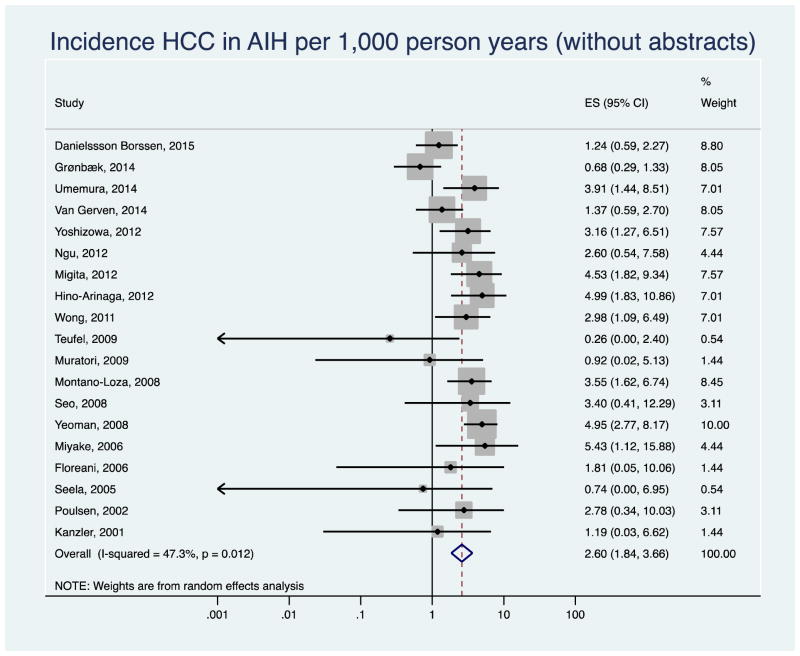
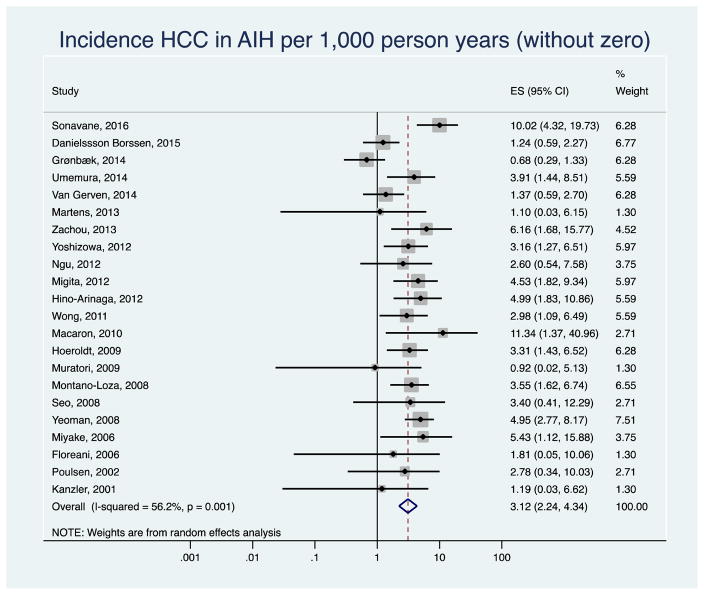
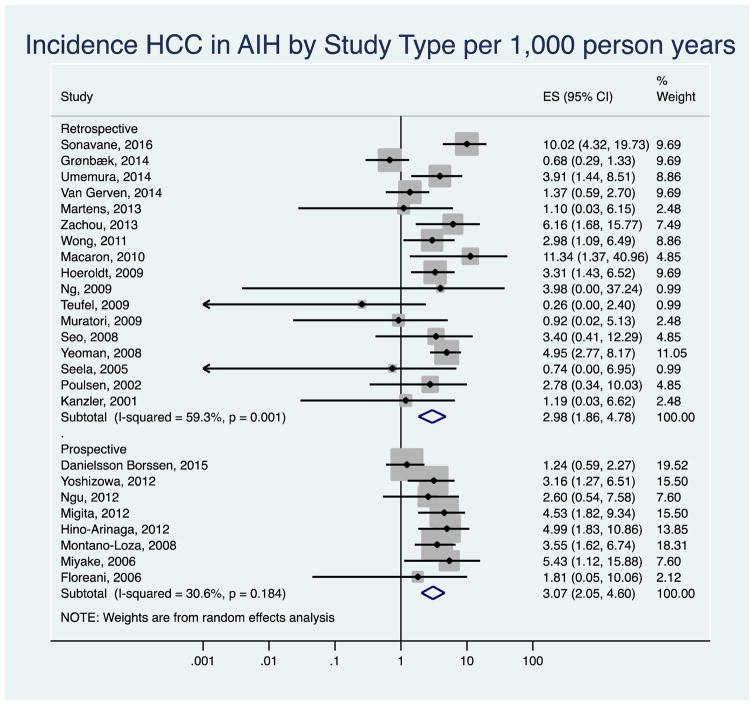
After excluding six studies published only as abstracts, the pooled annual HCC incidence rate decreased to 2.60 per 1,000 person-years (95% CI 1.84–3.66, I2=34.16) in the remaining 19 studies (Appendix Figure 1). The results did not change when we removed three studies with zero HCC counts (3.12 per 1,000 person years, 95% CI 2.24–4.34, I2=48.0%, Appendix Figure 2). The pooled HCC incidence rate was 2.98 per 1,000 person-years (95% CI 1.86–4.78) in retrospective, and 3.07 per 1,000 person-years (95% CI 2.05–4.60) in prospective or retrospective/prospective studies. There was low to moderate between-study heterogeneity among the studies (I2= 59.3% retrospective, I2= 30.6 % prospective, Appendix Figure 3).
Other determinants of HCC
