Abstract
Background:
Visceral leishmaniasis (VL) has strong links with poverty, substantial medical and veterinary impacts. This review aimed to focus in studies published during 1994–2016 on VL in southeastern Iran.
Methods:
The present review is based on expert knowledge and historical studies published during the past 23 yr (1994–2016) on VL in southeastern Iran. In addition, related literature found in PubMed by using the keywords such as visceral leishmaniasis, kala-azar, and Leishmania infantum are included.
Results:
Overall, 118 children aged 4.2 yr were detected as infected with human VL (HVL). The majority of the cases were from Orzoieh district (37.1%) in southwest of Kerman Province, followed by Sirjan (15.7%), Jiroft (14.8%), Kahnuj (9.3%) and to lesser extent from other areas. The male to female ratio was 1.7. The three most frequent clinical features were represented by fever (100.0%), anemia (95.0%) and splenomegaly (91.5%). Altogether, 42.0% of the VL cases developed secondary bacterial infections, the overall case-fatality rate was 3.4%, and majorities (88.0%) of the VL patients were undernourished. Overall, 733 dogs and wild canines were examined by different techniques with various seroprevalence ranges.
Conclusion:
In southeastern Iran, VL is endemic in Orzoieh district in Kerman Province. While the dogs are implicated as the main domestic reservoir of VL, wide range of wild canines can serve as a secondary potential reservoir host.
Keywords: Visceral leishmaniasis, Kala-azar, Leishmania infantum, Iran
Introduction
Leishmaniasis represents a group of parasitic disease with complex spectrum of clinical syndromes caused by the species of Genus Leishmania. The clinical manifestations of the disease vary significantly but are often classified into three distinct clinical forms including of cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL) and visceral leishmaniasis (VL) (1, 2). VL also known as kala–azar is the most severe and fatal form of leishmaniasis, widely distributed across the globe, mainly in tropical and sub-tropical countries (3).
This disease poses a serious clinical and veterinary impact in terms of morbidity and mortality. An estimate of 0.2 to 0.4 million cases suffer from potentially fatal VL, per year (4). Kala-azar is one of the most neglected and vector-borne diseases which has strong links with poverty exerting major impact and substantial health problems among the poor (5) and up to 40000 deaths per annum (4). Approximately, 90% of all VL cases occur in Indian subcontinent, East Africa and Brazil (4, 6).
Many factors play important roles in distribution of VL cases in each country and region including climatic condition, environmental changes, inadequate living conditions, malnourishment, presence of suitable reservoir hosts and principal biological vectors (7, 8). VL presents itself with various clinical features. It is often accompanied by other conditions leading to a fatality rate of 5%–15% and up to 95% if treatment is delayed or ignored (3). At present, the overall number of VL cases are rising globally, due to lack of efficacious vaccine, difficulty in controlling wide range of reservoir hosts and diverse phlebotomine sand fly species and emergence of resistance against drugs of choice (9).
In Iran, majority of VL cases are reported from two main endemic foci consisting of northwestern (East Azerbaijan and Ardabil provinces) and southern areas (Fars Province), and more recently to lesser extent from Bushehr, Qom, Kerman, northern Khorasan and other parts of the country (10–19). Over the past years, there has been an average of 100–300 new cases of VL reported annually (15). Most of the patients have been registered in remote areas, notably from rural and nomadic regions where living standards are poor and people dwell in close contact with the main animal reservoir, dogs (Canis familiaris). The causative agent is L. infantum, and over 90% of the cases have been found in children of up to 12 years of age (3,15).
In recent years, the incidence of Mediterranean VL caused by L. infantum has been decreasing in many foci worldwide and in Iran, mainly due to improvement of hygienic conditions and promotion of living standards (9). This is in contrast with kala-azar cases caused by L. donovani, which continues to rise and kill thousands of individuals each year in Indian Subcontinent and African countries (20).
The prevalence of canine VL (CVL) in endemic areas of Iran ranges between 14.2% and 17.4% (10, 21–27), although this range varies depending upon the geographical location, the method performed in the detection of CVL and also the availability of the major domestic and wild canine reservoir host and presence of specific species of vector (7, 8).
Methods
Literature search and Scope
The present review is based on expert knowledge and historical studies published during the past 23 yr (1994–2016) on VL in southeastern Iran. In addition, related literature found in PubMed by using the keywords such as visceral leishmaniasis, kala-azar, and Leishmania infantum are included. A review of other literature has been performed and almost all existing registered cases and reports have been presented. Issues such as clinical manifestations, epidemiological features, diagnostic methods, current treatment regimens and vector incrimination have been presented and discussed.
