Abstract
Objective
This study was designed to evaluate the efficacy and safety of preinjection of dezocine in preventing etomidate-induced myoclonus.
Methods
PubMed, Embase, The Cochrane Library, and China National Knowledge Infrastructure (CNKI) were searched to collect relevant randomized controlled trials (RCTs) from inception to July 2016 on the preinjection of dezocine in preventing etomidate-induced myoclonus. Two researchers independently screened literature, extracted data, and evaluated bias risks in accordance with inclusion and exclusion criteria, and then used RevMan 5.2 to perform the meta-analysis.
Results
A total of six RCTs were included in this study. The meta-analysis showed that 1) the preinjection of dezocine can reduce the incidence of etomidate-induced myoclonus (relative risk [RR] =0.25, 95% CI [0.13, 0.50], P<0.0001), which is consistent with the result of subgroup analysis; 2) the preinjection of dezocine can reduce the incidence of mild, moderate, and severe myoclonus; 3) dezocine was not related to an increasing incidence of etomidate-induced dizziness and nausea (RR =2.83, 95% CI [0.66, 12.08], P=0.6); and 4) dezocine did not reduce heart rates after the administration of etomidate (mean difference =1.06, 95% CI [−4.08, 6.19], P=0.69).
Conclusion
The preinjection of dezocine has the effect of both lowering the incidence of etomidate-induced myoclonus and easing the severity of myoclonus, but without increasing dizziness and nausea or affecting the heart rate.
Keywords: dezocine, etomidate, myoclonus, meta-analysis, RCTs
Introduction
Etomidate is a commonly used anesthetic induction drug. It has a limited effect on patients’ respiratory and circulatory systems, which is beneficial to maintaining hemodynamic stability. It also causes rapid results with smooth induction and quick revival.1 However, etomidate can cause myoclonus and injection pain. The issue of etomidate-induced injection pain has been resolved with etomidate fat milk, but the problem of etomidate-induced myoclonus has yet to be solved; its incidence rate can be as high as 80%.2 Myoclonus can increase the body’s oxygen consumption and accelerate metabolism, posing vital threats to patients with conditions such as coronary heart disease, hypertension, epilepsy, or intracranial aneurysm.3–5 Plenty of clinical studies on preventing etomidate-induced myoclonus have been conducted on opioids such as fentanyl, sufentanil, and remifentanil. Large doses of opioids have a high likelihood of controlling myoclonus, but at the same time, they can increase respiratory depression, chest wall stiffness, and other adverse reactions.6 As a new opioid receptor agonist–antagonist, full κ opioid receptor agonist, and partial δ opioid receptor agonist, dezocine makes a good effect in spinal analgesia and sedation; meanwhile, it has an antagonistic effect on μ-receptors, which are manifested as partially blocked, mild adverse reactions of the gastrointestinal tract, small respiratory depressions, low drug dependency, and high safety and tolerance.
In recent years, many studies have tested the efficacy of the preinjection of dezocine in preventing etomidate-induced myoclonus. This study conducted a meta-analysis of the efficacy and safety of the preinjection of dezocine in the prevention of etomidate-induced myoclonus.
Materials and methods
Inclusion and exclusion criteria
The studies included were randomized controlled trials (RCTs); regardless of whether allocation concealment or a blinding method was adopted, there were no language limitations. The studies excluded were RCT that did not use the efficacy outcome; studies with no full text and lacked detailed abstracts; randomization method was wrong; duplicate published articles, reviews, or lectures.
Study objects
Studies with patients who have received etomidate as an induction of anesthesia.
Interventions
Experimental group, dezocine; and control group, placebo.
Outcomes indicators
The main outcome indicator was incidence of myoclonus; the secondary outcome indicators were incidence of myoclonus at various degrees, incidence of dizziness, incidence of nausea, and increased heart rate.