A total of five studies used multivariable regression analyses to identify factors associated with the development of HCC in AIH.31,40,46–48 These studies identified older age, male gender, cirrhosis status at baseline, number of AIH relapses and alcohol use as independent factors associated with risk of HCC in patients with AIH (Appendix Table 3).
Two studies reported the risk of HCC in AIH patients with overlap syndrome with primary biliary cholangitis (PBC); the pooled HCC rate was 14.4 per 1,000 person years (95% CI 1.7–51.0).36,49 None of the 5 patients with AIH and primary sclerosing cholangitis overlap, reported in one study developed HCC.36 A total of six studies reported HCC surveillance but it was not possible to compare the incidence of HCC according to screening status because this information was not provided in most studies. 15,17,40,40,49,50
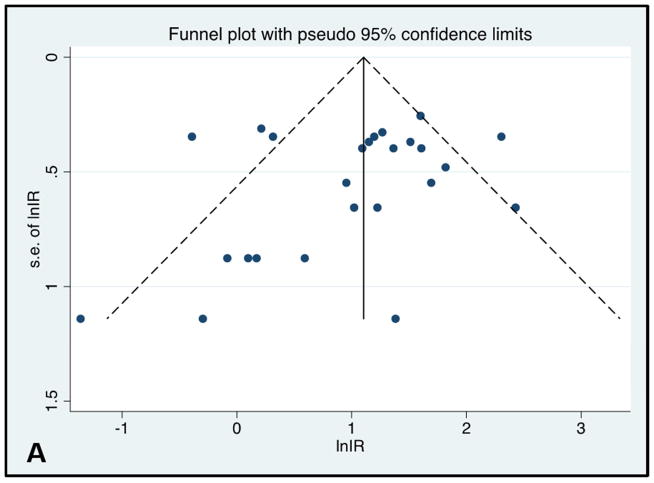
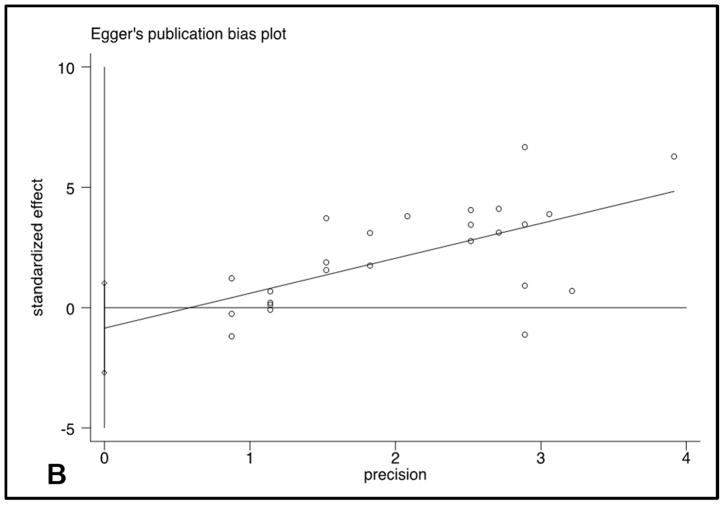
Publication bias
Egger’s plot and Begg’s plot did not demonstrate significant bias (p values 0.35 and 0.12, respectively). The funnel plot did not show significant asymmetry (Figure 5).