So far, no comprehensive review study has been published on VL from southeastern Iran. Therefore, presenting this narrative review work could help in better understanding of the clinical and veterinary impacts of the VL disease for planning possible future control strategies.
Study area
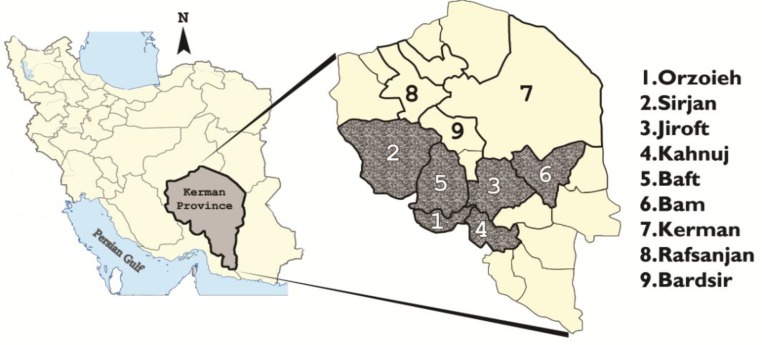
Province of Kerman covers 180726 km2 areas with approximately three million populations; located 1080 km from Tehran in southeastern Iran. It is the largest province of the country, representing 11.0% of the total land in 24 districts. An estimated 60% (1850000 individuals) of the population live as nomadic and rural life, generally in remote areas of the Province, more frequently in south and southwest. Although, attempt has been made to settle the nomad tribes’ population (nearly 100000 individuals) but they still travel back and forth through a distinct route from south to Kahnuj with altitude of 300 m above the sea level toward Jiroft, Orzoieh, Baft, Sirjon and Hajiabad, 1500 m above the sea level in mountainous areas toward north. Approximately, over 90.0% of the kala-azar cases belong to Orzoieh, Sirjan, Jiroft, Kahnuj, Baft, Bam and Kerman districts (Fig. 1). The Province possesses 25.0% of the total gardens including orchard of oranges, pistachios, palm trees, and walnuts in the country. A considerable number of population, more commonly from Sistan and Bluchestan and from neighboring cities of Hormozgan Provinces for easiness of accessibility seek medical treatment in Kerman Province; although, a similar attention is provided vice versa.
Fig. 1:
Spatial distribution of visceral leishmaniasis cases by districts in southeastern Iran
Results
Clinical manifestations Human
In general, the clinical manifestations of VL are similar in all endemic areas. Such clinical picture of VL is often indistinguishable from other infectious diseases. In acute cases, there may be an abrupt onset of high fever and chills that often mimics other infectious microbial diseases (28–30). The fever pattern typically shows two peaks per day, often used as a useful tool for clinical diagnosis (31). In chronic course, there is an insidious onset of fever, loss of appetite, weight loss, hepatosplenomegaly, anemia and pancytopenia causing abdominal distention and weakness. In endemic areas, low-grade symptoms may persist for few weeks to several months before progressing to fully developed VL manifestations, because these symptoms are relatively well tolerated and usually are not severe to seek medical attentions. The clinical picture of VL is multifactorial. Despite the complexity, Leishmania biology, genetic nature, host immunology, and physiology are major determinants of clinical outcome (8).
Dogs
Reticuloendothelial system (RES) is the most affected organs, with zoonotic visceral leishmaniasis (ZVL), characterized by an acute or chronic and proliferative inflammatory process. The broad spectrum of clinical syndrome depends on the stage of the infection. Presentations, such as alopecia around the eyes and ears, onychogryphosis, weight loss, hepatosplenomegaly, lymphadenopathy muscular weakness, anemia, renal failure and followed by cachexia are typical manifestations (22, 27).
Diagnostic methods
The diagnosis of VL is complicated by the fact that this disease shares its clinical manifestations with other parasitic and microbial infections.
Parasitological methods
Definitive diagnosis relies on demonstration of the parasites, amastigotes (Leishman bodies) in the bone marrow or in other tissues. In both human and principal reservoir host, dogs and wild canines, spleen puncture is definitely an effective method for detecting infection, but it is a risky task and may result in massive bleeding (3, 32). Liver puncture is much safer but is not productive. In routine medical practice, sternum bone marrow or iliac crest aspiration detects the organisms and is generally considered the method of choice; although, the sensitivity is low. In some occasion, buffy coat films, prepared from peripheral venous blood, could be used for demonstrations of amastigotes in mononuclear phagocyte cells, but with low sensitivity.
