Abstract
The Huntington’s disease protein Huntingtin (Htt) regulates axonal transport of dense-core vesicles (DCVs) containing neurotrophins and neuropeptides. DCVs travel down axons to reach nerve terminals where they are either captured in synaptic boutons to support later release or reverse direction to reenter the axon as part of vesicle circulation. Currently, the impact of Htt on DCV dynamics in the terminal is unknown. Here we report that knockout of Drosophila Htt selectively reduces retrograde DCV flux at proximal boutons of motoneuron terminals. However, initiation of retrograde transport at the most distal bouton and transport velocity are unaffected suggesting that synaptic capture rate of these retrograde DCVs could be altered. In fact, tracking DCVs shows that retrograde synaptic capture efficiency is significantly elevated by Htt knockout or knockdown. Furthermore, synaptic boutons contain more neuropeptide in Htt knockout larvae even though bouton size, single DCV fluorescence intensity, neuropeptide release in response to electrical stimulation and subsequent activity-dependent capture are unaffected. Thus, loss of Htt increases synaptic capture as DCVs travel by retrograde transport through boutons resulting in reduced transport toward the axon and increased neuropeptide in the terminal. These results therefore identify native Htt as a regulator of synaptic capture and neuropeptide storage.
Keywords: Huntingtin, axonal transport, neuromuscular junction, synapse
Introduction
Huntington’s disease is a dominantly inherited neurodegenerative disorder caused by an abnormal polyglutamine expansion in the N-terminal part of the Htt protein. Htt is a large scaffold protein that tethers many partners into complexes to coordinate different cellular processes including autophagy, transcription, vesicle trafficking, and endocytosis (Saudou and Humbert, 2016). The progression of Huntington’s disease is linked to toxic accumulation of mutant Htt, but loss of wild type Htt function might also contribute to neuronal cell death. Yet, although Htt is ubiquitously expressed and conserved from Drosophila to humans and its mutation is linked to Huntington’s disease, its native role is not fully understood.
Native Htt is predominantly found in cytoplasm where it binds to vesicles and microtubules. Growing evidence links Htt to regulation of fast axonal transport of various organelles including BDNF (brain-derived neurotrophic factor) and APP (amyloid precursor protein) vesicles (Gunawardena et al., 2003; Gauthier et al., 2004; Caviston et al., 2011; White et al., 2015). Htt can regulate axonal transport through binding to its adaptor Huntingtin-associated protein 1 (HAP1), which interacts with kinesin (McGuire et al., 2006) and dynein (Engelender et al., 1997; Li et al., 1995). A direct interaction between Htt and the dynein/dynactin complex has also been reported (Caviston et al., 2011). In addition, Htt scaffolds GAPDH (glyceraldhyde 3-phosphate dehydrogenase) on vesicles and Htt depletion induces detachment of GAPDH from vesicles leading to decreased axonal transport of BDNF and APP (Zala et al., 2013b). Finally, Htt may induce secondary effects on axonal transport by regulating dynein heavy chain expression (Weiss and Littleton, 2016).
Htt function in axonal transport is conserved between flies and mammals (Zala et al., 2013a). Therefore, Drosophila melanogaster is a powerful model system to study Htt function under physiological and pathological conditions. Recent observations revealed that knockout (KO) of Drosophila Htt leads to subtle changes in axonal transport of dense-core vesicles (DCVs) (i.e., modestly decreased anterograde and increased retrograde axonal flux) in specialized peptidergic neurons (Weiss and Littleton, 2016). DCVs travel by fast anterograde axonal transport to reach nerve terminals where they are either captured in synaptic boutons for many hours to support future neuropeptide release or reverse direction to travel by dynein/dynactin-dependent retrograde transport as part of DCV circulation (Shakiryanova et al., 2006, Wong et al., 2012). Notably, due to circulation of excess DCVs in strings of synaptic boutons, the main parameter that determines neuropeptide accumulation in nerve terminals is DCV capture efficiency, not the rates of DCV delivery or exit of resident DCVs (Wong et al., 2012, Bulgari et al., 2014). Yet, the impact of Htt on synaptic capture is not known.