Figure 5.
Funnel Plot and Egger’s publication bias plot
Discussion
In this systematic review and meta-analysis, we found that HCC developed at an overall incidence rate of 3.06 per 1,000 patient-years (95% CI 2.22–4.23) among patients with AIH. Cirrhosis was a predominant risk factor for HCC. The incidence of HCC in patients with cirrhosis at time of AIH diagnosis was 10.07 per 1,000 patient-years (95% CI 6.90–14.70). Although HCC was reported to occur in patients who did not have cirrhosis at time of AIH diagnosis, it was a relatively rare and not statistically significant scenario (1.14 per 1,000 patient-years, 95% CI 0.60–2.17).
AASLD guidelines suggest that HCC surveillance is cost-effective if the expected risk of HCC exceeds 1.5% per year.51–53 In this meta-analysis, the overall pooled incidence of patients with AIH cirrhosis was 1.007% per year (10.07 per 1,000 patient-years). However, the 95% CI nearly encompasses the recommended cut-off value of 1.5% and in five of the 16 studies, the annual incidence rate (that reported HCC incidence in patients with AIH cirrhosis) exceeded 1.5%.44,46–49 We also found that almost all patients (92 of 93) who developed HCC had progressed to cirrhosis during their follow up. Collectively, these data support the importance of regular monitoring of disease severity in AIH with initiation of HCC screening in patients who progress to cirrhosis despite medical management.
Based on published literature, in patients with hepatitis B, HCC incidence varies between 0–7 per 1,000 person-years in carriers, 1–10 per 1,000 person-years in chronic hepatitis and 9–54 per 1,000 person-years in patients with hepatitis B cirrhosis.54,55 The HCC incidence among patients with hepatitis C has been reported to be 1–8 per 1,000 person-years in patients with chronic hepatitis and 37–71 per 1,000 person-years in patients with cirrhosis.54 The incidence of HCC among patients with PBC has been reported to be 0–11 per 1,000 person-years and in patients with PBC cirrhosis has been reported to be 7–24 per 1,000 person-years.55–60 The incidence of HCC among patients with PSC is not known but likely very low.61 Based on our study findings, the risk of HCC in patients with AIH cirrhosis is lower than patients with cirrhosis related to hepatitis B, hepatitis C, or PBC.
Besides cirrhosis, we found several demographic and clinical factors associated with HCC risk. Older age at AIH diagnosis was associated with risk of HCC. Men with AIH had a slightly higher risk of developing HCC than women, but the difference was not statistically significant, likely due to sample size limitations (only 1138 men were included in this analysis and of these 26 developed HCC) and relatively younger age of men at time of AIH diagnosis compared to women. While the effect of AIH treatment was difficult to assess given the heterogeneity of disease severity in this population, two studies suggested that increased frequency of AIH relapses portend increased risk of HCC. Macaron et al. found that AIH patients with concomitant alcohol abuse had 6-fold higher risk of HCC than patients without alcohol use.40 These data support careful monitoring of patients with AIH, particularly older men, patients with multiple AIH relapses, and those with ongoing alcohol abuse. On the other hand, the concomitant presence of primary sclerosing cholangitis was associated with low HCC risk; similar findings were reported in studies of patients with primary sclerosing cholangitis alone.61 Asian populations have the highest incidence of HCC; in this study we also found a higher HCC risk in Asian patients with AIH than patients in other continents.14 This increased risk may be related to the older mean age of patients or from other factors such as aflatoxin exposure. 62,63 Additionally, we found the incidence rates varied by source population. Studies using single tertiary center data found higher rates of HCC than studies using multicenter or country data. This may reflect increased risk of HCC in patients with more complicated or severe disease that were referred to those tertiary centers.