Culturing and animal inoculation
Whenever possible, culturing of materials obtained from RES, using biphasic, medium (NNN) and further subculturing into monophasic culture media such as RPMI1640 and maintaining at ambient temperature, 22 °C–26 °C, could be used (3). However, in general, because of low infection rate, the chance of growth is negligible and often inconclusive. Touch smear preparation of spleen spices followed by methanol fixation and Giemsa staining could reveal amastigotes by high dry microscopy. For research purposes, intraperitoneal inoculation of golden hamsters, a standard biological method of choice, might be performed.
Serological methods
A number of serological techniques using different antigens and assays are available. Currently, ELISA, indirect fluorescent antibody test (IFAT), direct agglutination test (DAT) and rK39 (recombinant K39 antigen) are widely used tests and have demonstrated excellent sensitivity and specificity for the diagnosis of VL (17, 33–35).
Molecular methods
For identification and characterization of the causative agent to species and strain level, molecular techniques employing DNA-based methods, more frequently PCR techniques followed by sequencing are routinely used (35, 36).
Vector incrimination
Interestingly, in the main endemic areas in Orzoieh district in the Province of Kerman (south to the Baft district) two Leishmania species coexist; L.major, the cause of zoonotic cutaneous leishmaniasis (ZCL) and L.infantum, the causative agent of kala-azar. Only one study has been performed by sticky traps and aspiratory methods in the closely related localities within the district (37). The predominant sand fly species were Phlebotomus papatasi (33.7%) and P. alexandri (29.8%) during the peak activity of the sand fly species. Based on the results of blood-fed index by ELISA test and using dog and human antisera, they were positive for human blood. At present, the role of P. alexandri as being implicated in the transmission of L. infantum in the area is not well clear. However, due to high index of human blood feeding, it is assumed that this species might be a probable vector for kala-azar in this locality. P. alexandri species has been implicated as principal phlebotomine sand fly vector of kala-azar in some parts of the world including China (38).
Current therapy
For many years, pentavalent antimony drugs particularly meglumine antimoniate (Glucan-time®, Sanofi-Aventis, France) have been used as the first-line drugs to treat human VL nationally and also in Kerman Province (39). The recommended regimen is 50 mg /kg/day of Glucantime® for 3–4 wk. The drug is given intramuscularly (IM). In some instances, amphotericin B has been used in patients with compromised immune system.
Epidemiology
A total of 118 children aged 4.2 yr (range; 0.2–13 yr) comprising 75 boys (63.6%) and 43 girls (36.4%) were diagnosed and confirmed by combination of parasitological, serological and molecular methods as HVL patients between 1994 and 2011 in southeastern Iran (Table 1). The majority of the cases were from Orzoieh district in southwest of Kerman Province (37.1%), followed by Sirjan (15.7%), Jiroft (14.8%), Kahnuj (9.3%), Hajiabad in Hormozgan Province (7.4%), Baft (6.5%), Bam and Kerman (3.7%, each) and Rafsanjan and Bardsir (0.9%, each) (Table 2). In Mediterranean kala-azar, the proportion of male to female is significant mainly due to higher exposure of boys to the source of infection and incomplete dressing.
Table 1:
Reported cases of human visceral leishmaniasis in Kerman Province, southeastern Iran, 1981–2011
| Cases(No) | Mean age (yr) | Reported years | Diagnostic Method | Reference |
|---|---|---|---|---|
| 40 | 4.2(0.5–12) | 1981–1992 | BMA | 32 |
| 68 | 4.3(0.5–13) | 1993–2006 | BMA | 29 |
| 10 | 3.5(0.2–7) | 2007–2011 | BMA and nested PCR | 44 |
BMA; bone marrow aspiration
Table 2:
Distribution of human visceral leishmaniasis cases by district, southeastern Iran
| Orzoieh | Sirjan | Jiroft | Kahnuj | Hajiabad |
| 37.1% | 157% | 14.8% | 9.3% | 74% |
| Baft | Bam | Kerman | Rafsanjani | Bardsir |
| 6.5% | 3.7% | 3.7% | 0.9% | 0.9% |
Various clinical presentations are given in Table 3. As the time passes, infiltration of infected white blood cells particularly mononuclear phagocytes and proliferation of these cells, frequently in RES such as spleen, liver, lymph nodes and bone marrow results in massive enlargement of these organs (29). Bone marrow cells become infected with amastigotes and patients develop pancytopenia (depression of erythrocytes, leukocytes, and platelets) and eventually suppression of immune responses (30), which make kala-azar patients susceptible to microbial super-infections. Therefore, 42.0% of the patients developed secondary bacterial co-infections of the urinary tract, blood respiratory and gastro-intestinal tracts and skin as presented in Table 4. Unfortunately, such underlying infections may complicate the syndrome, produce unusual clinical features (29, 40, 41) and persist for weeks to several months (average 32 d in this review before visiting a physician).