Here, to gain further insight into Htt function, we studied neuropeptide-containing DCVs in motoneuron terminals of Htt deficient Drosophila. Htt KO neuromuscular junctions (NMJs) display a selective reduction in retrograde DCV transport at proximal boutons. This effect is shown to be an indirect consequence of enhanced retrograde synaptic capture, which promotes neuropeptide storage in the terminal. These experiments therefore identify a role for Htt in synaptic capture and furthermore illustrate how capture in the terminal influences retrograde axonal transport.
Results and Discussion
Htt KO and retrograde DCV flux in type Is terminals
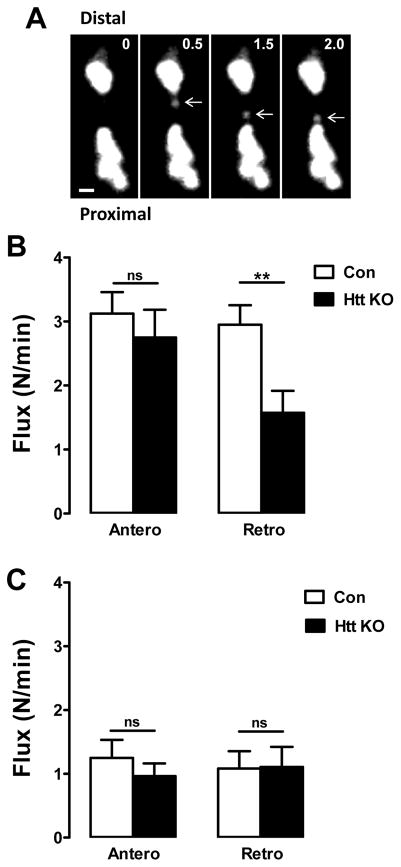
Transport of DCVs was imaged in type Is terminals at muscle 6/7 of recombinant larvae expressing atrial natriuretic factor tagged with GFP (AnfGFP) driven by the pan-neuronal driver elav-Gal4 (Rao et al., 2001). Because of their small size, type Is boutons are fully contained in confocal depth of field and DCVs moving through boutons stay in focus during imaging (Cavolo et al., 2016). Therefore, DCVs were tracked as they moved between neighboring boutons (Fig. 1A). In Htt KO larvae DCV retrograde flux was decreased by proximal boutons, while anterograde flux was unaffected (Fig. 1B). Thus, data from the type Is motoneuron terminal differ from findings previously obtained in axons of CCAP-containing peptidergic neurons (Weiss and Littleton, 2016). Furthermore, tracking of DCVs through photobleached proximal boutons showed that Htt KO does not affect DCV transport velocity: for anterograde transport, DCVs moved at 0.89 ± 0.09 μm/s in controls and 0.93 ± 0.10 μm/s in Htt KOs, while for retrograde transport, DCVs moved at 0.81 ± 0.07 μm/s in controls and 0.83 ± 0.08 μm/s in Htt KOs (Control, n = 13; Htt KO, n = 16). Thus, Htt KO reduces proximal retrograde flux without altering transport speed. This implies that there are fewer DCVs undergoing retrograde transport through proximal boutons in the Htt KOs.
Figure 1.
Htt depletion in type Is synaptic boutons attenuates DCV retrograde flux. A. Contrast-enhanced time-lapse images of type Is boutons expressing AnfGFP show a DCV (arrows) traveling by retrograde transport between adjacent boutons. The time interval between frames is 0.5 seconds. Scale bar 1 μm. B. Htt knockout decreases retrograde DCV flux in proximal boutons expressing AnfGFP. Control (Con), n = 21; Htt KO, n = 20. **p < 0.01, Unpaired t-test. C. Retrograde flux is not affected at the most distal boutons in Htt knockout larvae expressing AnfGFP. Con, n = 6; Htt KO, n = 14. Unpaired t-test shows that there is no significant (ns) change.