Search strategy
We searched the related RCTs on the preinjection of dezocine in preventing etomidate-induced myoclonus from inception to July 2016. We searched PubMed, Embase, The Cochrane Library, and China National Knowledge Infrastructure (CNKI) with search terms such as “dezocine,” “etomidate,” and “myoclonus.” PubMed: [(myoclonus) OR (myoclonic movements)] AND (dezocine) AND (etomidate). A combination of subject terms and free words were used for the search.
Literature screening and data extraction
Two reviewers independently screened literature and extracted data, and then cross-checked each other. When there was a disagreement, the two discussed or consulted a third party. Extensive efforts were made to contact authors to supplement any incomplete data. The data extraction included baseline information on participants, sample size, key elements of quality assessment, outcome indicators, results, data, and notes.
Risk of bias assessment of the included studies
Quality assessment
We used the Jadad scale to evaluate the methodological quality of the included literature. Articles with 1–3 points were deemed to be of low quality, and those with 4–7 points were deemed to be of high quality.
Generation of allocation sequences
Items could be scored as adequate when random numbers had been generated by a computer or similar technology (2 points); items could be scored as unclear when a randomized trial had been conducted, but the method of random allocation was not described (1 point); items could be scored as inadequate when alternate allocation methods had been applied, for instance, using odd and even numbers (0 point).
Concealment of allocation sequences
Items could be scored as adequate if a research center or pharmacy had controlled the allocation plan, had employed numbered containers with identical sequence numbers or a field computer, or had applied opaque and sealed envelopes or other random allocation methods unknown to clinicians and participants (2 points); items could be scored as unclear if random number tables or other random allocation plans had been used (1 point); items could be scored as inadequate if alternate allocation, case number, the number of Sundays, open random number table, sealed envelopes, or any other predictability measures that could not prevent grouping (0 point); unused (0 point).
Blinding
Items could be scored as adequate if identical placebo tablets or the like were adopted (2 points); items could be scored as clear if a test statement had employed a blinding method without describing the means (1 point); items could be scored as inadequate if double blinding was not used or if the blind method had been inappropriate (0 point).
Withdrawals and dropouts
Numbers of and reasons for withdrawal and dropouts are given (1 point); numbers of and reasons for withdrawal and dropouts are not given (0 point).
Statistical analysis
We used RevMan 5.2.3 to conduct the meta-analysis. For identical numeric variables, weights and measures were expressed with mean difference (MD); for dichotomous data, relative risk (RR) and odds ratios (ORs) were applied to express the effect size with a 95% CI. Heterogeneity of the included studies was analyzed using a χ2 test (significance level was α=0.1), and the significance of heterogeneity was evaluated by combining this with I2. A fixed-effects model was used to conduct the meta-analysis if no significant heterogeneity had been found among any study results, whereas a random-effects model was adopted to conduct the meta-analysis after excluding the impact of significant clinical heterogeneity, if it had been found. We used subgroup analysis or sensitivity analysis or descriptive analysis to analyze significant clinical heterogeneity. The significance level for the meta-analysis was α=0.05.
Results
Literature search results
Initially, 508 articles were retrieved. Of them, six7–12 were eventually included in the meta-analysis after step-by-step screening. Figure 1 shows the screening process and results.
Figure 1.
Flow diagram.
Abbreviation: RCTs, randomized controlled trials.
Basic characteristics and methodological quality of enrolled studies
The basic characteristics of included studies are shown in Table 1.
Table 1.