Table 3:
Clinical manifestations accompanying human visceral leishmaniasis, southeastern Iran
| Fever | Anemia | Splenomegaly | Hepatomegaly | Anorexia |
| 100.0% | 95.5% | 91.5% | 77.1% | 47.4% |
| Paleness | Weight loss | Dysentery/Diarrhea | Chills | Hemorrhage |
| 46.2% | 24.4% | 23.1% | 14.7% | 5.9% |
Table 4:
Secondary bacterial coninfections with visceral leishmaniasis in children, southeastern Iran
| Site | Children (%) | Etiology |
|---|---|---|
| Urinary tract | 36 | Escherichia coli |
| Enterobacter spp. | ||
| Klebsiella spp. | ||
| Proteus spp. | ||
| Blood (Septicemia) | 28 | Staphylococcus aureus |
| coagulase-negative | ||
| Pseudomnas spp. | ||
| Respiratory tract | 16 | ND |
| Gastro-intestinal tract | 12 | Shigella spp. |
| E.coli | ||
| Skin | 8 | S. aureus |
ND; not determined. Table 4 is taken from reference 41
Seroprevalence of HVL infection showed different ranges at different time intervals depending upon the tests performed, spatial distribution and selection of samples in southeastern Iran (Table 5). It varied sharply among the districts.
Table 5:
Seroprevalence of human visceral infection in southeastern Iran
| District | Examined No. | Method performed | Seroprevalence No. (%) | Reference |
|---|---|---|---|---|
| Baft 1994 | 1304 | IFAT | 39 (3.0) | 34 |
| Jiroft | 950 | IFAT | 18 (1.9) | 17 |
| 2000–2001 | 950 | ELISA | 171 (18.3) | 17 |
| Orzoieh 2009–2010 | 1476 | DAT | 14 (0.95) | 16 |
Mortality data were considerably sparse and limited generally to hospital-based deaths, only. The overall case-fatality rate was four patients (3.4%) out of 118 cases of children. Most of the patients were poor and majority from rural and nomadic tribes (83.0%) in remote areas of the country, who are not able to seek medical treatment at the earliest time. The majority (88.0%) of the HVL patients were undernourished with moderate to severe malnourishment (29). Some patients, due to passive case detection approaches go underreported and the degree of unnoticed HVL cases in official surveillance data is not clearly known, but it varies country to country. It ranges between 1.3–1.7 fold in Brazil (42) and 4–8 folds in India (43), which are lower than the actual incidence rate found by active case detection strategies.
Evaluation of drug efficacy indicated that most of the cases responded well to meglumine antimoniate (Glucantime®) and seemed to be successful in treatment of patients. However, few cases (1.7%) had relapsed to the initial course of therapy treated with the drug (Glucantime®), consequently. This rate is much less than the range (10%) previously reported from other countries (3, 9). Similarly, in one case, with viscerotropic strains of L. tropica (the cause of anthroponotic cutaneous leishmaniasis), the patient was unresponsive, to Glucantime (44).
Although dogs have been implicated as the major domestic reservoir of L. infantum, two dogs of the total 471 stray dogs were infected with L. tropica, presenting typical manifestations resembling VL affected dogs due to L. infantum (45). Combination of parasitological, molecular, serological and biological methods detected significant numbers of asymptomatic infections and could help properly in discrimination between L. infantum and L. tropica (22). A case of viscerotopic manifestions due to L. tropica was found in a 5 yr old child who was unresponsive to conventional dosages of Glucantime® (44).
Prevalence of CVL infection by different tests is presented in Table 6. Most of the studies were performed from the districts in which HVL cases were reported (21–23, 26, 35, 46). Overall, 733 dogs (domestic and stray) and wild canids (jackals and foxes) were examined by various techniques. CVL infection was officially first reported in 1994 (46). VL was endemic in Orzoieh district and at that time Orzoieh was a part of Baft district, where currently it is segregated in form of separate district.