To determine the basis of this retrograde specific effect, traffic in and out of the most distal bouton was studied. Anterograde traffic there, like at proximal boutons, was unaffected by Htt KO (Fig. 1C). The unaffected drop in anterograde flux as DCVs are transported away from the axon further supports the conclusion that capture of DCVs during anterograde transport through boutons is unaffected by the Htt KO. Time-lapse imaging also showed that Htt KO does not affect retrograde flux at the most distal bouton (Fig. 1C) where the dynein-dynactin complex initiates retrograde DCV transport (Wong et al., 2012; Lloyd et al., 2012). Therefore, the reduced number of DCVs undergoing retrograde transport in Htt KO proximal boutons is not due to a change in the initiation of retrograde transport.
Htt KO increases DCV retrograde capture efficiency
One possible explanation for the above results is that synaptic capture of DCVs during retrograde transport through boutons is enhanced in Htt KO animals. Therefore, synaptic capture was measured for DCVs moving through photobleached boutons (Fig. 2A) (Shakiryanova et al., 2006). Specifically, after photobleaching the second most proximal bouton in a string, capture efficiency was quantified as the fractional difference in retrograde flux (R) of DCVs entering and leaving the photobleached region (i.e., for retrograde DCVs, (Rin−Rout)/Rin as described previously) (Cavolo et al., 2016). In Htt KO larvae DCV capture efficiency was elevated during retrograde transport (Fig. 2B). To independently verify the effect of Htt on capture efficiency, Htt in motoneurons was targeted by RNAi-mediated knockdown. Because these experiments entailed single crosses, DCVs were labeled in recombinants expressing Drosophila insulin-like peptide 2 tagged with GFP (Dilp2GFP) driven by OK6-Gal4, which generates a robust signal (Wong et al., 2012, 2015). Again, these experiments demonstrated an increase of capture efficiency for DCVs undergoing retrograde transport (Fig. 2C). Therefore, KO and knockdown of Htt are sufficient to increase retrograde synaptic capture efficiency.
Figure 2.
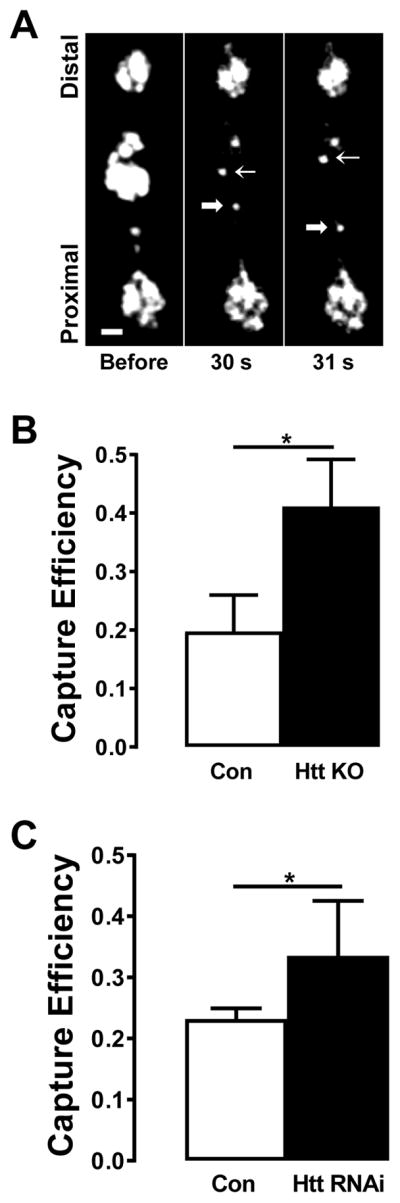
Htt loss increases DCV retrograde synaptic capture efficiency. A. Detection of anterograde and retrograde DCV movement through a photobleached bouton. Contrast-enhanced images of type Is boutons expressing AnfGFP before, 30 s and 31 s after photobleaching of bouton. Note the DCVs moving through photobleached area in anterograde (thin arrow) and retrograde (bold arrow) direction. Scale bar 1 μm. B. Retrograde capture efficiency is increased in Htt knockouts expressing AnfGFP. Control (Con), n = 10; Htt KO, n = 12. C. Htt RNAi-mediated knockdown increases retrograde capture efficiency in boutons expressing Dilp2GFP. Con, n = 12; Htt RNAi, n = 7. *p < 0.05, Unpaired t-test.