Characteristics of the studies included in the meta-analysis
| Author (year published) | Country | Headcount | Grouping | Surgical setting | Jadad score | Blinded | Allocation concealment | Random | Exit |
|---|---|---|---|---|---|---|---|---|---|
| He et al7 2015 | China | 108 | Dezocine 0.1 mg/kg Normal saline |
Elective surgery | 6 | 2 | 1 | 2 | 1 |
| Lv et al8 2014 | China | 80 | Dezocine 0.1 mg/kg Normal saline |
Elective surgery | 6 | 2 | 1 | 2 | 1 |
| Wu and Zhou10 2012 | China | 100 | Dezocine 0.1 mg/kg Normal saline |
Laparoscopic cholecystectomy | 5 | 1 | 1 | 2 | 1 |
| Zhang et al12 2014 | China | 100 | Dezocine 0.05 mg/kg Dezocine 0.075 mg/kg Dezocine 0.1 mg/kg Normal saline |
Elective surgery | 5 | 2 | 1 | 1 | 1 |
| Zhang et al11 2015 | China | 150 | Dezocine 0.1 mg/kg Normal saline Butorphanol 15 μg/kg |
Elective surgery | 6 | 2 | 1 | 2 | 1 |
| Zhou et al9 2015 | China | 120 | Dezocine 0.1 mg/kg Normal saline |
Elective surgery | 4 | 1 | 1 | 1 | 1 |
Meta-analysis results
Incidence of myoclonus
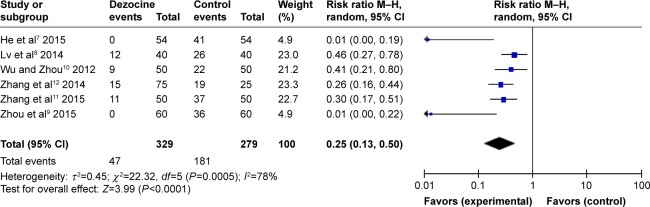
A total of six RCTs were included in this meta-analysis. Heterogeneity was found among the studies (I2=78%). Therefore, a random-effects model was applied to conduct the meta-analysis. The results showed that dezocine in combination with etomidate has a significantly lower incidence of myoclonus, compared with etomidate alone. This difference was found to be statistically significant (RR =0.25, 95% CI [0.13, 0.50], P<0.0001) (Figure 2). As the heterogeneity was significant, a subgroup analysis was performed.
Figure 2.
Forest plot of weighed risk ratio. The dezocine and placebo groups in the prevention of etomidate-induced myoclonus with a 95% CI.
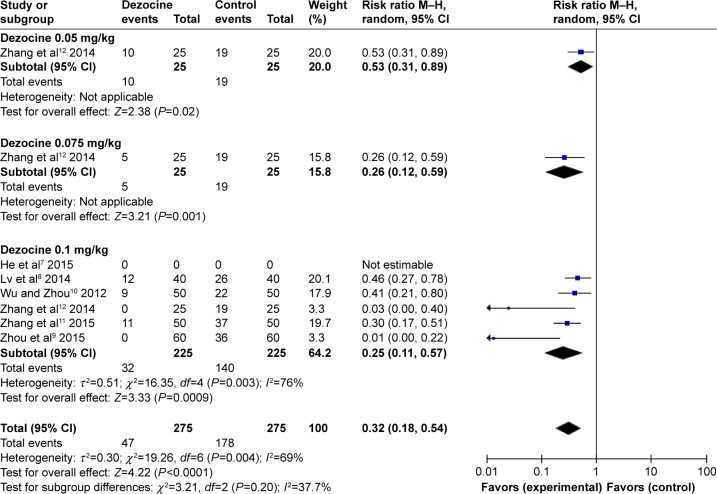
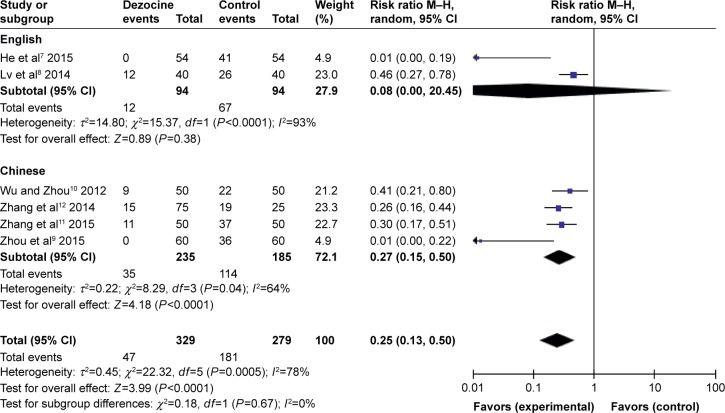
According to the operation type (Figure 3) and the dose of dezocine (Figure 4), the subgroup analyses had no effect on pooled results; all of these analyses were affected by heterogeneity.