Table 6:
Seroprevalence and molecular identification of canine visceral infection in southeastern Iran
| District | Animal | Examined (No.) | Seroprevalence No. (%) | Method Performed | Reference |
|---|---|---|---|---|---|
| Orzoieh 1994 | Domestic dogs | 83 | 15(18.0) 12(14.5) | IFAT | 51 |
| Domestic dogs Domestic dogs | 83 | 1(1.2) | ELISA | 51 | |
| 83 | 8(25.0) | BMA | 51 | ||
| Jackals | 32 | 7(22.0) | IFAT | 51 | |
| 32 | 1(9.0) | ELISA | 51 | ||
| Foxes | 11 | 2(18.0) | IFAT | 51 | |
| 11 | ELISA | 51 | |||
| Orzoieh 2009–2010 | Domestic dogs Domestic dogs | 30 | 7(23.0) | DAT & Nested-PCR | 16 |
| 30 | 5(16.7) | 16 | |||
| Orzoieh 2009–2011 | Stray dogs | 32 | 6(18.8) | ELISA | 26 |
| Orzoieh 2013–2014 | Homestead dogs | 59 | 18(30.5) 22(37.3) | IFAT | 23 |
| 59 | Nested-PCR | 23 | |||
| Bam 2009–2011 | Stray dogs | 57 | 13(22.8) | ELISA | 26 |
| Zabol 2009–2011 | Stray dogs | 45 | 7(15.6) | ELISA | 26 |
| Kerman 2009–2011 | Stray dogs | 67 | 5(7.5) | ELISA | 26 |
| Kerman 2011 | Domestic dogs | 128 | 9(7.0) | ELISA | 21 |
| Kerman 2009–2010 | Stray dogs | 80 | 9(11.3) | DAT | 22 |
| 9 | 2(22.2)* | PCR | 22 | ||
| Jiroft 2013–2014 | Domestic dogs | 100 | 14(14.0) | ELISA | 27 |
| 18 | 17(94.4) | Nested-PCR | 27 |
IFAT; indirect fluorescent antibody test, ELISA; enzyme-linked immunoabsorbent assay, BMA; bone marrow aspiration; DAT; direct agglutination test.
Of two VL dogs, one was coinfected with L. tropica.
Various seroprevalence ranges were reported in dogs, depending on the location and type of the test performed (21–22, 26, 27, 46). While the seroprevalence was the lowest (7.0%) by ELISA in Kerman district, it was the highest by IFAT or nested – PCR in Orzoieh district. In general, there was no significant difference between male and female dogs, while the VL infection was significantly higher in older age group than younger ones. In addition to dogs, as principal and peridomestic reservoir of VL, a wide range of wild canids such as Jackals and foxes are found near human habitations (25, 46).
Discussions
The clinical features of HVL in this location were considerably similar to those patients from other parts of the country and even the world (3, 40). Overall, 3.4% died due to poverty, undernourishment, seeking late medical treatment and secondary bacterial superinfections. The death rate was significantly lower than the tentative estimates of HVL deaths in global range (3). In rare cases viscerotopic L. tropica might cause VL, presenting typical manifestations. These cases are usually unresponsive to conventional therapy by Glucantime. Physicians should be aware of such clinical presentation in order to manage the patient properly. Similar viscerotropic syndrome due to L. tropica was reported among American troops who participated in Operation Desert Storm (47).
Dogs are the main reservoir host for dissemination of infection to human. In principal reservoir, canines and less well-adapted hosts including humans, VL can produce a wide range of manifestations including asymptomatic condition to very serious visceral form. Asymptomatic dogs and humans could constitute a major source of infection for the vector and in turn transmission to humans (31, 48–51), that is why dogs represent a significant public health threat to humans. A high proportion of seropositive dogs (over 50%) is asymptomatic and may propagate infection with no clinical manifestations for several years or even throughout life (25, 50). Moreover, such asymptomatic dogs are as infective to the vector as symptomatic ones (52). Transmission of VL infection among wildlife, domestic animals, and human is of great concern to public health. They can harbor the infection and serve as secondary potential reservoirs for transmission of the disease (53).
Conclusion
Clinical and epidemiological data on the spatial distribution and prevalence of HVL and CVL are essential for planning and implementing appropriate control strategies.
VL in Orzoieh district is endemic, while it is sporadic to some extent in other districts in southeastern Iran.
Early detection of HVL cases, prompt treatment and integrated vector management by active and passive case detection approaches through an effective health surveillance system should receive high priority.
Since stray and feral dogs pose serious human and welfare problems, veterinary services should play a leading role in dog population control; to improve health and welfare of owned and stray dog population and to reduce numbers of stray dogs to an acceptable level.
A combination of applicable parasitological, serological and molecular methods are curtailed for the detection of the extent of infection in human and dogs in endemic areas, particularly those with latent and/or apparent manifestations in order to understand their role in epidemiology of ZVL.
Detection and surveillance of VL status in immunocompromised patients should receive special attention in future planning.
Acknowledgments
The authors declare that there is no conflict of interests.