Our study has several limitations related to the available primary studies. All studies had small numbers of patients who developed HCC (n=0–15), and therefore the incidence rate estimates are likely not precise, particularly in the subgroup analyses. Most studies lacked a comparison cohort and followed patients for relatively short durations. Inconsistent reporting limited our ability to assess the influence of various risk factors. We were also unable to measure the effects of HCC surveillance in this population because this information was not included in most studies.
We used a comprehensive systematic search strategy that included hand searches and searches for unpublished reports. We did not find any evidence of publication bias, providing support to the decision to incorporate gray literature in our study. Additionally, we conducted several subgroup and sensitivity analyses to evaluate HCC incidence rates in various subgroups. The between study heterogeneity was reduced in the subgroups including prospective and single tertiary centers studies, suggesting that some of the heterogeneity in the HCC incidence rates resulted from differences in study design and source population.
In conclusion, patients with AIH related cirrhosis are at an increased risk for HCC. HCC surveillance might be cost effective in this population. On the other hand, AIH patients without cirrhosis at index diagnosis, particularly those identified from general populations, are at an extremely low risk of HCC.
Acknowledgments
GRANT SUPPORT: Aylin Tansel is supported by 5T32DK083266-07 grant.
APPENDIX
Appendix Table 1.
Search strategy for PubMed and Embase.
PubMed
|
|
Embase autoimmune AND hepatitis AND 'hepatocellular carcinoma' |
Appendix Table 2.
Modified New Castle Ottawa.
| Comparative studies | ||||
|---|---|---|---|---|
| Study | Selection | Comparability | Outcome | Total |
| Danielsson Borssen (2015) | 4 | 1 | 2 | 7 |
| Umemura (2014) | 4 | 1 | 2 | 7 |
| Grønbæk (2014) | 3 | 1 | 2 | 6 |
| Van Gerven (2014) | 4 | 0 | 2 | 6 |
| Ngu (2012) | 3 | 1 | 2 | 6 |
| Macaron (2010) | 1 | 1 | 2 | 4 |
| Poulson (2002) | 3 | 1 | 2 | 6 |
| Kanzler (2001) | 3 | 1 | 2 | 6 |
| Non-comparative studies | ||||
|---|---|---|---|---|
| Study | Selection | Outcome | Total | |
| Sonavane (2016) | 1 | 2 | 3 | |
| Martens (2013) | 2 | 2 | 4 | |
| Zachou (2013) | 2 | 2 | 4 | |
| Yoshizowa (2012) | 3 | 3 | 6 | |
| Migita (2012) | 3 | 2 | 5 | |
| Hino-Arinaga (2012) | 4 | 3 | 7 | |
| Wong (2011) | 2 | 2 | 4 | |
| Hoeroldt (2009) | 3 | 2 | 5 | |
| Ng (2009) | 2 | 2 | 4 | |
| Teufel (2009) | 3 | 2 | 5 | |
| Muratori (2009) | 3 | 2 | 5 | |
| Montano-Loza (2008) | 3 | 3 | 6 | |
| Seo (2008) | 3 | 2 | 5 | |
| Yeoman (2008) | 3 | 3 | 6 | |
| Miyake (2006) | 3 | 2 | 5 | |
| Floreani (2006) | 3 | 3 | 6 | |
| Seela (2005) | 3 | 2 | 5 | |
Appendix Table 3.
Risk factors for hepatocellular cancer in autoimmune hepatitis. Table displays risk factors identified as significant based on multivariable regression models. HR=hazard ratio; OR; OR=odds ratio
| Risk factors | Study | Statistics |
|---|---|---|
| Demographics | ||
| Older age | Macaron40 | HR 1.1, p < 0.001 |
| Hoeroldt64 | HR not reported, p = 0.034 | |
|
| ||
| Clinical factors | ||
| Two or more relapses | Yoshizawa46 | HR 9.1, p = 0.007 |
| High relapse rate | Hoeroldt64 | HR not reported, p = 0.034 |
| Cirrhosis | Migita47 | HR 11.47, p = 0.005 |
| Hino-Arinago48 | OR 4.08, p = 0.01 | |
| Alcohol consumption | Macaron40 | HR 5.9, p < 0.001 |
Appendix Figure 1.