Figure 3.
Forest plot of weighed risk ratio. The dezocine and placebo groups in the prevention of etomidate-induced myoclonus with a 95% CI (subgroup analyses of operation type).
Figure 4.
Forest plot of weighed risk ratio. The dezocine and placebo groups in the prevention of etomidate-induced myoclonus with a 95% CI (subgroup analyses of dezocine doses).
Severity of etomidate-induced myoclonus
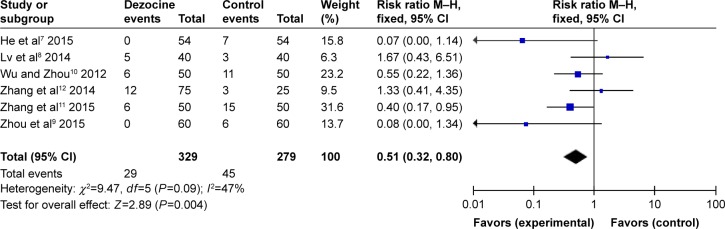
Mild myoclonus
All RCTs the reported the degrees of mild myoclonus induced by etomidate. There was no statistical heterogeneity among the study results (I2=47%), so a random-effects model was employed to perform the meta-analysis. The results indicated that there was a significant difference between the dezocine group and the control group in the incidence of etomidate-induced mild myoclonus (RR =0.51, 95% CI [0.32, 0.80], P=0.004) (Figure 5).
Figure 5.
Forest plot of weighed risk ratio. The dezocine and placebo groups in reducing the intensity of etomidate-induced myoclonus with a 95% CI: mild myoclonus.
Moderate myoclonus
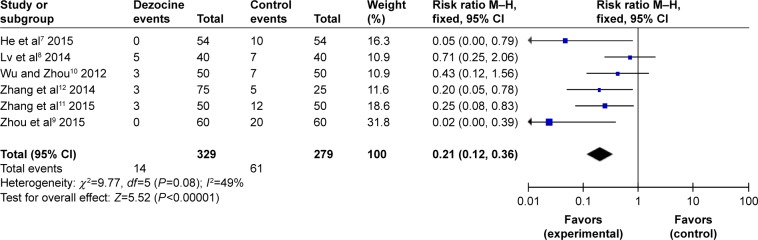
All RCTs reported the degrees of etomidate-induced moderate myoclonus. No statistical heterogeneity was found among the study results (I2=49%); thus, a fixed-effects model was used to conduct the meta-analysis. It showed that there was a significant difference between the dezocine group and the control group in the incidence of etomidate-induced moderate myoclonus (RR =0.21, 95% CI [0.12, 0.36], P<0.00001) (Figure 6).
Figure 6.
Forest plot of weighed risk ratio. The dezocine and placebo groups in reducing the intensity of etomidate-induced myoclonus with a 95% CI: moderate myoclonus.