References
- 1. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004; 27 (5): 305– 18. [DOI] [PubMed] [Google Scholar]
- 2. Postigo JA. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int J Antimicrob Agents. 2010; 36 (Suppl 1): S62– 5. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO) ( 2010). Control of the leishmaniases. Report of a meeting of the WHO Ex-pert Committee on the Control of Leishmaniases, WHO Technical Report Series 949, Geneva, pp 1 –187. [Google Scholar]
- 4. Alvar J, Velez ID, Bern C, Herrero M, Desjeux Ph, Cano J, Jannin J, den Boer M. WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012; 7 (5): e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006; 22 (12): 552– 7. [DOI] [PubMed] [Google Scholar]
- 6. Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007; 5(11): 873– 82. [DOI] [PubMed] [Google Scholar]
- 7. Desjeux P. The increase of risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001; 95 (3): 239– 43. [DOI] [PubMed] [Google Scholar]
- 8. Bañuls AL, Hide M, Prugnolle F. Leishmania and the leishmaniases: a parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans. Adv Parasitol. 2007; 64: 1– 109. [DOI] [PubMed] [Google Scholar]
- 9. Ready Paul D. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014; 6: 147– 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bokai S, Mobedi I, Edrissian GH, Nadim A. Seroepidemiological study of canine visceral leishmaniasis in Meshkin Shahr, northwest of Iran. Arch Inst Razi. 1998; 41 (6): 48– 9. [Google Scholar]
- 11. Edrissian GhH, Nadim A, Alborzi AV, Ardehali S. Visceral leishmaniasis: the Iranian experiences. Arch Iran Med. 1998; 1: 22– 6. [Google Scholar]
- 12. Fakhar M, Rahmati B, Gohardehi S, Mohebali M, Akhoundi B, Sharif M, Ali Mahdavi S. Molecular and seroepidemiological survey of visceral leishmaniasis among humans and domestic dogs in Mazandaran Province, north of Iran. Iran J Parasitol. 2011; 6 (4): 51– 59. [PMC free article] [PubMed] [Google Scholar]
- 13. Fakhar M, Asadi Kia A, Gohardehi S, Sharif M, Mohebali M, Akhoundi B, Pagheh A, Dadimoghadam Y, Cheraghali F. Emergence of a new focus of visceral leishmaniasis due to Leishmania infantum in Golestan Province, northeastern of Iran. J Parasit Dis. 2014. :38 (3); 255 –9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohebali M, Edrissian GH, Shirzadi MR, et al. An observational study on the current distribution of visceral leishmaniasis in different geographical zones of Iran and implication to health policy. Travel Med Infect Dis. 2011; 9 (2): 67– 74. [DOI] [PubMed] [Google Scholar]
- 15. Mohebali M. Visceral leishmaniasis in Iran: review of the epidemiological and clinical features. Iran J Parasitol. 2013; 8 (3): 348– 58. [PMC free article] [PubMed] [Google Scholar]
- 16. Mahmoudvand H, Mohebali M, Sharifi I, Keshavarz H, Hajjaran H, Akhoundi B, Jahanbakhsh S, Zarean M, Javadi A. Epidemiological aspects of visceral leishmaniasis in Baft district, Kerman province, Southeast of Iran. Iran J Parasitol. 2011; 6 (1): 1– 11. [PMC free article] [PubMed] [Google Scholar]
- 17. Mohammadi M, Sharifi I, Keshavarz H. Seroepidemiology of visceral leishmaniasis in children under 12 years of age in nomads of Jiroft district using indirect immunoabsorbent assay, 2001. 4th National Iranian Congress of Parasitology and Parasitic Diseases Oct 13–16, 2003 Mashhad, Iran. [Google Scholar]
- 18. Rakhshanpour A, Mohebali M, Akhondi B, Rahimi MT, Rokni MB. Serological survey and associated risk factors of visceral leishmaniasis in Qom Province, Central Iran. Iran J Public Health. 2014; 43 (1): 50– 55. [PMC free article] [PubMed] [Google Scholar]
- 19. Sarkari B, Pedram N, Mohebali M, Moshfe AA, Zargar MA, Akhoundi B, Shirzadi MR. Seroepidemiological study of visceral leishmaniasis in Booyerahmad district, south-west Islamic Republic of Iran. East Mediterr Health J. 2010; 16 (11): 1133– 6. [PubMed] [Google Scholar]