Elevated retrograde DCV capture increases neuropeptide content in boutons
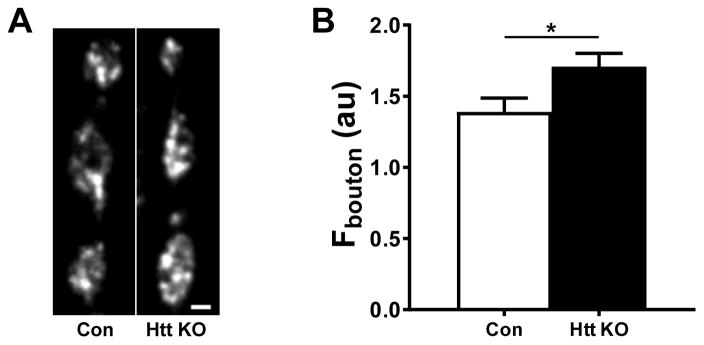
Elevated capture efficiency could lead to accumulation of DCVs in nerve terminals. To test this hypothesis, we quantified GFP fluorescence in boutons of Htt KO and wild type larvae expressing AnfGFP. These measurements demonstrated that type Is boutons of Htt KO larvae contained 22.8 ± 6.7% more neuropeptide than wild type animals (Fig. 3A, B).
Figure 3.
Htt knockout elevates fluorescence in synaptic boutons expressing AnfGFP. A. Representative images of type Is boutons in control (Con) and Htt KO NMJs expressing AnfGFP. Scale bar equals 2 μm. B. Quantification of fluorescence intensity in boutons. Con, n = 45; Htt KO, n = 83. *p < 0.05, Unpaired t-test.
In principle, the increased fluorescence in Htt deficient boutons could also be due to changes in bouton size or single DCV fluorescence. However, comparison of bouton size (measured in terms of area in images) and DCV fluorescence (measured as DCVs traveled between boutons) in Htt KO and wild type synaptic boutons did not reveal any differences (Fig. 4A, B). Increased neuropeptide accumulation in Htt KO boutons could also result from reduced DCV-mediated release. Recent studies demonstrate involvement of Htt adaptor protein HAP1 in insulin release from pancreatic beta cells (Pan et al., 2016). HAP1 is also important in several steps of exocytosis in adrenal chromaffin cells (Mackenzie et al., 2014). Therefore, to test the role of Htt on DCV-mediated release, we examined neuropeptide secretion by synaptic boutons in response to 70 Hz stimulation. However, neuropeptide release, measured by the evoked percent decrease in AnfGFP fluorescence, was not altered with Htt KO (Fig. 4C). As these results failed to uncover an alternative mechanism, the above experiments demonstrate that accumulation of DCVs in Htt KO boutons and the accompanying reduction in retrograde transport are likely produced by enhanced DCV capture during retrograde transport in the terminal.
Figure 4.