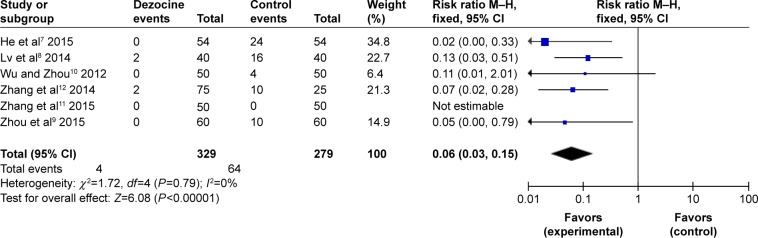
Severe myoclonus
All RCTs reported the degrees of etomidate-induced severe myoclonus. No statistical heterogeneity was found among the study results (I2=0%); therefore, a fixed-effects model was applied to perform the meta-analysis, implying that there was a significant difference between the dezocine group and the control group in the incidence of etomidate-induced severe myoclonus (RR =0.0.06, 95% CI [0.03, 0.15], P<0.00001) (Figure 7).
Figure 7.
Forest plot of weighed risk ratio. The dezocine and placebo groups in reducing the intensity of etomidate-induced myoclonus with a 95% CI: severe myoclonus.
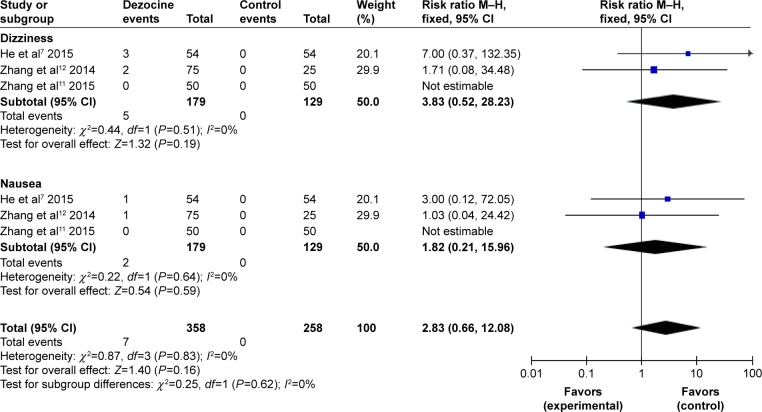
Incidence of nausea and dizziness
A total of three RCTs were included. As no statistical heterogeneity was found within the study (I2=0%), a fixed-effects model was employed to do the meta-analysis, suggesting that there was no significant difference between the dezocine group and the control group in etomidate-induced nausea and dizziness (dizziness: RR =3.83, 95% CI [0.52, 28.23], P=0.19; nausea: RR =1.82, 95% CI [0.21, 15.96], P=0.59) (Figure 8).
Figure 8.
Forest plot of weighed risk ratio: adverse effects after pretreatment with dezocine: nausea and dizziness.
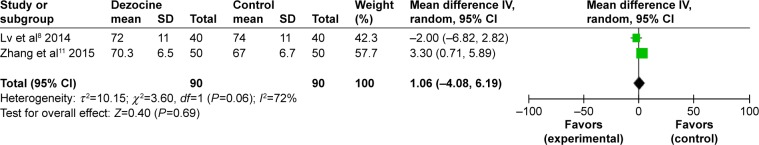
Heart rate
A total of two RCTs were included. As no statistical heterogeneity was found in the study (I2=72%), a random-effects model was used to perform the meta-analysis, showing that there was no significant difference between the dezocine group and the control group in the heart rate after administering etomidate (MD =1.06, 95% CI [−4.08, 6.19], P=0.19) (Figure 9).
Figure 9.
Forest plot of weighed risk ratio: the effect of pretreatment with dezocine on the heart rate.
Discussion
This study performed a meta-analysis comparing the efficacy and safety of etomidate-induced myoclonus in a dezocine group and a control group. The meta-analysis indicated that dezocine can reduce the incidence of etomidate-induced myoclonus and lower the incidence of etomidate-induced mild, moderate, and severe myoclonus. In addition, no significant difference was found between the dezocine group and the control group in the incidence of nausea and dizziness, and no significant difference was found between the dezocine group and the control group in the heart rate after administrating etomidate.