- 20. Desjeux P, Ghosh RS, Dhalaria P, Strub-Wourgaft N, Zijlstra EE. Report of the post kala-azar dermal leishmaniasis (PKDL), Consortium Meeting, New Delhi, India, 27–29 June 2012. Parasit Vectors. 2013; 6: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aflatoonian MR, Akhtardanesh B, Sharifi I, Mostafavi M, Aflatoonian B, Khalili M, Ghanbarpour R, Baniaasadi M. Seroepidemiology of canine visceral leishmaniasis in Kerman city, 2011. J Kerman Univ Med Sci. 2012; 19 (6): 531– 539. [Google Scholar]
- 22. Bamorovat M, Sharifi I, Mohammadi MA, et al. Canine visceral leishmaniasis in Kerman, southeast of Iran: a seroepidemiological, histo-pathological and molecular study. Iran J Parasitol. 2014; 9 (3): 342– 9. [PMC free article] [PubMed] [Google Scholar]
- 23. Kamiabi IT, Sharifi I, Daneshvar H, Mohammodi Z. Comparative study for diagnosis of canine visceral leishmaniasis using IFA and nested PCR in the endemic area of Iran. Proceedings of 2nd International and 9th National Congress of Parasitology and Parasitic Diseases of Iran (NICOPA9) Iran J Parasitol, 2015. ; 10 (1 ): 192. [Google Scholar]
- 24. Gavgani AS, Mohite H, Edrissian GH, Mohebali M, Davies CR. Domestic dog ownership in Iran is a risk factor for human infection with Leishmania infantum. Am J Trop Med Hyg. 2002; 67 (5): 511– 5. [DOI] [PubMed] [Google Scholar]
- 25. Mohebali M, Hajjaran H, Hamzavi Y, Mobedi I, Arshi S, Zarei Z, Akhoundi B, Naeini KM, Avizeh R, Fakhar M. Epidemiological aspects of canine visceral leishmaniasis in the Islamic Republic of Iran. Vet Parasitol. 2005; 129 (3–4): 243– 251. [DOI] [PubMed] [Google Scholar]
- 26. Mahshid M, Baharak A, Iraj S, Sina K, Javad K, Mehdi B. Seroprevalence of canine visceral leishmaniasis in southeast of Iran. J Parasit Dis. 2014; 38 (2): 218– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naderi A, Sharifi I, Babaei Z, Dabiri S, Mashayekhi J, Bamorovat M, Mostafavi M. Epidemiology and clinical manifestations of visceral leishmaniasis and species identity in domestic digs using ELISA, nested-PCR and histopatological techniques in southeastern districts of Kerman province, Iran in 2013. Proceedings of 2nd International and 9th National Congress of Parasitology and Parasitic Diseases of Iran (NICOPA9) Iran J Parasitol. 2015; 10 (1): 178. [Google Scholar]
- 28. Andrade TM., Carvalho EM, Rocha H. Bacterial infections in patients with visceral leishmaniasis. J Infect Dis. 1990; 162 (6): 1354– 9. [DOI] [PubMed] [Google Scholar]
- 29. Barati M, Daie Parizi HM, Sharifi I. Epidemiological and clinical aspects of kala-azar in hospitalized children of Kerman province, during 1991–2006. J Kerman Univ Med Sci. 2008; 15 (2): 148– 155. [Google Scholar]
- 30. McGwire BS, Satoskar AR. Leishmaniasis: clinical syndromes and treatment. QJM. 2014; 107 (1): 7– 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riera C, Fisa R, Udina M, Gállego M, Portus M. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic Islands, Spain) by different diagnostic methods. Trans R Soc Trop Med Hyg. 2004; 98 (2): 102– 10. [DOI] [PubMed] [Google Scholar]
- 32. Niknafs P, Daie Parizi M, Ahmadi A. Report of 40 cases of Kala-azar from Kerman province. J Kerman Univ of Med Sci. 1994; 1: 30– 37. [Google Scholar]
- 33. Harith A el, Slappendel RJ, Reiter I, van Knapen F, de Korte P, Huigen E, Kolk AH. Application of a direct agglutination test for detection of specific anti-Leishmania antibodies in the canine reservoir. J Clin Microbiol. 1989; 27 (10): 2252– 2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keshavarz H, Zangiabadi M, Mamishi S. Epidemiology of visceral leishmaniasis in children under 12 years old in Baft district by indirect immunofluorescent antibody test (IFAT), 1994. 2nd National Congress of Parasitic Diseases Oct 19–22, 1997 Tehran, Iran , P88. [Google Scholar]
- 35. Mohammadiha A, Haghighi A, Mohebali M, et al. Canine visceral leishmaniasis: a comparative study of real-time PCR, conventional PCR, and direct agglutination on sera for the detection of Leishmania infantum infection. Vet Parasitol. 2013; 192 (1–3): 83– 90. [DOI] [PubMed] [Google Scholar]