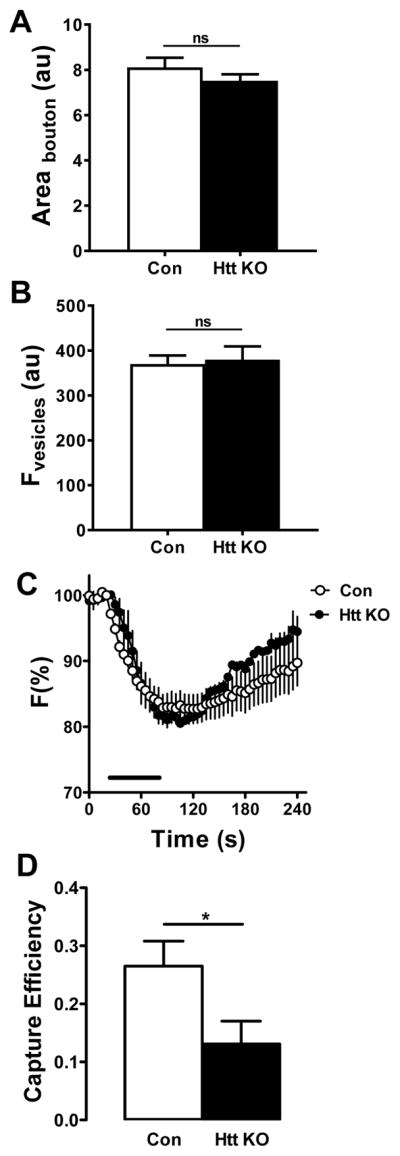
Elevated fluorescence in Htt depleted boutons is not induced by a change of boutons size, DCV fluorescence, neuropeptide release or anterograde capture. A. Quantification of bouton area in larvae expressing AnfGFP. Control (Con), n = 45; Htt knockout, n = 83. Unpaired t-test shows there is no significant change between wild type and Htt depleted larvae. B. Quantification of single DCV fluorescence in boutons expressing AnfGFP shows no difference in wild type and Htt depleted boutons. Con, n = 68; Htt KO, n = 51. Unpaired t-test shows there is no significant change. C. Htt depletion does not induce statistically significant changes in neuropeptide release and subsequent replenishment by activity-dependent capture in boutons expressing AnfGFP. Con, n = 6; Htt KO, n = 4. Bar indicates 70 Hz stimulation. D. Anterograde capture efficiency is decreased in Htt knockouts expressing AnfGFP. Control (Con), n = 10; Htt KO, n = 12. *p < 0.05, Unpaired t-test.
Recently, it was shown that activity induces selective capture of anterograde DCVs to replenish neuropeptide stores following release (Cavolo et al., 2016). Furthermore, it was established that, as a consequence of increased anterograde capture and vesicle circulation, activity indirectly decreases retrograde flux (Shakiryanova et al., 2006, Cavolo et al., 2016). Therefore, reduced axonal retrograde DCV flux in Htt KOs might result in part from enhanced capture of anterograde DCVs. However, as noted earlier, the drop in anterograde flux between proximal and distal boutons, which would reflect anterograde capture, was unaffected by the Htt KO (Fig. 1). Furthermore, initial experiments showed that there is not a statistically significant effect of Htt KO on the recovery of fluorescence following release that is indicative of activity-dependent anterograde capture (Fig. 4C). Also, basal anterograde capture efficiency measured in single Htt KO boutons was reduced, not increased (Fig. 4D). The latter effect might seem surprising given that anterograde flux was unaffected (Fig. 1). However, our measurement of capture efficiency (i.e., the imbalance of flux in and out of boutons over minutes) reflects both brief (<2 minutes) visits and long lasting capture (Wong et al., 2012). Therefore, only brief visits that minimally affect steady state anterograde flux may dominate in Figure 4D, while long-lasting capture may dominate in Figure 1. More relevant here, anterograde transport measurements verify that the reduction in retrograde DCV flux and the increase in bouton neuropeptide content with Htt loss are due to selectively enhanced capture of retrograde DCVs.
Conclusions
Many studies of axonal transport have focused on regulation of the motors responsible for microtubule-mediated transport of organelles without considering the fate of motor cargoes in the nerve terminal. With this approach, Htt dependent changes in retrograde flux of DCVs might be assumed to reflect regulation of the dynein-dynactin complex in the axon. However, analysis of circulating DCVs in the NMJ shows that loss of native Htt reduces retrograde transport indirectly because of enhanced synaptic capture during retrograde transport through en passant synaptic boutons. Thus, in Htt KOs there are fewer DCVs to travel back toward the soma in the axon because more DCVs are captured in boutons to elevate neuropeptide stores.