Etomidate is a non-barbiturate intravenous induction agent that plays a major role in the GABA receptor. It has the effect of sedation and hypnosis, rather than analgesia and muscle relaxation. Etomidate-induced myoclonus may be the result of inhibition of the cerebral cortex, depression of subcortical structures (including the extrapyramidal system), or competitive inhibition of endogenous dopamine receptors. Several studies13 have shown that the mechanisms behind etomidate-induced myoclonus may be similar to several of the mechanisms behind epilepsy. Loacker et al14 reported that κ-opioid receptor agonist can produce a strong anticonvulsant effect. κ-opioid receptor agonist’s anticonvulsant effect mechanism is not only related to κ-opioid receptors but also to N-methyl-d-aspartate channel, γ-aminobutyric acid A–benzodiazepine–chloride ion channel, and γ-aminobutyric acid receptors.15 That dezocine can effectively inhibit etomidate-induced myoclonus may be the result of the interaction with these receptors; that dezocine can activate κ receptors may be relevant to its effectiveness on inhibiting etomidate-induced myoclonus.
Several limitations of this meta-analysis should also be taken into consideration. First, the sample size was small. In addition, there was a deficiency of unpublished data and other nontraditional sources of evidence. Moreover, there was also heterogeneity. All these factors may have affected the objectivity and reliability of the meta-analysis.
In summary, this study showed that the preinjection of dezocine can reduce the incidence of etomidate-induced myoclonus and can mitigate the severity of myoclonus without adverse reactions.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Patel S. Cardiovascular effects of intravenous anesthetics. Int Anesthesiol Clin. 2002;40(1):15–33. doi: 10.1097/00004311-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Doenicke AW, Roizen MF, Kugler J, Kroll H, Foss J, Ostwald P. Reducing myoclonus after etomidate. Anesthesiology. 1999;90(1):113–119. doi: 10.1097/00000542-199901000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Gultop F, Akkaya T, Bedirli N, Gumus H. Lidocaine pretreatment reduces the frequency and severity of myoclonus induced by etomidate. J Anesth. 2010;24(2):300–302. doi: 10.1007/s00540-010-0869-6. [DOI] [PubMed] [Google Scholar]
- 4.Mizrak A, Koruk S, Bilgi M, et al. Pretreatment with dexmedetomidine or thiopental decreases myoclonus after etomidate: a randomized, double-blind controlled trial. J Surg Res. 2010;159(2):e11–e16. doi: 10.1016/j.jss.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Ri HS, Shin SW, Kim TK, Baik SW, Yoon JU, Byeon GJ. The proper effect site concentration of remifentanil for prevention of myoclonus after etomidate injection. Korean J Anesthesiol. 2011;61(2):127–132. doi: 10.4097/kjae.2011.61.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hueter L, Schwarzkopf K, Simon M, Bredle D, Fritz H. Pretreatment with sufentanil reduces myoclonus after etomidate. Acta Anaesthesiologica Scandinavica. 2003;47(4):482–484. doi: 10.1034/j.1399-6576.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 7.He L, Ding Y, Chen H, Qian Y, Li Z. Dezocine pretreatment prevents myoclonus induced by etomidate: a randomized, double-blinded con-trolled trial. J Anesth. 2015;29(1):143–145. doi: 10.1007/s00540-014-1854-2. [DOI] [PubMed] [Google Scholar]