- 36. Solano-Gallego L, Morell P, Arboix M, Alberola J, Ferrer L. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J Clin Microbiol. 2001; 39 (2): 560– 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aghaei Afshar A, Rasi Y, Ebaei MR, Aghaei Afshar M. Determination of fauna and monthly activity of sandflies in the south of Baft district, Kerman province in 2004. J Kerman Univ of Med Sci. 2005; 12 (2): 136– 41. [Google Scholar]
- 38. Guan LR, Xu YX, Li BS, Dong J. The role of Phlebotomus alexandri Sinton, 1928 in the transmission of kala-azar. Bull World Health Organ. 1986; 64 (1): 107– 112. [PMC free article] [PubMed] [Google Scholar]
- 39. Freitas-Junior LH, Chatelain E, Kim HA, Siqueira-Neto JL. Visceral leishmaniasis treatment: what do we have, what do we need and how to deliver it? Int J Parasitol Drugs Drug Resist. 2012; 2: 11– 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tofighi Naeem A, Mahmoudi S, Sabouni F, Hajjaran H, Pourakbari B, Mohebali M, Zarkesh MR, Mamishi S. Clinical features and laboratory findings of visceral leishmaniasis in children referred to Children Medical Center Hospital, Tehran, Iran during 2004–2011. Iran J Parasitol. 2014; 9 (1): 1– 5. [PMC free article] [PubMed] [Google Scholar]
- 41. Barati M, Sharifi I, Daie Parizi M, Fasihi Harandi M. Bacterial infections in children with visceral leishmaniasis: observations made in Kerman province, southern Iran, between 1997 and 2007. Ann Trop Med Parasitol. 2008; 102 (7): 635– 41. [DOI] [PubMed] [Google Scholar]
- 42. Maia-Elkhoury AN, Carmo EH, Sousa-Gomes ML, Mota E. Analysis of visceral leishmaniasis reports by the capture-recapture method. Rev Saude Publica. 2007; 41 (6): 931– 7. [DOI] [PubMed] [Google Scholar]
- 43. Singh VP, Ranjan A, Topno RK, et al. Estimation of under-reporting of visceral leishmaniasis cases in Bihar, India. Am J Trop Med Hyg. 2010; 82 (1): 9– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hosseininasab A, Sharifi I, Daei MH, et al. Causes of pediatric visceral leishmaniasis in southeastern Iran. Iran J Parasitol. 2014; 9 (4): 584– 587. [PMC free article] [PubMed] [Google Scholar]
- 45. Bamorovat M, Sharifi I, Dabiri SH, et al. Leishmania tropica in stray dogs in southeast Iran. Iran J Public Health. 2015; 44 (10): 1359– 1366. [PMC free article] [PubMed] [Google Scholar]
- 46. Sharifi I, Daneshvar H. The prevalence of visceral leishmaniasis in sus-pected canine reservoirs in southeastern Iran. Iran J Med Sci. 1998; 21 (3): 130– 4. [Google Scholar]
- 47. Magill AJ, Grogl M, Gasser RA, Jr, Sun W, Oster CN. Visceral infection caused by Leishmania tropica in veterans of Operation Desert Storm. N Engl J Med. 1993; 328 (19): 1383– 7. [DOI] [PubMed] [Google Scholar]
- 48. Costa CH, Stewar JM, Gomes RB, Garcez LM, Ramos PK, et al. Asymptomatic carriers of Leishmania chagasi. Am J Trop Med Hyg. 2002; 66 (4): 334– 7. [DOI] [PubMed] [Google Scholar]
- 49. Fakhar M, Motazedian MH, Hatam GR, Asgari Q, Kalantari M, Mohebali M. Asymptomatic human carriers of Leishmania infantum: possible reservoirs for Mediterranean visceral leishmaniasis in southern Iran. Ann Trop Med Parasitol. 2008; 102 (7): 577– 83. [DOI] [PubMed] [Google Scholar]
- 50. Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002; 18(9: 399– 405. [DOI] [PubMed] [Google Scholar]
- 51. Moshfe A, Mohebali M, Edrissian G, Zarei Z, Akhoundi B, Kazemi B, Jamshidi S, Mahmoodi M. Canine visceral leishmaniasis: asymptomatic infected dogs as a source of L. infantum infection. Acta Trop. 2009; 112 (2): 101– 5. [DOI] [PubMed] [Google Scholar]
- 52. Molina R, Amela C, Nieto J, San-Andrés M, González F, Castillo JA, Lucientes J, Alvar J. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg. 1994; 88 (4): 491– 3. [DOI] [PubMed] [Google Scholar]
- 53. Millán J, Ferroglio E, Solano-Gallego L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol Res. 2014; 113 (6): 2005– 14. [DOI] [PubMed] [Google Scholar]