These data therefore add Htt to the factors that regulate synaptic capture with a directional bias; recently, it was shown that activity acts in an Fmr1-sensitive manner to selectively increase capture of DCVs undergoing anterograde transport through boutons (Cavolo et al., 2016). Differential control of anterograde and retrograde capture suggests that distinct signals exist to induce kinesin and dynein motors to release their vesicle cargos in synaptic boutons where they can take up long lasting residence (Shakiryanova et al., 2006). For example, Htt might influence retrograde capture by a variety of dynein-related mechanisms (see Introduction). The results with Fmr1 (the fragile X syndrome protein) and Htt further illustrate that synaptic capture mechanisms are regulated by disease-related genes. Therefore, in the context of Huntington’s disease, it will be of interest to determine the molecular basis of synaptic capture and its regulation by Huntington’s disease-associated Htt mutations.
Materials and Methods
In vivo imaging experiments were performed at type Is synaptic boutons at muscle 6/7 on third instar Drosophila NMJ as described previously (Levitan et al., 2007). AnfGFP (Han et al., 1999, Rao et al., 2001) was expressed in motoneurons using the elav-Gal4 driver, while Dilp2GFP (Wong et al., 2012) expression was driven with OK6-Gal4. Htt KO flies (Zhang et al., 2009) were kindly provided by N. Perrimon and the Htt RNAi line (#44550) was obtained from the Bloomington stock center. 3rd instar larvae were filleted in HL3 saline, which contains (in mM) 70 NaCl, 5 KCl, 1.5 CaCl2, 20 MgCl2, 10 NaHCO3, 5 trehalose, 115 sucrose, and 5 sodium HEPES, pH 7.2, or HL3 saline in which Ca2+ was replaced with 0.5 mM EGTA (0 Ca2+ HL3) and viewed under 60X Olympus objectives with 1.1 numerical aperture on a Fluoview 1000 scanning confocal microscope or an Olympus microscope equipped with a Yokogawa Spinning disk confocal module and a Hamamatsu EMCCD camera. Time-lapse images were acquired for 4 minutes at 0.5 or 1 Hz. Nerve stimulation at 70 Hz was performed in HL3 supplemented with 10 mM glutamate to prevent muscle contraction as described previously (Levitan et al., 2007). Images were analyzed in ImageJ (NIH), while graphing and statistics were performed with Graphpad Prism. For all statistical tests, two-tailed t-test p values were set at < 0.05 as the threshold for statistical significance.
Acknowledgments
This study was supported by NIH grant R01NS032385 to ESL. The authors declare no competing financial interests. We thank N. Perrimon (Harvard University) for the Htt KO flies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bulgari D, Zhou C, Hewes RS, Deitcher DL, Levitan ES. Vesicle capture, not delivery, scales up neuropeptide storage in neuroendocrine terminals. Proc Natl Acad Sci U S A. 2014;111:3597–3601. doi: 10.1073/pnas.1322170111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Zajac AL, Tokito M, Holzbaur EL. Huntingtin coordinates the dynein-mediated dynamic positioning of endosomes and lysosomes. Mol Biol Cell. 2011;22:478–492. doi: 10.1091/mbc.E10-03-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavolo SL, Bulgari D, Deitcher DL, Levitan ES. Activity induces Fmr1-sensitive synaptic capture of anterograde circulating neuropeptide vesicles. J Neurosci. 2016;36:11781–11787. doi: 10.1523/JNEUROSCI.2212-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavolo SL, Zhou C, Kethcham SA, Suzuki MM, Ukalovic K, Silverman MA, Schroer TA, Levitan ES. Mycalolide B dissociates dynactin and abolishes retrograde axonal transport of dense-core vesicles. Mol Biol Cell. 2015;26:2664–2672. doi: 10.1091/mbc.E14-11-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelender S, Sharp AH, Colomer V, Tokito MK, Lanahan A, Worley P, Holzbaur EL, Ross CA. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet. 1997;6:2205–2212. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gunawardena S, Her LS, Brush RG, Laymon RA, Niesman IR, Gordesky-Gold B, Sintasath L, Bonini NM, Goldstein LS. Disruption of axonal transport by loss of Huntingtin or expression of pathogenic polyQ protein in Drosophila. Neuron. 2003;40:25–40. doi: 10.1016/s0896-6273(03)00594-4. [DOI] [PubMed] [Google Scholar]