Sensitivity analysis: Forest plot of incidence of hepatocellular cancer (HCC) in autoimmune hepatitis (AIH) patients after excluding abstracts and low-quality studies.
Appendix Figure 2.
Sensitivity analysis: Incidence of hepatocellular cancer (HCC) in autoimmune hepatitis (AIH) patients after excluding studies with zero HCC cases.
Appendix Figure 3.
Sensitivity Analysis: Forest plot of incidence of hepatocellular cancer in autoimmune hepatitis patients by study design. Incidence rate reported per 1,000 person-years.
Footnotes
AUTHOR CONTRIBUTION: Aylin Tansel, study concept and design; acquisition of data; interpretation of data; writing manuscript; Lior Katz, acquisition of data; study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content; Hashem B. El-Serag, study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content; Aaron Thrift, interpretation of data; critical revision of the manuscript for important intellectual content; Mayur Parepally, acquisition of data; critical revision of the manuscript for important intellectual content; Mohammad H. Shakhatreh, study concept and design; critical revision of the manuscript for important intellectual content; Fasiha Kanwal, study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content; study supervision.
CONFLICT OF INTEREST: No conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354(1):54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, Vogel A. Autoimmune hepatitis, from mechanisms to therapy. Hepatology. 2006;43(2 Suppl 1):S132–44. doi: 10.1002/hep.21059. [DOI] [PubMed] [Google Scholar]
- 3.Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36(2):479–497. doi: 10.1053/jhep.2002.34944. [DOI] [PubMed] [Google Scholar]
- 4.Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 5.Zachou K, Muratori P, Koukoulis GK, et al. Review article: autoimmune hepatitis -- current management and challenges. Aliment Pharmacol Ther. 2013;38(8):887–913. doi: 10.1111/apt.12470. [DOI] [PubMed] [Google Scholar]
- 6.Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology. 2010;139(1):58–72. e4. doi: 10.1053/j.gastro.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 7.Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis--Update 2015. J Hepatol. 2015;62(1 Suppl):S100–11. doi: 10.1016/j.jhep.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Vierling JM. Autoimmune Hepatitis and Overlap Syndromes: Diagnosis and Management. Clin Gastroenterol Hepatol. 2015;13(12):2088–2108. doi: 10.1016/j.cgh.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Poulsen LO, Thulstrup AM, Mellemkjaer L, et al. Mortality and causes of death in patients with “lupoid hepatitis.” A long-term follow-up study in Denmark. Dan Med Bull. 2002;49(3):263–265. [PubMed] [Google Scholar]
- 10.Hino T, Kumashiro R, Ide T, et al. Predictive factors for remission and death in 73 patients with autoimmune hepatitis in Japan. Int J Mol Med. 2003;11(6):749–755. [PubMed] [Google Scholar]
- 11.Danielsson Borssén Å, Almer S, Prytz H, et al. Hepatocellular and extrahepatic cancer in patients with autoimmune hepatitis – a long-term follow-up study in 634 Swedish patients. Scand J Gastroenterol. 2015;50(2):217–223. doi: 10.3109/00365521.2014.983154. [DOI] [PubMed] [Google Scholar]
- 12.Zachou K, Gatselis N, Gabeta S, Saitis AI, Papadamou G, Rigopoulou EI, Koukoulis GKDGN. Autoimmune hepatitis natural history: A single Greek center experience focused on long-term effect of mycophenolate mofetil as first line treatment. Suppl. Vol. 58. Washington, DC, United States: Hepatology; 2013. pp. 563A–571A. [DOI] [Google Scholar]