- 8.Lv Z, Fang J, Zhu J, et al. Intravenous dezocine pretreatment reduces the incidence and intensity of myoclonus induced by etomidate. J Anesth. 2014;28(6):944–947. doi: 10.1007/s00540-014-1842-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhou XL, Chen JT, Yu W, Ma XS, Zhang W. The clinical research in the influence of dezocine on the heart rate and mean arterial pressure during the inhibition of myoclonus induced by etomidate. [Accessed July 04, 2017];Progress in Modern Biomedicine. 2015 15(13):2491–2494. Available from: http://en.oversea.cnki.net/kcms/detail/detail.aspx?QueryID=15&CurRec=1&dbCode=CJFD&filename=SWCX201513025&dbname=CJFDLAST2015. [Google Scholar]
- 10.Wu WH, Zhou YT. Effect of pretreatment with dezocine on etomidate lipid emulsion induced myoclonus and awakening quality. [Accessed July 04, 2017];Occup and Health. 2012 28(23):3007–3008. Available from: http://www.cnki.net/KCMS/detail/detail.aspx?QueryID=0&CurRec=1&filename=ZYJK201223068&dbname=CJFD2012&dbcode=CJFQ&pr=&urlid=&yx=&uid=WEEvREcwSlJHSldRa1FhdXNXYXJwcGY1MHdVcVVOTmpvQ2EyRzZrU1JTYz0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4ggI8Fm4gTkoUKaID8j8gFw!!&v=MjA2NTZiSVI4ZVgxTHV4WVM3RGgxVDNxVHJXTTFGckNVUkwyZlllUm9GQ2poVXIzTVB6VE-JaYkc0SDlQT3JJOUQ= [Google Scholar]
- 11.Zhang Q, Liu L, Lv GY. Comparison of the effects of intravenous pre-treatment of Butorphanol and Dezocine on prevention of Etomidate-induced myoclonus. [Accessed July 04, 2017];Tianjin Med J. 2015 43(12):1450–1452. Available from: http://www.cnki.net/KCMS/detail/detail.aspx?QueryID=2&CurRec=1&filename=TJYZ201512029&dbname=CJFDLAST2016&dbcode=CJFQ&pr=&urlid=&yx=&uid=WEEvREcwSlJHSldRa1FhdXNXYXJwcGY1MHdVcVVOTmpvQ2EyRzZrU1JTYz0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4ggI8Fm4gTkoUKaID8j8gFw!!&v=MjEyNDJmU2RMRzRIOVROclk5SGJZUjhlWDFMdXhZUzdEaDFUM3-FUcldNMUZyQ1VSTDJmWWVSb0ZDamhVTHJQTVM= [Google Scholar]
- 12.Zhang Q, Liang He, Liu CM. Preventive effect of dezocine pretreatment on etomidate-induced myolonu. [Accessed July 04, 2017];Jiang su Med J. 2014 40(21):2601–2603. Available from: http://www.cnki.net/KCMS/detail/detail.aspx?QueryID=1&CurRec=1&filename=YIYA201421028&dbname=CJFD2014&dbcode=CJFQ&pr=&urlid=&yx=&uid=WEEvREcwSlJHSldRa1FhdXNXYXJwcGY1MHdVcVVOTmpvQ2EyRzZrU1JTYz0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKe-nsW4ggI8Fm4gTkoUKaID8j8gFw!!&v=MjgxMzc3ek9QQ1RTYjdHNEg5WE9ybzlIYklSOGVYMUx1eFlTN0RoMVQzcVRyV00xRnJDVVJMMmZZZVJvRkNqaFU= [Google Scholar]
- 13.Herrera-Peco I, Wix-Ramos R, Dominguez-Gadea L, et al. Changes in cerebral perfusion induced by etomidate in patients with temporal lobe epilepsy. Rev Neurol. 2009;49(11):561–565. [PubMed] [Google Scholar]
- 14.Loacker S, Sayyah M, Wittmann W, Herzog H, Schwarzer C. Endogenous dynorphin in epileptogenesis and epilepsy: anticonvulsant net effect via kappa opioid receptors. Brain. 2007;130(4):1017–1028. doi: 10.1093/brain/awl384. [DOI] [PubMed] [Google Scholar]
- 15.Manocha A, Mediratta PK, Sharma KK. Studies on the anticonvulsant effect of U50488H on maximal electroshock seizure in mice. Pharmacol Biochem Behav. 2003;76(1):111–117. doi: 10.1016/s0091-3057(03)00218-1. [DOI] [PubMed] [Google Scholar]