- Han W, Ng YK, Axelrod D, Levitan ES. Neuropeptide release by efficient recruitment of diffusing cytoplasmic secretory vesicles. Proc Natl Acad Sci U S A. 1999;96:14577–14582. doi: 10.1073/pnas.96.25.14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan ES, Lanni F, Shakiryanova D. In vivo imaging of vesicles and release at the Drosophila neuromuscular junction. Nature Protocols. 2007;2:1117–1125. doi: 10.1038/nprot.2007.142. [DOI] [PubMed] [Google Scholar]
- Li XJ, Li SH, Sharp AH, Nucifora FC, Jr, Schilling G, Lanahan A, Worley P, Snyder SH, Ross SA. Interaction of Huntingtin-associated protein enriched in brain with implications for pathology. Nature. 1995;378:398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- Lloyd TE, et al. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron. 2012;74:344–360. doi: 10.1016/j.neuron.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie KD, Duffield MD, Peiris H, Phillips L, Zanin MP, Teo EH, Zhou XF, Keating DJ. Huntingtin-associated protein 1 regulates exocytosis, vesicle docking, readily releasable pool size and fusion pore stability in mouse chromaffin cells. J Physiol. 2014;592:1505–1518. doi: 10.1113/jphysiol.2013.268342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcguire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem. 2006;281:3552–3559. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- Pan JY, Yuan S, Yu T, Su CL, Liu XL, He J, Li H. Regulation of L-type Ca2+ Channel activity and insulin secretion by Huntingtin-associated protein 1. J Biol Chem. 2016 doi: 10.1074/jbc.M116.727990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C, Lang C, Levitan ES, Deitcher DL. Visualization of neuropeptide expression, transport and exocytosis in Drosophila melanogaster. J Neurobiol. 2001;49:159–172. doi: 10.1002/neu.1072. [DOI] [PubMed] [Google Scholar]
- Saudou F, Humbert S. The biology of Huntingtin. Neuron. 2016;89:910–926. doi: 10.1016/j.neuron.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Shakiryanova D, Tully A, Levitan ES. Activity-dependent synaptic capture of transiting peptidergic vesicles. Nat Neurosci. 2006;9:896–900. doi: 10.1038/nn1719. [DOI] [PubMed] [Google Scholar]
- Weiss KR, Littleton JT. Characterization of axonal transport defects in Drosophila Huntingtin mutants. J Neurogenetics. 2016 Jul;22:1–10. doi: 10.1080/01677063.2016.1202950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, 2nd, Anderson E, Zimmerman K, Zheng RR, Gunawardena S. Huntingtin differentially regulates the axonal transport of a sub-set of Rab-containing vesicles in vivo. Human Mol Genetics. 2015;24:7182–7195. doi: 10.1093/hmg/ddv415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Zhou C, Shakiryanova D, Lloyd TE, Deitcher DL, Levitan ES. Neuropeptide delivery to synapses by long range vesicle circulation and sporadic capture. Cell. 2012;148:1029–1038. doi: 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Cavolo SL, Levitan ES. Synaptic neuropeptide release by dynamin-dependent partial release from circulating vesicles. Mol Biol Cell. 2015;26:2466–2474. doi: 10.1091/mbc.E15-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala D, Hinckelmann MV, Saudou F. Huntingtin’s function in axonal transport is conserved in Drosophila melanogaster. PLoS One. 2013a;8:e60162. doi: 10.1371/journal.pone.0060162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelieres FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for axonal transport. Cell. 2013b;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Zhang S, Feany MB, Saraswati S, Littleton JT, Perrimon N. Inactivation of Drosophila Huntingtin affects long-term adult functioning and the pathogenesis of a Huntington’s disease model. Dis Model Mech. 2009;2:247–266. doi: 10.1242/dmm.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]