- 13.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 14.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol. 2015;13(12):2140–2151. doi: 10.1016/j.cgh.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montano-loza AJ, Carpenter HA, Czaja AJ. Predictive factors for hepatocellular carcinoma in type 1 autoimmune hepatitis. Am J Gastroenterol. 2008;103(8):1944–1951. doi: 10.1111/j.1572-0241.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 18.Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60(3):612–617. doi: 10.1016/j.jhep.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Yeoman AD, Al-chalabi T, Karani JB, et al. Evaluation of Risk Factors in the Development of Hepatocellular Carcinoma in Autoimmune Hepatitis: Implications for Follow-up and Screening. Hepatology. 48(3):863–870. doi: 10.1002/hep.22432. [DOI] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune Hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 23.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-Analysis in Medical Research. Wiley; London: 2000. [Google Scholar]
- 24.Sutton AJ, Abrams KR, Jones DR, et al. Methods for Meta-Analysis in Medical Research. J Wiley. 2002 [Google Scholar]
- 25.Werner M, Almer S, Prytz H, et al. Hepatic and extrahepatic malignancies in autoimmune hepatitis . A long-term follow-up in 473 Swedish patients q. J Hepatol. 2009;50(2):388–393. doi: 10.1016/j.jhep.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Danielsson Borssén Å, Lindgren S, Bergquist A, et al. Risk for Hepatocellular Carcinoma in Autoimmune Hepatitis - Is there an Indication for Surveillance? Journal of gastroenterology. 2012;47(5):S382–S383. doi: 10.1016/S0168-8278(13)60929-0. [DOI] [Google Scholar]
- 27.Tatsuya TH, Ryoko I, Ichiro K. Risk factors for hepatocellular carcinoma in Japanese patients with autoimmune hepatitis type 1. 2012:569–576. doi: 10.1007/s00535-011-0519-2. [DOI] [PubMed] [Google Scholar]
- 28.Arinaga-Hino T, Ide T, Miyajima I, Ogata K, Kuwahara R, Amano K, Noguchi K, Ishii K, Fukuizumi K, Yonemoto KTT. Carcinogenesis in autoimmune hepatitis type 1 patients with a long-term follow-up in Japan. 66th Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting; 2015; San Francisco, CA, United States: Hepatology; 2015. pp. 363A–370A. [DOI] [Google Scholar]
- 29.Al-Chalabi T, Underhill JA, Portmann BC, et al. Impact of gender on the long-term outcome and survival of patients with autoimmune hepatitis. J Hepatol. 2008;48(1):140–147. doi: 10.1016/j.jhep.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Hoeroldt B, MacFarlane E, Basumani P, et al. Hepatocellular carcinoma in autoimmune hepatitis. J Hepatol. 2009;50:S290. doi: 10.1016/S0168-8278(09)60795-9. [DOI] [Google Scholar]
- 31.Hoeroldt B, McFarlane E, Dube A, et al. Long-term outcomes of patients with autoimmune hepatitis managed at a nontransplant center. Digestive DIseases and Sciences. 2011;45(10):1980–1989. doi: 10.1053/j.gastro.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 32.Park SZ, Nagorney DM, Czaja AJ. Hepatocellular Carcinoma in Autoimmune Hepatitis. 2000;45(10):1944–1948. doi: 10.1023/a:1005638500236. [DOI] [PubMed] [Google Scholar]
- 33.Czaja AJ, Carpenter HA. Distinctive clinical phenotype and treatment outcome of type 1 autoimmune hepatitis in the elderly. Hepatology. 1988;8(6):1679–1683. doi: 10.1002/hep.21074. [DOI] [PubMed] [Google Scholar]
- 34.Wang KK, Czaja AJ. Hepatocellular carcinoma in corticosteroid-treated severe autoimmune chronic active hepatitis. PLoS One. 2014 Jun 23;9(6):e100565. doi: 10.1002/hep.1840080635. [DOI] [PubMed] [Google Scholar]
- 35.Umemura T, Katsuyama Y, Yoshizawa K, et al. Human Leukocyte Antigen Class II Haplotypes Affect Clinical Characteristics and Progression of Type 1 Autoimmune Hepatitis in Japan. 2014;9(6) doi: 10.1371/journal.pone.0100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Gerven NMF, Verwer BJ, Witte BI, et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Hepatology. 2012 Feb;55(2):522–9. doi: 10.3109/00365521.2014.946083. [DOI] [PubMed] [Google Scholar]
- 37.Ngu JH, Gearry RB, Frampton CM, et al. Mortality and the Risk of Malignancy in Autoimmune Liver Diseases: A Population-Based Study in Canterbury, New Zealand. 2011:522–529. doi: 10.1002/hep.24743. [DOI] [PubMed] [Google Scholar]
- 38.Poulsen LO, Thulstrup AM, Mellemkjaer L, et al. Mortality and causes of death in patients with "lupoid hepatitis." A long-term follow-up study in Denmark. Dan Med Bull. 2002;49(3):263–265. [PubMed] [Google Scholar]
- 39.Kanzler S, Löhr H, Gerken G, et al. Long-term management and prognosis of autoimmune hepatitis (AIH): a single center experience. Z Gastroenterol. 2010;138(5) 1:S–809. doi: 10.1055/s-2001-13708. [DOI] [PubMed] [Google Scholar]
- 40.Macaron C, Hanouneh I, Lopez RZNN. Incidence and risk factors of hepatocellular carcinoma in patients with primary biliary cirrhosis and autoimmune hepatitis. Gastroenterology. 2010;3(1):S809. doi: 10.1002/hep.24005. [DOI] [PubMed] [Google Scholar]
- 41.Ng TL, Li F, Lao WC, Leung CM, Leong IS, Kung KN, Li WH, Luk YW, Tang WLCKF. Immunosuppresive therapy in autoimmune hepatitis in Hong Kong Chinese: 10-Year follow-up of a cohort. Hepatology International. 2009;(Suppl):86–220. doi: 10.1007/s12072-009-9123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seela S, Sheela H, Boyer JL. Autoimmune hepatitis type 1: safety and efficacy of prolonged medical therapy. Liver Int. 2005;25(4):734–739. doi: 10.1111/j.1478-3231.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 43.Miyake Y, Iwasaki Y, Terada R, et al. Persistent elevation of serum alanine aminotransferase levels leads to poor survival and hepatocellular carcinoma development in type 1 autoimmune hepatitis. Aliment Pharmacol Ther. 2006;24(August):1197–1205. doi: 10.1111/j.1365-2036.2006.03113.x. [DOI] [PubMed] [Google Scholar]
- 44.Seo S, Toutounjian R, Conrad A, et al. Favorable outcomes of autoimmune hepatitis in a community clinic setting. Liver Int. 2012 May;32(5):837–44. doi: 10.1111/j.1478-3231.2011.02734.x. Epub 2011 Dec 30. [DOI] [PubMed] [Google Scholar]
- 45.Migita K, Watanabe Y, Jiuchi Y, et al. Hepatocellular carcinoma and survival in patients with autoimmune hepatitis ( Japanese National Hospital Organization-autoimmune hepatitis prospective study ) Hepatology. 2012 Aug;56(2):668–76. doi: 10.1002/hep.25658. Epub 2012 Jul 6. [DOI] [PubMed] [Google Scholar]
- 46.Yoshizawa K, Matsumoto A, Ichijo T, et al. Long-term Outcome of Japanese Patients With Type 1 Autoimmune Hepatitis. doi: 10.1002/hep.25658. [DOI] [PubMed] [Google Scholar]
- 47.Migita K, Watanabe Y, Jiuchi Y, et al. Hepatocellular carcinoma and survival in patients with autoimmune hepatitis (Japanese National Hospital Organization-autoimmune hepatitis prospective study) Liver Int. 2012;32(5):837–844. doi: 10.1111/j.1478-3231.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 48.Hino-Arinaga T, Ide T, Kuromatsu R, et al. Risk factors for hepatocellular carcinoma in Japanese patients with autoimmune hepatitis type 1. J Gastroenterol. 2012;47(5):569–576. doi: 10.1007/s00535-011-0519-2. [DOI] [PubMed] [Google Scholar]
- 49.Wong RJ, Gish R, Frederick T, et al. Development of Hepatocellular Carcinoma in Autoimmune Hepatitis Patients: A Case Series. Dig Dis Sci. 2011;56(2):578–585. doi: 10.1007/s10620-010-1444-6. [DOI] [PubMed] [Google Scholar]
- 50.Teufel A, Weinmann A, Centner C, et al. Hepatocellular carcinoma in patients with autoimmune hepatitis. World J Gastroenterol. 2009;15(5):578–582. doi: 10.3748/wjg.15.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101(4):422–434. doi: 10.1016/S0002-9343(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 52.Arguedas MR, Chen VK, Eloubeidi MA, et al. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003;98(3):679–690. doi: 10.1111/j.1572-0241.2003.07327.x. [DOI] [PubMed] [Google Scholar]
- 53.Lin OS, Keeffe EB, Sanders GD, et al. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther. 2004;19(11):1159–1172. doi: 10.1111/j.1365-2036.2004.01963.x. [DOI] [PubMed] [Google Scholar]
- 54.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Papatheodoridis GV, Chan HL-Y, Hansen BE, et al. Risk of hepatocellular carcinoma in chronic hepatitis B: Assessment and modification with current antiviral therapy. J Hepatol. 2015;62(4):956–967. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X-X, Wang L-F, Jin L, et al. Primary biliary cirrhosis-associated hepatocellular carcinoma in Chinese patients: incidence and risk factors. World J Gastroenterol. 2015;21(12):3554–3563. doi: 10.3748/wjg.v21.i12.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ngu JH, Gearry RB, Frampton CM, et al. Mortality and the risk of malignancy in autoimmune liver diseases: a population-based study in Canterbury, New Zealand. Hepatology. 2012;55(2):522–529. doi: 10.1002/hep.24743. [DOI] [PubMed] [Google Scholar]
- 58.Jackson H, Solaymani-Dodaran M, Card TR, et al. Influence of ursodeoxycholic acid on the mortality and malignancy associated with primary biliary cirrhosis: a population-based cohort study. Hepatology. 2007;46(4):1131–1137. doi: 10.1002/hep.21795. [DOI] [PubMed] [Google Scholar]
- 59.Trivedi PJ, Lammers WJ, van Buuren HR, et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gastroenterol Res Pract. 2013;2013:168012. doi: 10.1155/2013/168012. Published online 2013 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hosonuma K, Sato K, Yanagisawa M, et al. Incidence, mortality, and predictive factors of hepatocellular carcinoma in primary biliary cirrhosis. Gastroenterol Res Pract. 2013;2013:168012. doi: 10.1155/2013/168012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zenouzi R, Weismüller TJ, Hübener P, et al. Low risk of hepatocellular carcinoma in patients with primary sclerosing cholangitis with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(10):1733–1738. doi: 10.1016/j.cgh.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31(1):71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2016;10(S1):1–506. [PubMed] [Google Scholar]
- 64.Sonavane AD, ADN Hepatocellular carcinoma in autoimmune hepatitis: The Western India experience. Hepatol Int. 2013;58(S1):563A–571A. doi: 10.1007/s12072-016-9707-8. [DOI] [Google Scholar]
- 65.Martens B, Schlosser B, Biermer M, Van Boemmel F, Fueloep B, Schott E, Wiedenmann B, Mueller TBT. Evaluation of long-term outcome in 170 patients with well-characterized autoimmune hepatitis (AIH) in a German single university center cohort. Hepatology. 2012 Feb;55(2):522–9. doi: 10.1002/hep.26851. [DOI] [Google Scholar]
- 66.Zealand N, Ngu JH, Gearry RB, et al. Mortality and the Risk of Malignancy in Autoimmune Liver Diseases: A Population-Based Study in Canterbury, New Zealand. 2011:522–529. doi: 10.1002/hep.24743. [DOI] [PubMed] [Google Scholar]
- 67.Muratori P, Granito A, Quarneti C, et al. Autoimmune hepatitis in Italy: The Bologna experience. J Hepatol. 2009;50(6):1210–1218. doi: 10.1016/j.jhep.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 68.Floreani A, Niro G, Rosa Rizzotto E, et al. Type I autoimmune hepatitis: clinical course and outcome in an Italian multicentre study. Aliment Pharmacol Ther. 2006;24(7):1051–1057. doi: 10.1111/j.1365-2036.2006.03104.x. [DOI] [PubMed] [Google Scholar]